Abstract
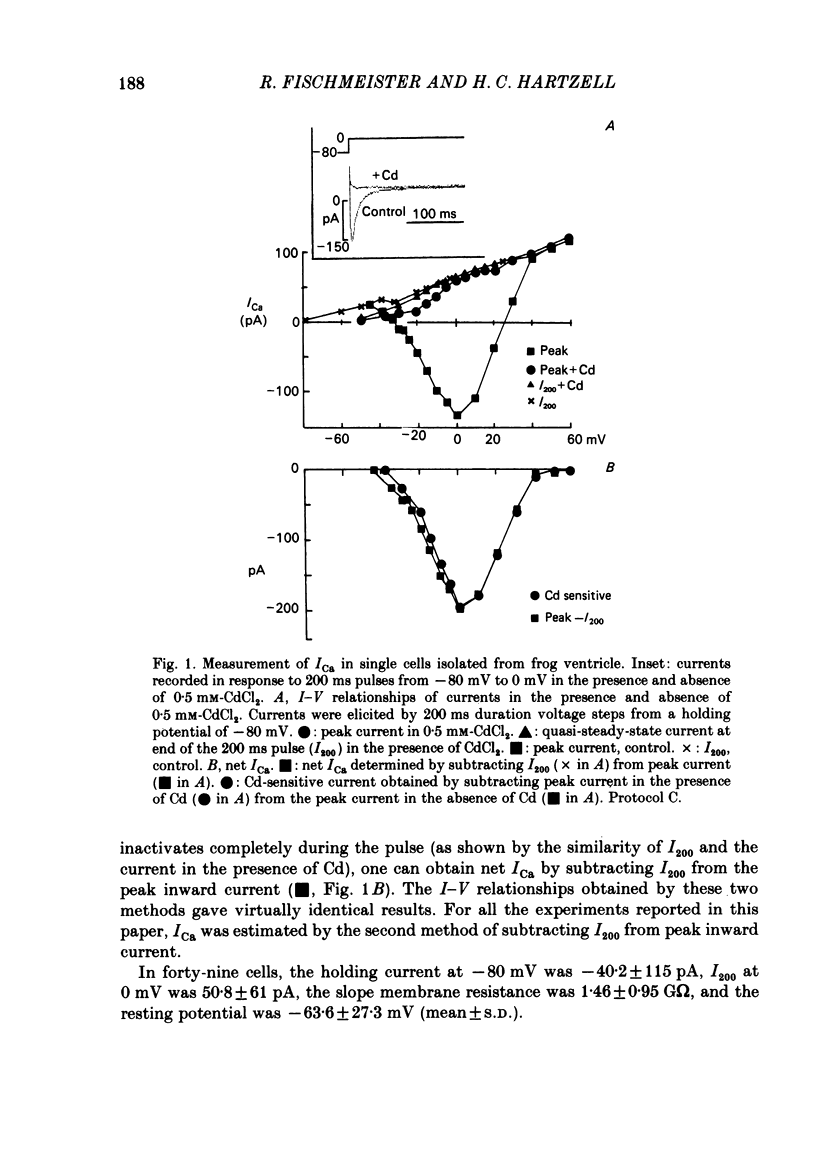
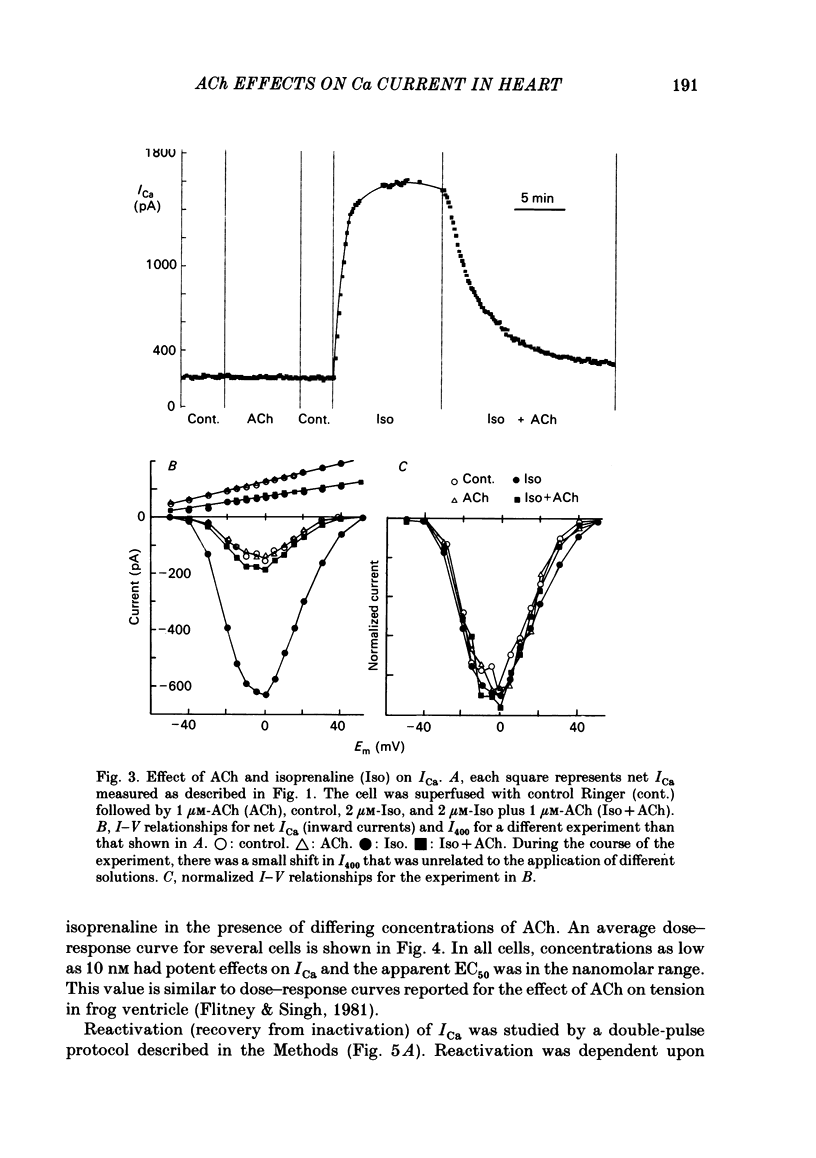
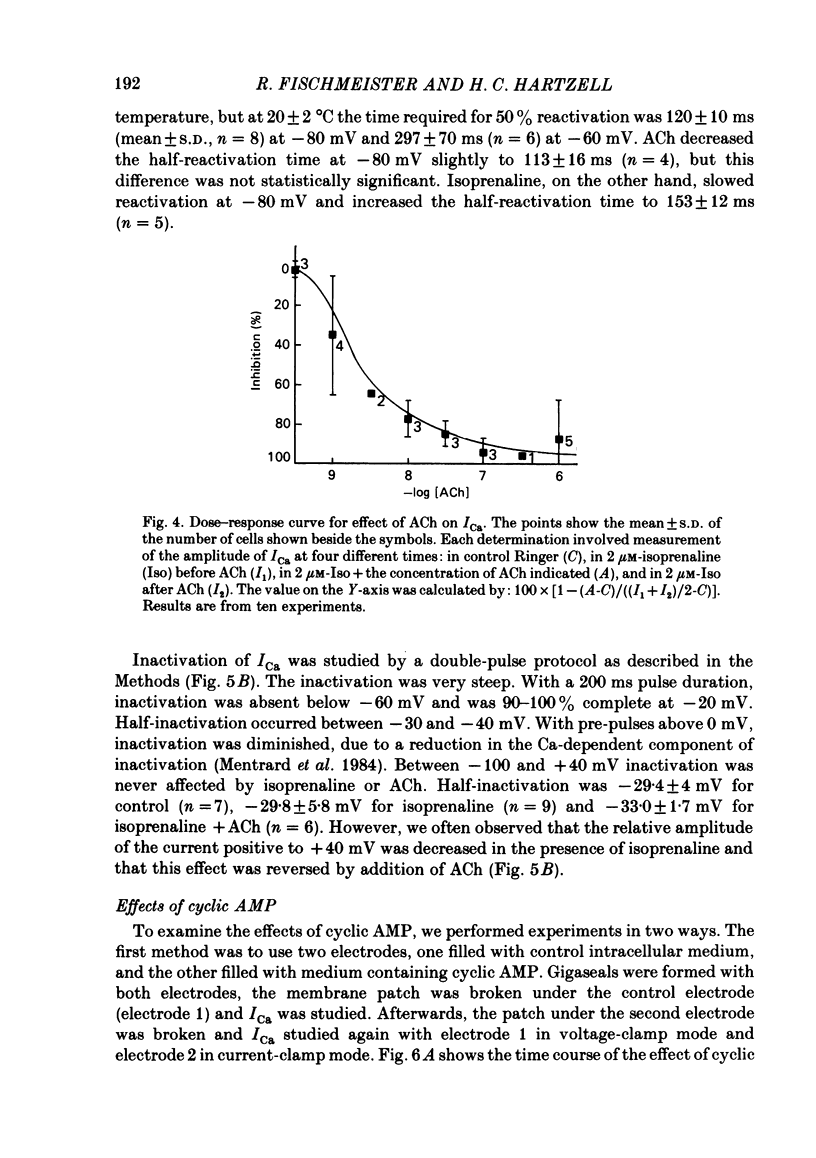
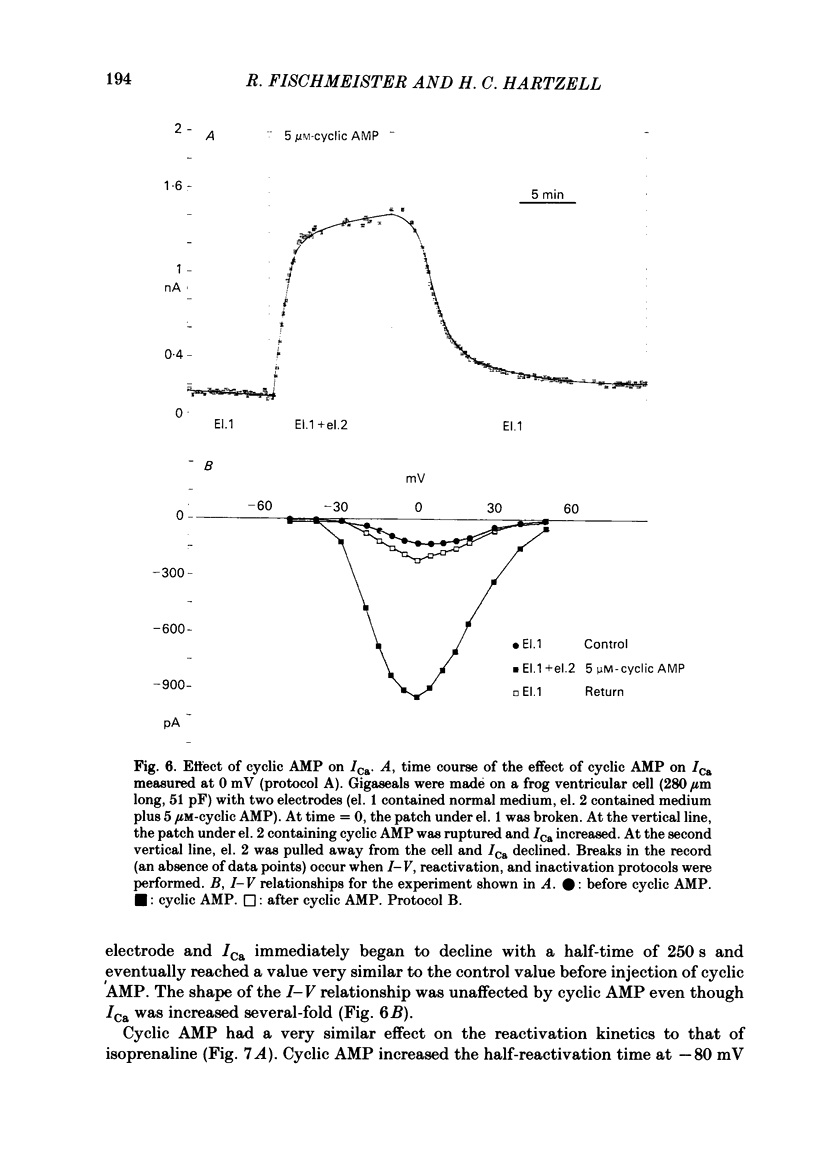
Ca currents (ICa) were measured by whole-cell patch clamp in single cells isolated from frog ventricle in which K currents were blocked with intracellular (120 mM) and extracellular (20 mM) Cs. Inward currents elicited by depolarizing voltage steps from a holding potential of -80 mV were blocked completely by 0.5 mM-Cd. The quality of the voltage clamp was assessed using two patch electrodes on a single cell. One electrode was used in the voltage-clamp mode to measure membrane currents and the other in current-clamp to measure membrane potential. Ca currents as large as 2 nA were well clamped in cells as long as 210 micron. Acetylcholine (ACh) had no effect on ICa in the absence of beta-adrenergic stimulation but reduced to control levels ICa elevated by isoprenaline. Nanomolar concentrations of ACh were able to reduce significantly ICa elevated by 2 microM-isoprenaline. ACh had no effect on the shape of the I-V curve, on the reactivation (recovery from inactivation), or on the inactivation of ICa. Although isoprenaline increased ICa by an average of 6.5-fold, it had no effect on the shape of the I-V curve or on the inactivation at test potentials negative to +40 mV. However, isoprenaline slowed the half-reactivation time from a control value of 120 +/- 10 ms (mean +/- S.D.) to 153 +/- 12 ms at -80 mV. The effect of cyclic AMP on ICa was investigated using two patch electrodes, one filled with cyclic AMP. Maximal effects of cyclic AMP were observed with 5 microM-cyclic AMP in the pipette. Maximal ICa was recorded several minutes after breaking the patch with the second electrode. After removing the cyclic-AMP-containing electrode, ICa declined to control levels after approximately 10 min. 5 microM-cyclic AMP in the patch electrode increased ICa by an average of 6.9-fold, but had no effect on the shape of the I-V curve or on inactivation. Cyclic AMP had a slowing effect on reactivation (half-reactivation time = 155 +/- 24 ms) similar to that of isoprenaline. ACh (1-10 microM) did not reduce ICa elevated with cyclic AMP (0.1-20 microM-cyclic AMP in the pipette). With low concentrations of cyclic AMP in the pipette (0.1 microM), isoprenaline augmented ICa, but with 5 microM-cyclic AMP in the pipette, isoprenaline was incapable of increasing ICa further. These results suggest that the decrease of ICa produced by ACh can be explained solely by decreases in cyclic AMP levels.
