Abstract
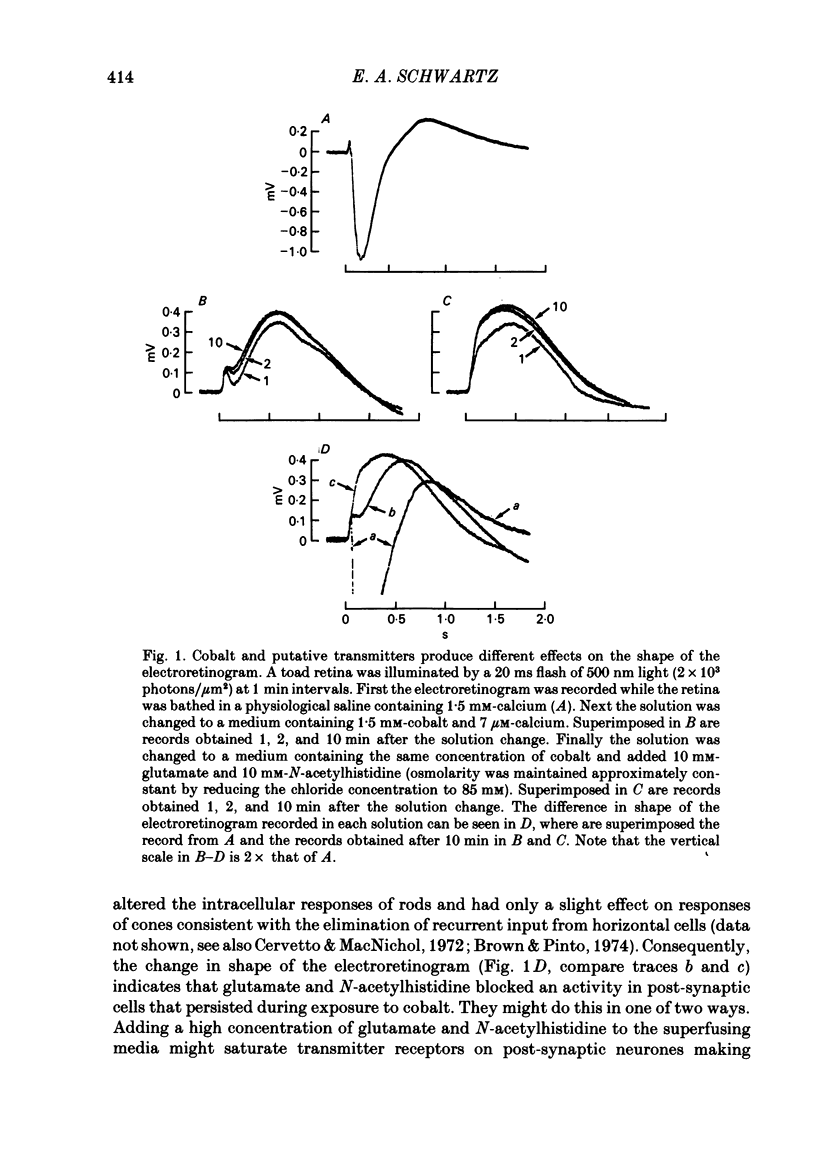
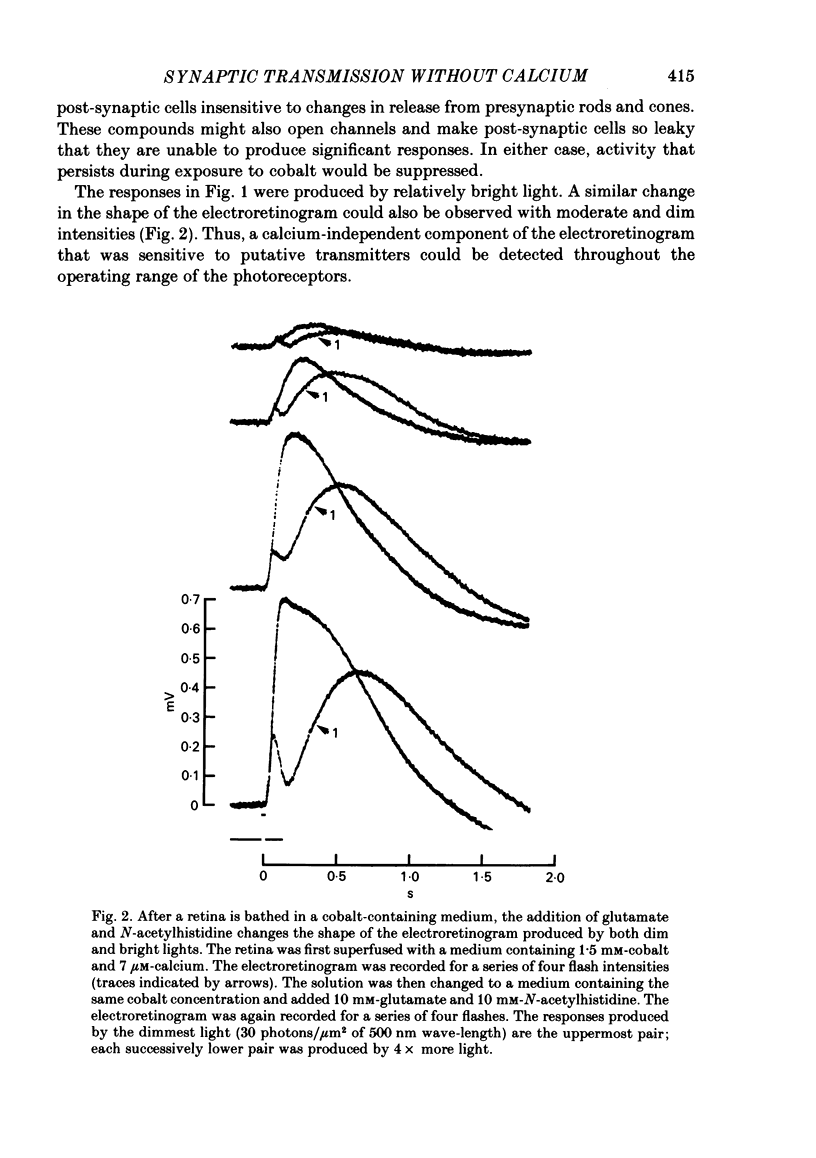
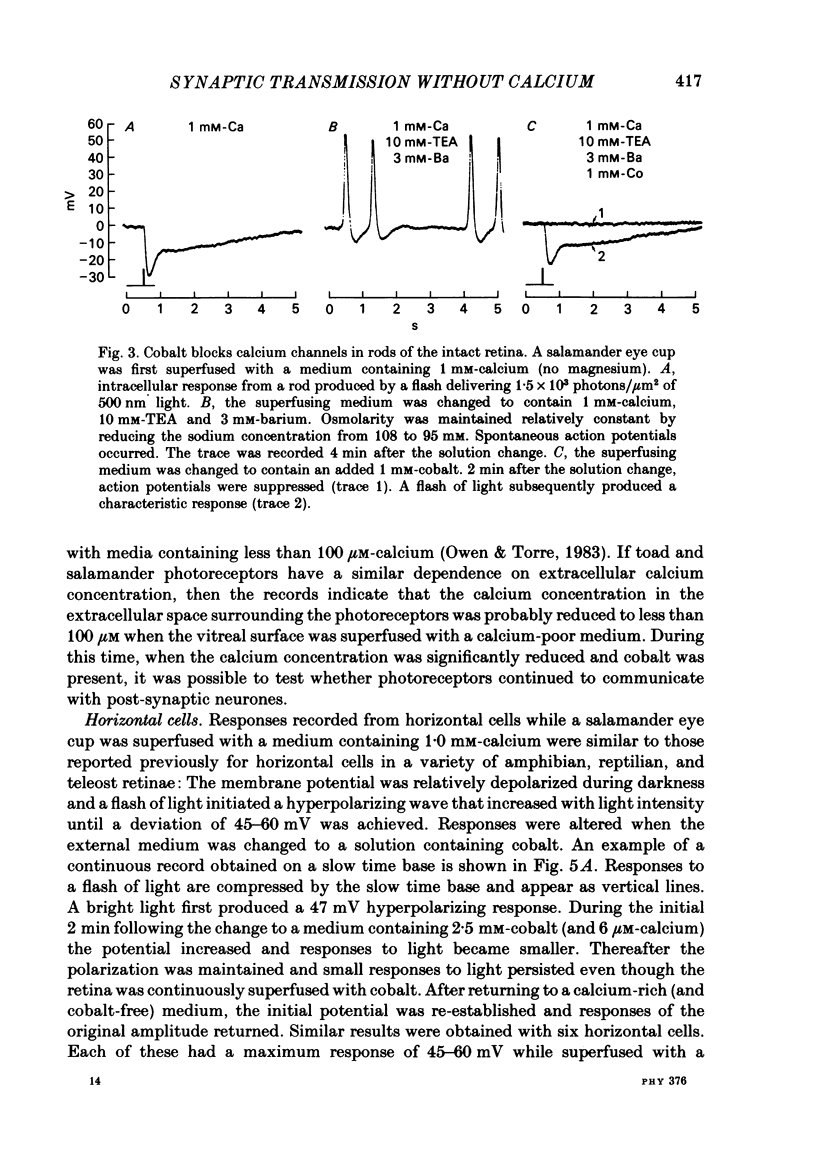
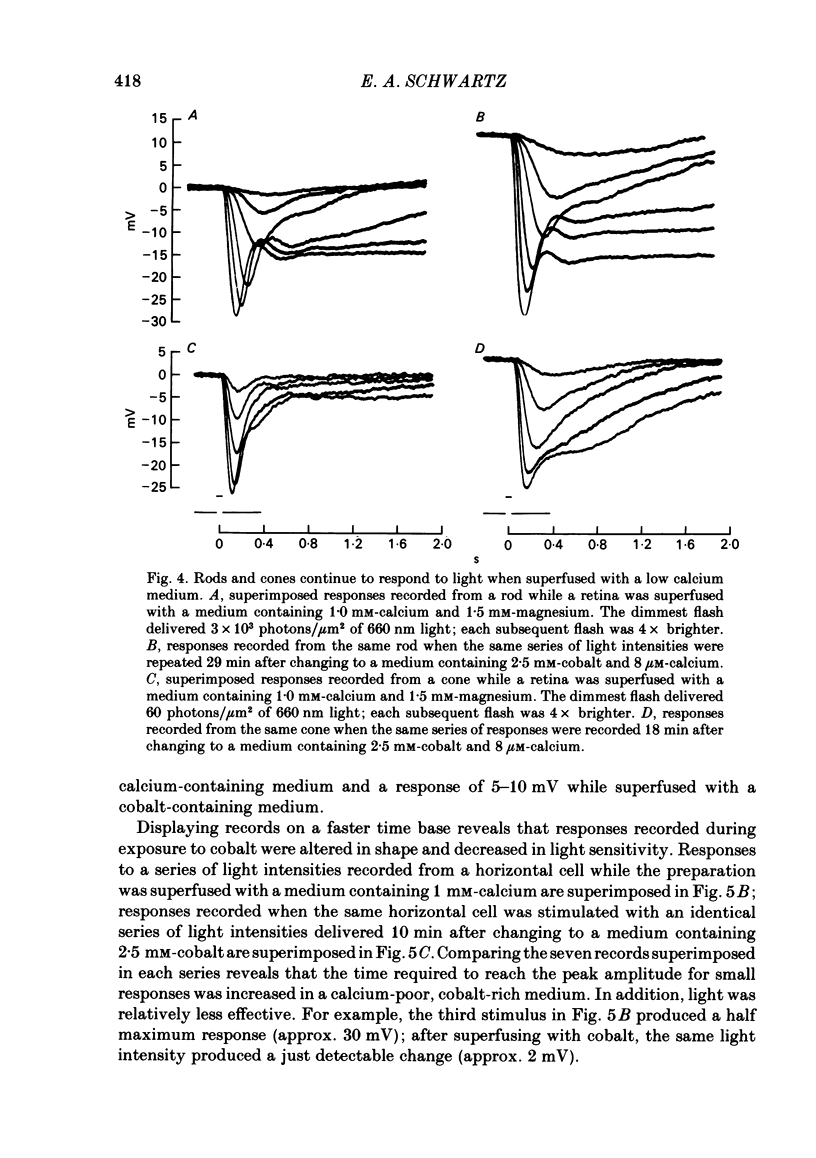
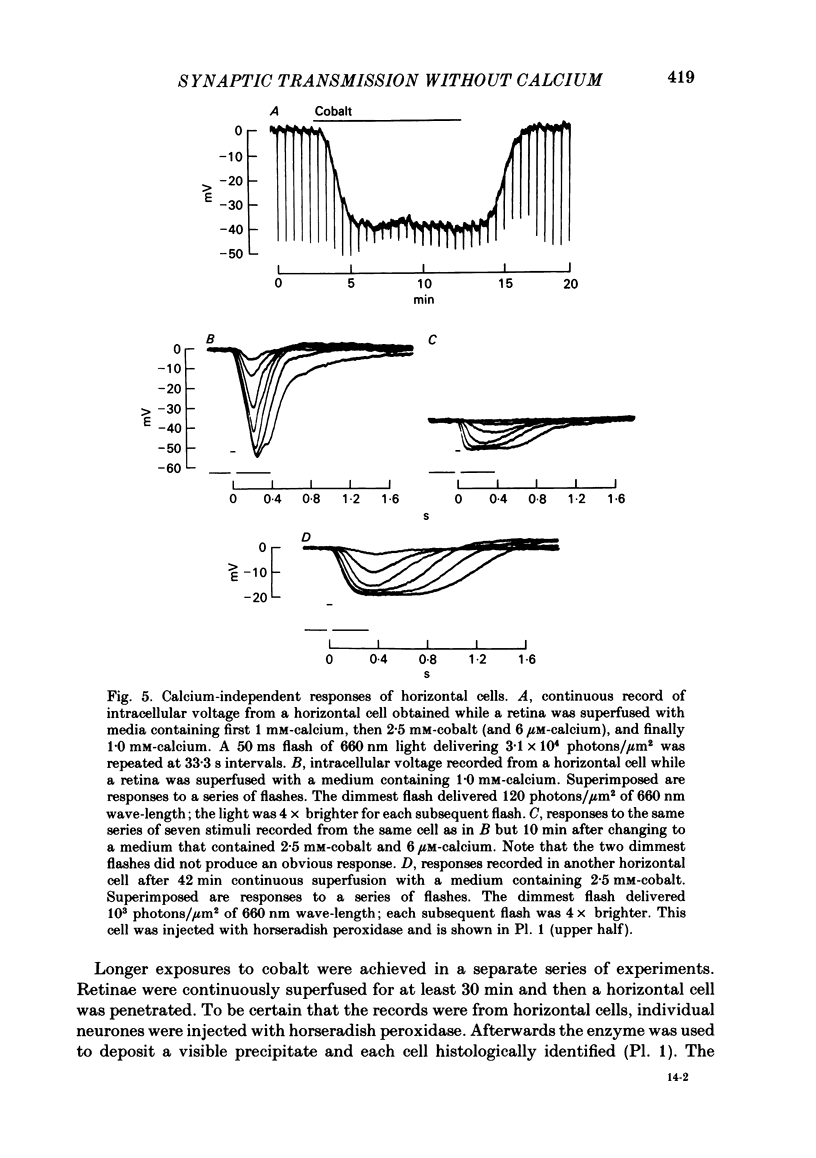
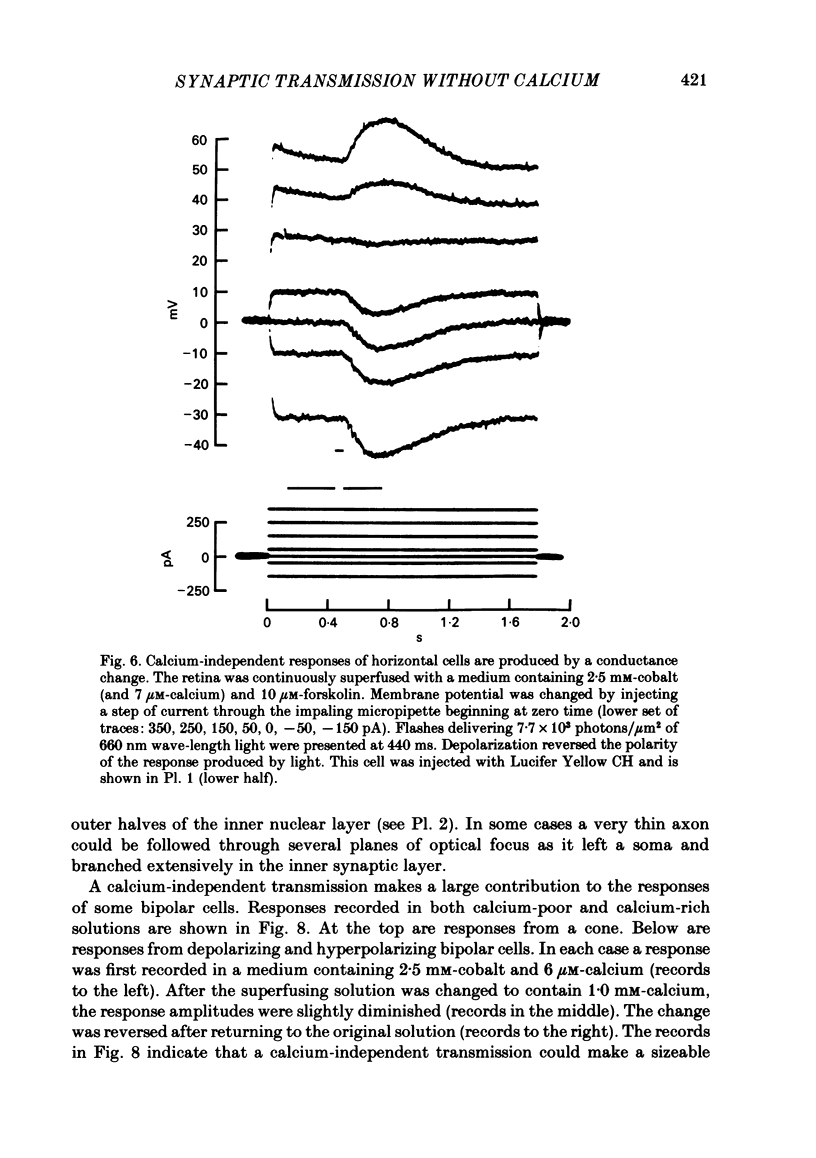
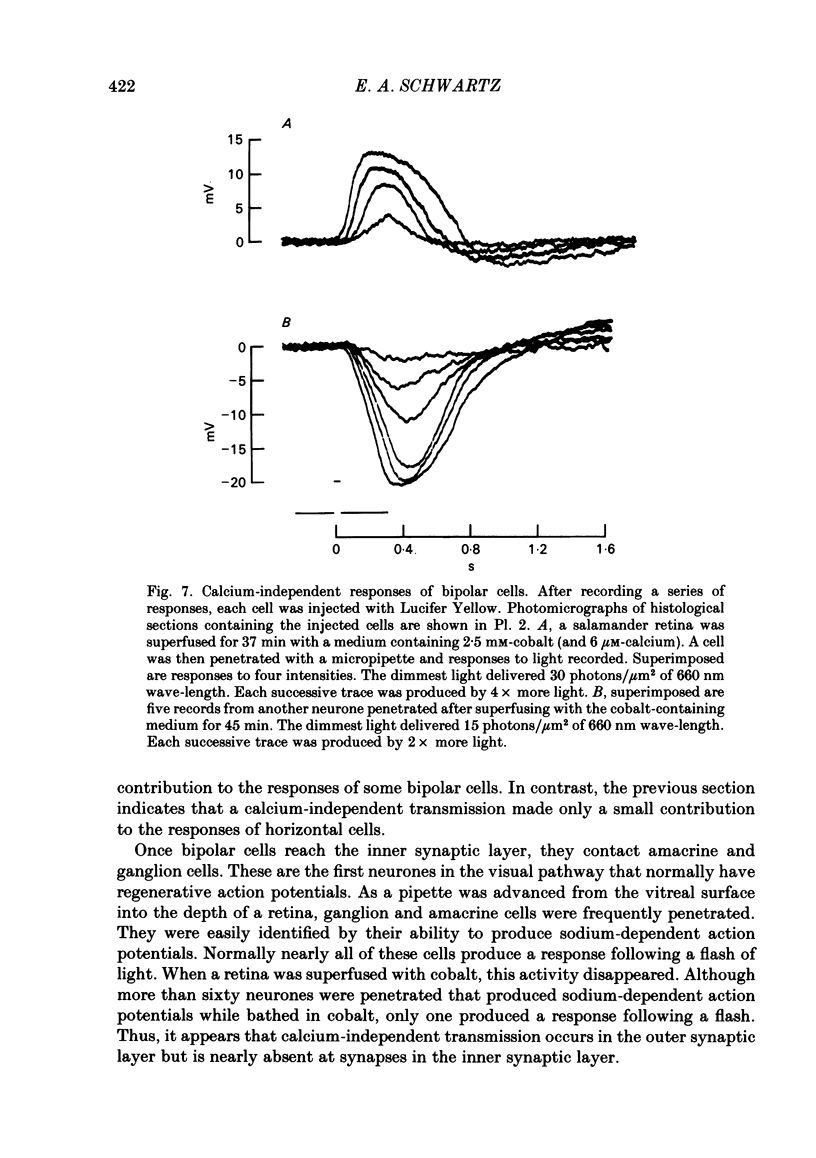
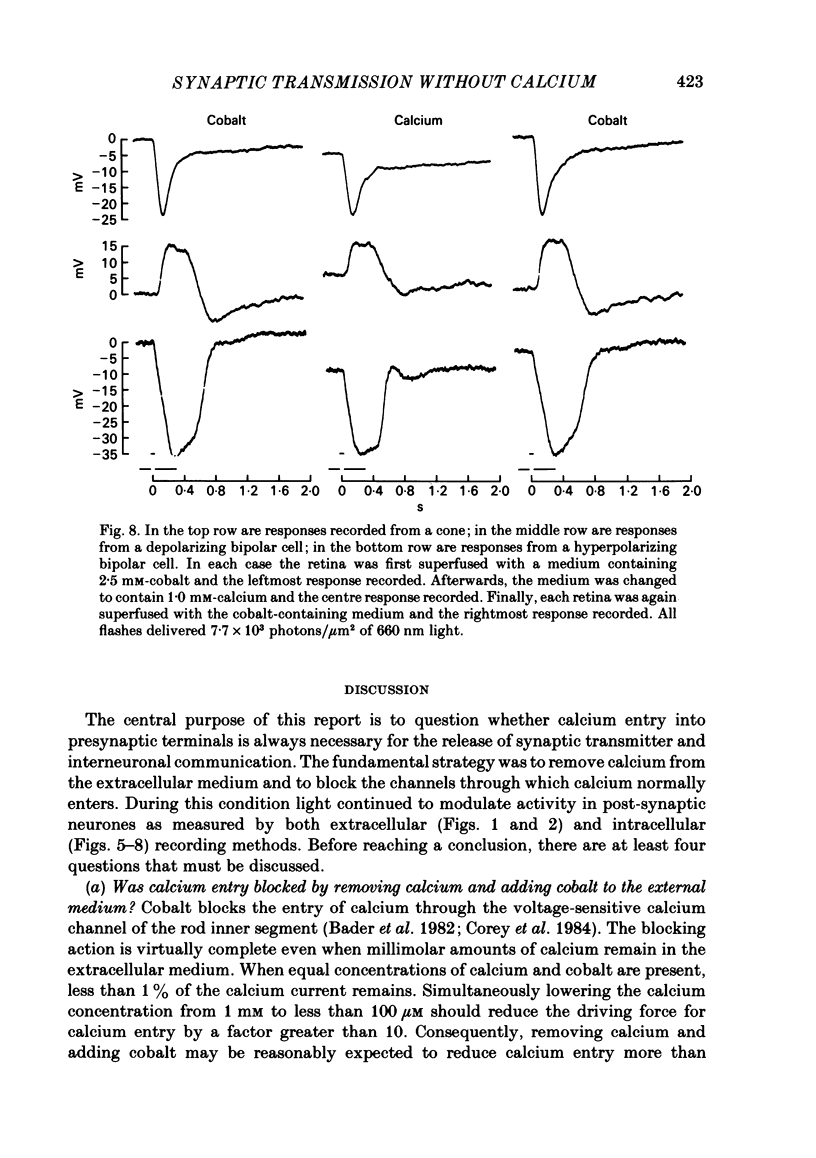
Toad (Bufo marinus) retinae were peeled from the pigment epithelium and superfused over the photoreceptor surface with a calcium-poor, cobalt-rich medium. The shape of the electroretinogram indicated that post-synaptic neurones received synaptic input. Adding the putative transmitters glutamate and N-acetylhistidine changed the shape of the electroretinogram. The change suggests that an excess of the putative transmitters blocked a component of synaptic transmission that persisted when a retina was bathed in cobalt. Salamander (Ambystomatigrinum) retinae in hemisected eye cups were superfused over their vitreal surface. Intracellular responses were recorded from photoreceptors. Reducing the calcium concentration in the superfusing medium from 1 mM to less than 10 microM slowly changed responses produced by light. The change indicates that the calcium concentration in the extracellular space surrounding photoreceptors fell to less than 100 microM. When retinae were superfused with a medium containing 1 mM-calcium, 3 mM-barium, and 10 mM-tetraethylammonium (TEA), rods produced action potentials that were later blocked by adding 1 mM-cobalt. Blocking calcium channels with cobalt and lowering the extracellular calcium concentration should together block calcium-dependent synaptic transmission. Intracellular responses were recorded from horizontal cells. After replacing external calcium with cobalt the membrane potential hyperpolarized and responses produced by light became smaller but did not entirely disappear. The responses that remained were less sensitive to light and had an altered shape. The change was reversible. Similar responses could be recorded after prolonged (30-120 min) exposure to cobalt. Electrical synapses between horizontal cells were uncoupled by adding 10 microM-forskolin to the cobalt medium. The polarity of a response could then be reversed if a cell was depolarized by injecting current. The observation of a reversal potential demonstrates that the response was produced by a conductance change. Intracellular responses were recorded from depolarizing and hyperpolarizing bipolar cells while retinae were superfused with cobalt-rich medium. After changing to a cobalt-free medium containing 1 mM-calcium, responses produced by light were slightly smaller. Large responses were recorded after superfusing with cobalt-rich, calcium-poor medium for 30-120 min. The results indicate that synaptic transmission by photoreceptors continues during conditions expected to block the entry of calcium into their presynaptic terminals.