Full text
PDF














Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antoni H., Rotmann M. Zum Mechanismus der negative inotropen Acetylcholin-Wirkung auf das isolierte Froschmyokard. Pflugers Arch Gesamte Physiol Menschen Tiere. 1968;300(2):67–86. [PubMed] [Google Scholar]
- Bean B. P., Nowycky M. C., Tsien R. W. Beta-adrenergic modulation of calcium channels in frog ventricular heart cells. 1984 Jan 26-Feb 1Nature. 307(5949):371–375. doi: 10.1038/307371a0. [DOI] [PubMed] [Google Scholar]
- Bean B. P. Two kinds of calcium channels in canine atrial cells. Differences in kinetics, selectivity, and pharmacology. J Gen Physiol. 1985 Jul;86(1):1–30. doi: 10.1085/jgp.86.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biegon R. L., Epstein P. M., Pappano A. J. Muscarinic antagonism of the effects of phosphodiesterase inhibitor (methylisobutylxanthine) in embryonic chick ventricle. J Pharmacol Exp Ther. 1980 Nov;215(2):348–356. [PubMed] [Google Scholar]
- Biegon R. L., Pappano A. J. Dual mechanism for inhibition of calcium-dependent action potentials by acetylcholine in avian ventricular muscle. Relationship to cyclic AMP. Circ Res. 1980 Mar;46(3):353–362. doi: 10.1161/01.res.46.3.353. [DOI] [PubMed] [Google Scholar]
- Bolton T. B. Intramural nerves in the ventricular myocardium of the domestic fowl and other animals. Br J Pharmacol Chemother. 1967 Oct;31(2):253–268. doi: 10.1111/j.1476-5381.1967.tb01996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown H. F., McNaughton P. A., Noble D., Noble S. J. Adrenergic control of cardian pacemaker currents. Philos Trans R Soc Lond B Biol Sci. 1975 Jun 10;270(908):527–537. doi: 10.1098/rstb.1975.0029. [DOI] [PubMed] [Google Scholar]
- Brown J. H. Cholinergic inhibition of catecholamine-stimulable cyclic AMP accumulation in murine atria. J Cyclic Nucleotide Res. 1979 Dec;5(6):423–433. [PubMed] [Google Scholar]
- Brum G., Flockerzi V., Hofmann F., Osterrieder W., Trautwein W. Injection of catalytic subunit of cAMP-dependent protein kinase into isolated cardiac myocytes. Pflugers Arch. 1983 Jul;398(2):147–154. doi: 10.1007/BF00581064. [DOI] [PubMed] [Google Scholar]
- Brum G., Osterrieder W., Trautwein W. Beta-adrenergic increase in the calcium conductance of cardiac myocytes studied with the patch clamp. Pflugers Arch. 1984 Jun;401(2):111–118. doi: 10.1007/BF00583870. [DOI] [PubMed] [Google Scholar]