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayoub G. S., Lam D. M. The release of gamma-aminobutyric acid from horizontal cells of the goldfish (Carassius auratus) retina. J Physiol. 1984 Oct;355:191–214. doi: 10.1113/jphysiol.1984.sp015414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader C. R., Bertrand D., Schwartz E. A. Voltage-activated and calcium-activated currents studied in solitary rod inner segments from the salamander retina. J Physiol. 1982 Oct;331:253–284. doi: 10.1113/jphysiol.1982.sp014372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader C. R., Macleish P. R., Schwartz E. A. A voltage-clamp study of the light response in solitary rods of the tiger salamander. J Physiol. 1979 Nov;296:1–26. doi: 10.1113/jphysiol.1979.sp012988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. E., Pinto L. H. Ionic mechanism for the photoreceptor potential of the retina of Bufo marinus. J Physiol. 1974 Feb;236(3):575–591. doi: 10.1113/jphysiol.1974.sp010453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervetto L., MacNichol E. F., Jr Inactivation of horizontal cells in turtle retina by glutamate and aspartate. Science. 1972 Nov 17;178(4062):767–768. doi: 10.1126/science.178.4062.767. [DOI] [PubMed] [Google Scholar]
- Cervetto L., Piccolino M. Synaptic transmission between photoreceptors and horizontal cells in the turtle retina. Science. 1974 Feb 1;183(4123):417–419. doi: 10.1126/science.183.4123.417. [DOI] [PubMed] [Google Scholar]
- Corey D. P., Dubinsky J. M., Schwartz E. A. The calcium current in inner segments of rods from the salamander (Ambystoma tigrinum) retina. J Physiol. 1984 Sep;354:557–575. doi: 10.1113/jphysiol.1984.sp015393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacheux R. F. Connections of the small bipolar cells with the photoreceptors in the turtle. An electron microscope study of Golgi-impregnated, gold-toned retinas. J Comp Neurol. 1982 Feb 10;205(1):55–62. doi: 10.1002/cne.902050106. [DOI] [PubMed] [Google Scholar]