- Cachelin A. B., de Peyer J. E., Kokubun S., Reuter H. Ca2+ channel modulation by 8-bromocyclic AMP in cultured heart cells. Nature. 1983 Aug 4;304(5925):462–464. doi: 10.1038/304462a0. [DOI] [PubMed] [Google Scholar]
- Fields J. Z., Roeske W. R., Morkin E., Yamamura H. I. Cardiac muscarinic cholinergic receptors. Biochemical identification and characterization. J Biol Chem. 1978 May 10;253(9):3251–3258. [PubMed] [Google Scholar]
- Fischmeister R., Brocas-Randolph M., Lechêne P., Argibay J. A., Vassort G. A dual effect of cardiac glycosides on Ca current in single cells of frog heart. Pflugers Arch. 1986 Mar;406(3):340–342. doi: 10.1007/BF00640925. [DOI] [PubMed] [Google Scholar]
- Flitney F. W., Singh J. Evidence that cyclic GMP may regulate cyclic AMP metabolism in the isolated frog ventricle. J Mol Cell Cardiol. 1981 Nov;13(11):963–979. doi: 10.1016/0022-2828(81)90472-7. [DOI] [PubMed] [Google Scholar]
- Galper J. B., Klein W., Catterall W. A. Muscarinic acetylcholine receptors in developing chick heart. J Biol Chem. 1977 Dec 10;252(23):8692–8699. [PubMed] [Google Scholar]
- Garnier D., Nargeot J., Ojeda C., Rougier O. The action of acetylcholine on background conductance in frog atrial trabeculae. J Physiol. 1978 Jan;274:381–396. doi: 10.1113/jphysiol.1978.sp012154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles W., Noble S. J. Changes in membrane currents in bullfrog atrium produced by acetylcholine. J Physiol. 1976 Sep;261(1):103–123. doi: 10.1113/jphysiol.1976.sp011550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillo M. A. Electron microscopy of sympathetic tissues. Pharmacol Rev. 1966 Mar;18(1):387–399. [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hartzell H. C. Distribution of muscarinic acetylcholine receptors and presynaptic nerve terminals in amphibian heart. J Cell Biol. 1980 Jul;86(1):6–20. doi: 10.1083/jcb.86.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzell H. C., Titus L. Effects of cholinergic and adrenergic agonists on phosphorylation of a 165,000-dalton myofibrillar protein in intact cardiac muscle. J Biol Chem. 1982 Feb 25;257(4):2111–2120. [PubMed] [Google Scholar]
- Hino N., Ochi R. Effect of acetylcholine on membrane currents in guinea-pig papillary muscle. J Physiol. 1980 Oct;307:183–197. doi: 10.1113/jphysiol.1980.sp013430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima T., Irisawa H., Kameyama M. Membrane currents and their modification by acetylcholine in isolated single atrial cells of the guinea-pig. J Physiol. 1985 Feb;359:485–501. doi: 10.1113/jphysiol.1985.sp015598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto Y., Goto M. Effects of ACh on slow inward current and tension components of the bullfrog atrium. J Mol Cell Cardiol. 1977 Apr;9(4):313–326. doi: 10.1016/s0022-2828(77)80037-0. [DOI] [PubMed] [Google Scholar]
- Ingebretsen C. G. Interaction between alpha and beta adrenergic receptors and cholinergic receptors in isolated perfused rat heart: effects on cAMP-protein kinase and phosphorylase. J Cyclic Nucleotide Res. 1980;6(2):121–132. [PubMed] [Google Scholar]
- Inoue D., Hachisu M., Pappano A. J. Acetylcholine increases resting membrane potassium conductance in atrial but not in ventricular muscle during muscarinic inhibition of Ca++-dependent action potentials in chick heart. Circ Res. 1983 Aug;53(2):158–167. doi: 10.1161/01.res.53.2.158. [DOI] [PubMed] [Google Scholar]
- Inui J., Imamura H. Effects of acetylcholine on calcium-dependent electrical and mechanical responses in the guinea-pig papillary muscle partially depolarized by potassium. Naunyn Schmiedebergs Arch Pharmacol. 1977 Aug;299(1):1–7. doi: 10.1007/BF00508630. [DOI] [PubMed] [Google Scholar]
- Irisawa H., Kokubun S. Modulation by intracellular ATP and cyclic AMP of the slow inward current in isolated single ventricular cells of the guinea-pig. J Physiol. 1983 May;338:321–337. doi: 10.1113/jphysiol.1983.sp014675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobs K. H., Aktories K., Schultz G. GTP-dependent inhibition of cardiac adenylate cyclase by muscarinic cholinergic agonists. Naunyn Schmiedebergs Arch Pharmacol. 1979 Dec;310(2):113–119. doi: 10.1007/BF00500275. [DOI] [PubMed] [Google Scholar]
- Josephson I., Sperelakis N. On the ionic mechanism underlying adrenergic-cholinergic antagonism in ventricular muscle. J Gen Physiol. 1982 Jan;79(1):69–86. doi: 10.1085/jgp.79.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kameyama M., Hofmann F., Trautwein W. On the mechanism of beta-adrenergic regulation of the Ca channel in the guinea-pig heart. Pflugers Arch. 1985 Oct;405(3):285–293. doi: 10.1007/BF00582573. [DOI] [PubMed] [Google Scholar]
- Kass R. S., Wiegers S. E. The ionic basis of concentration-related effects of noradrenaline on the action potential of calf cardiac purkinje fibres. J Physiol. 1982 Jan;322:541–558. doi: 10.1113/jphysiol.1982.sp014054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keely S. L., Jr, Lincoln T. M., Corbin J. D. Interaction of acetylcholine and epinephrine on heart cyclic AMP-dependent protein kinase. Am J Physiol. 1978 Apr;234(4):H432–H438. doi: 10.1152/ajpheart.1978.234.4.H432. [DOI] [PubMed] [Google Scholar]
- Linden J., Brooker G. The questionable role of cyclic guanosine 3':5'-monophosphate in heart. Biochem Pharmacol. 1979 Dec 1;28(23):3351–3360. doi: 10.1016/0006-2952(79)90072-8. [DOI] [PubMed] [Google Scholar]
- Löffelholz K., Pappano A. J. The parasympathetic neuroeffector junction of the heart. Pharmacol Rev. 1985 Mar;37(1):1–24. [PubMed] [Google Scholar]
- MURAD F., CHI Y. M., RALL T. W., SUTHERLAND E. W. Adenyl cyclase. III. The effect of catecholamines and choline esters on the formation of adenosine 3',5'-phosphate by preparations from cardiac muscle and liver. J Biol Chem. 1962 Apr;237:1233–1238. [PubMed] [Google Scholar]
- Manalan A. S., Jones L. R. Characterization of the intrinsic cAMP-dependent protein kinase activity and endogenous substrates in highly purified cardiac sarcolemmal vesicles. J Biol Chem. 1982 Sep 10;257(17):10052–10062. [PubMed] [Google Scholar]
- McAfee D. A., Whiting G. J., Siegel B. Neurotransmitter and cyclic nucleotide modulation of frog cardiac contractility. J Mol Cell Cardiol. 1978 Aug;10(8):705–716. doi: 10.1016/0022-2828(78)90405-4. [DOI] [PubMed] [Google Scholar]
- Mentrard D., Vassort G., Fischmeister R. Calcium-mediated inactivation of the calcium conductance in cesium-loaded frog heart cells. J Gen Physiol. 1984 Jan;83(1):105–131. doi: 10.1085/jgp.83.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morad M., Sanders C., Weiss J. The inotropic actions of adrenaline on frog ventricular muscle: relaxing versus potentiating effects. J Physiol. 1981 Feb;311:585–604. doi: 10.1113/jphysiol.1981.sp013606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nargeot J., Garnier D. The action of muscarinic agonists and antagonists on frog atrial fibers. Electrophysiological studies. J Pharmacol. 1982 Jul-Sep;13(3):431–445. [PubMed] [Google Scholar]
- Nargeot J. Mécanisme d'action des neuromédiateurs sympathique et parasympathique au niveau de la cellule cardiaque: utilisation de molécules photosensibles. J Pharmacol. 1985;16 (Suppl 1):89–118. [PubMed] [Google Scholar]
- Nargeot J., Nerbonne J. M., Engels J., Lester H. A. Time course of the increase in the myocardial slow inward current after a photochemically generated concentration jump of intracellular cAMP. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2395–2399. doi: 10.1073/pnas.80.8.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B., Hess P., Lansman J. B., Tsien R. W. A novel type of cardiac calcium channel in ventricular cells. Nature. 1985 Aug 1;316(6027):443–446. doi: 10.1038/316443a0. [DOI] [PubMed] [Google Scholar]
- Noma A., Kotake H., Irisawa H. Slow inward current and its role mediating the chronotropic effect of epinephrine in the rabbit sinoatrial node. Pflugers Arch. 1980 Oct;388(1):1–9. doi: 10.1007/BF00582621. [DOI] [PubMed] [Google Scholar]
- Noma A., Trautwein W. Relaxation of the ACh-induced potassium current in the rabbit sinoatrial node cell. Pflugers Arch. 1978 Nov 30;377(3):193–200. doi: 10.1007/BF00584272. [DOI] [PubMed] [Google Scholar]
- Osterrieder W., Brum G., Hescheler J., Trautwein W., Flockerzi V., Hofmann F. Injection of subunits of cyclic AMP-dependent protein kinase into cardiac myocytes modulates Ca2+ current. Nature. 1982 Aug 5;298(5874):576–578. doi: 10.1038/298576a0. [DOI] [PubMed] [Google Scholar]
- Pappano A. J., Carmeliet E. E. Epinephrine and the pacemaking mechanism at plateau potentials in sheep cardiac Purkinje fibers. Pflugers Arch. 1979 Oct;382(1):17–26. doi: 10.1007/BF00585899. [DOI] [PubMed] [Google Scholar]
- Pappano A. J., Hartigan P. M., Coutu M. D. Acetylcholine inhibits positive inotropic effect of cholera toxin in ventricular muscle. Am J Physiol. 1982 Sep;243(3):H434–H441. doi: 10.1152/ajpheart.1982.243.3.H434. [DOI] [PubMed] [Google Scholar]
- Prokopczuk A., Lewartowski B., Czarnecka M. On the cellular mechanism of the inotropic action of acetylcholine on isolated rabbit and dog atria. Pflugers Arch. 1973;339(4):305–316. doi: 10.1007/BF00594166. [DOI] [PubMed] [Google Scholar]
- Reuter H. Calcium channel modulation by neurotransmitters, enzymes and drugs. Nature. 1983 Feb 17;301(5901):569–574. doi: 10.1038/301569a0. [DOI] [PubMed] [Google Scholar]
- Reuter H. Localization of beta adrenergic receptors, and effects of noradrenaline and cyclic nucleotides on action potentials, ionic currents and tension in mammalian cardiac muscle. J Physiol. 1974 Oct;242(2):429–451. doi: 10.1113/jphysiol.1974.sp010716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter H., Scholz H. The regulation of the calcium conductance of cardiac muscle by adrenaline. J Physiol. 1977 Jan;264(1):49–62. doi: 10.1113/jphysiol.1977.sp011657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard S., Nerbonne J. M., Nargeot J., Lester H. A., Garnier D. Photochemically produced intracellular concentration jumps of cAMP mimic the effects of catecholamines on excitation-contraction coupling in frog atrial fibers. Pflugers Arch. 1985 Mar;403(3):312–317. doi: 10.1007/BF00583606. [DOI] [PubMed] [Google Scholar]
- Rinaldi M. L., Capony J. P., Demaille J. G. The cyclic AMP-dependent modulation of cardiac sarcolemmal slow calcium channels. J Mol Cell Cardiol. 1982 May;14(5):279–289. doi: 10.1016/0022-2828(82)90206-1. [DOI] [PubMed] [Google Scholar]
- Rinaldi M. L., Le Peuch C. J., Demaille J. G. The epinephrine-induced activation of the cardiac slow Ca2+ channel is mediated by the cAMP-dependent phosphorylation of calciductin, a 23 000 Mr sarcolemmal protein. FEBS Lett. 1981 Jul 6;129(2):277–281. doi: 10.1016/0014-5793(81)80183-4. [DOI] [PubMed] [Google Scholar]
- Sakmann B., Noma A., Trautwein W. Acetylcholine activation of single muscarinic K+ channels in isolated pacemaker cells of the mammalian heart. Nature. 1983 May 19;303(5914):250–253. doi: 10.1038/303250a0. [DOI] [PubMed] [Google Scholar]
- Shimoni Y., Raz S., Gotsman M. S. Two potentially arrhythmogenic mechanisms of adrenaline action in cardiac muscle. J Mol Cell Cardiol. 1984 May;16(5):471–478. doi: 10.1016/s0022-2828(84)80618-5. [DOI] [PubMed] [Google Scholar]
- Soejima M., Noma A. Mode of regulation of the ACh-sensitive K-channel by the muscarinic receptor in rabbit atrial cells. Pflugers Arch. 1984 Apr;400(4):424–431. doi: 10.1007/BF00587544. [DOI] [PubMed] [Google Scholar]
- Ten Eick R., Nawrath H., McDonald T. F., Trautwein W. On the mechanism of the negative inotropic effect of acetylcholine. Pflugers Arch. 1976 Feb 24;361(3):207–213. doi: 10.1007/BF00587284. [DOI] [PubMed] [Google Scholar]
- Trautwein W., Taniguchi J., Noma A. The effect of intracellular cyclic nucleotides and calcium on the action potential and acetylcholine response of isolated cardiac cells. Pflugers Arch. 1982 Feb;392(4):307–314. doi: 10.1007/BF00581624. [DOI] [PubMed] [Google Scholar]
- Tsien R. W. Adrenaline-like effects of intracellular iontophoresis of cyclic AMP in cardiac Purkinje fibres. Nat New Biol. 1973 Sep 26;245(143):120–122. doi: 10.1038/newbio245120a0. [DOI] [PubMed] [Google Scholar]
- Tsien R. W. Cyclic AMP and contractile activity in heart. Adv Cyclic Nucleotide Res. 1977;8:363–420. [PubMed] [Google Scholar]
- Tsien R. W., Giles W., Greengard P. Cyclic AMP mediates the effects of adrenaline on cardiac purkinje fibres. Nat New Biol. 1972 Dec 6;240(101):181–183. doi: 10.1038/newbio240181a0. [DOI] [PubMed] [Google Scholar]
- Tsien R. W., Weingart R. Inotropic effect of cyclic AMP in calf ventricular muscle studied by a cut end method. J Physiol. 1976 Aug;260(1):117–141. doi: 10.1113/jphysiol.1976.sp011507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji Y., Inoue D., Pappano A. J. beta-Adrenoceptor agonist accelerates recovery from inactivation of calcium-dependent action potentials. J Mol Cell Cardiol. 1985 May;17(5):517–521. doi: 10.1016/s0022-2828(85)80057-2. [DOI] [PubMed] [Google Scholar]
- Vassort G., Rougier O., Garnier D., Sauviat M. P., Coraboeuf E., Gargouïl Y. M. Effects of adrenaline on membrane inward currents during the cardiac action potential. Pflugers Arch. 1969;309(1):70–81. doi: 10.1007/BF00592283. [DOI] [PubMed] [Google Scholar]
- Vogel S., Sperelakis N. Induction of slow action potentials by microiontophoresis of cyclic AMP into heart cells. J Mol Cell Cardiol. 1981 Jan;13(1):51–64. doi: 10.1016/0022-2828(81)90228-5. [DOI] [PubMed] [Google Scholar]
- Watanabe A. M., Besch H. R., Jr Interaction between cyclic adenosine monophosphate and cyclic gunaosine monophosphate in guinea pig ventricular myocardium. Circ Res. 1975 Sep;37(3):309–317. doi: 10.1161/01.res.37.3.309. [DOI] [PubMed] [Google Scholar]
- Watanabe A. M., Lindemann J. P., Fleming J. W. Mechanisms of muscarinic modulation of protein phosphorylation in intact ventricles. Fed Proc. 1984 Aug;43(11):2618–2623. [PubMed] [Google Scholar]
- Watanabe A. M., McConnaughey M. M., Strawbridge R. A., Fleming J. W., Jones L. R., Besch H. R., Jr Muscarinic cholinergic receptor modulation of beta-adrenergic receptor affinity for catecholamines. J Biol Chem. 1978 Jul 25;253(14):4833–4836. [PubMed] [Google Scholar]
- Winegrad S. Regulation of cardiac contractile proteins. Correlations between physiology and biochemistry. Circ Res. 1984 Nov;55(5):565–574. doi: 10.1161/01.res.55.5.565. [DOI] [PubMed] [Google Scholar]
- Yellen G. Single Ca2+-activated nonselective cation channels in neuroblastoma. Nature. 1982 Mar 25;296(5855):357–359. doi: 10.1038/296357a0. [DOI] [PubMed] [Google Scholar]