- Dick E., Miller R. F. Light-evoked potassium activity in mudpuppy retina: its relationship to the b-wave of the electroretinogram. Brain Res. 1978 Oct 13;154(2):388–394. doi: 10.1016/0006-8993(78)90711-4. [DOI] [PubMed] [Google Scholar]
- Dowling J. E., Boycott B. B. Organization of the primate retina: electron microscopy. Proc R Soc Lond B Biol Sci. 1966 Nov 15;166(1002):80–111. doi: 10.1098/rspb.1966.0086. [DOI] [PubMed] [Google Scholar]
- Dowling J. E. Synaptic organization of the frog retina: an electron microscopic analysis comparing the retinas of frogs and primates. Proc R Soc Lond B Biol Sci. 1968 Jun 11;170(1019):205–228. doi: 10.1098/rspb.1968.0034. [DOI] [PubMed] [Google Scholar]
- Fain G. L., Gerschenfeld H. M., Quandt F. N. Calcium spikes in toad rods. J Physiol. 1980 Jun;303:495–513. doi: 10.1113/jphysiol.1980.sp013300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershengorn M. C., Geras E., Purrello V. S., Rebecchi M. J. Inositol trisphosphate mediates thyrotropin-releasing hormone mobilization of nonmitochondrial calcium in rat mammotropic pituitary cells. J Biol Chem. 1984 Sep 10;259(17):10675–10681. [PubMed] [Google Scholar]
- Joseph S. K., Thomas A. P., Williams R. J., Irvine R. F., Williamson J. R. myo-Inositol 1,4,5-trisphosphate. A second messenger for the hormonal mobilization of intracellular Ca2+ in liver. J Biol Chem. 1984 Mar 10;259(5):3077–3081. [PubMed] [Google Scholar]
- Kaneko A. Electrical connexions between horizontal cells in the dogfish retina. J Physiol. 1971 Feb;213(1):95–105. doi: 10.1113/jphysiol.1971.sp009370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasansky A. Contacts between receptors and electrophysiologically identified neurones in the retina of the larval tiger salamander. J Physiol. 1978 Dec;285:531–542. doi: 10.1113/jphysiol.1978.sp012587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe D. A., Richardson N. P., Taylor P., Donatsch P. Increasing intracellular sodium triggers calcium release from bound pools. Nature. 1976 Mar 25;260(5549):337–338. doi: 10.1038/260337a0. [DOI] [PubMed] [Google Scholar]
- Mariani A. P. A diffuse, invaginating cone bipolar cell in primate retina. J Comp Neurol. 1981 Apr 20;197(4):661–671. doi: 10.1002/cne.901970408. [DOI] [PubMed] [Google Scholar]
- Miller A. M., Schwartz E. A. Evidence for the identification of synaptic transmitters released by photoreceptors of the toad retina. J Physiol. 1983 Jan;334:325–349. doi: 10.1113/jphysiol.1983.sp014497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriarty C. M., Leuschen M. P. Role of calcium in acute stimulated release of prolactin from neoplastic GH3 cells. Am J Physiol. 1981 Jun;240(6):E705–E711. doi: 10.1152/ajpendo.1981.240.6.E705. [DOI] [PubMed] [Google Scholar]
- Naka K. I., Rushton W. A. The generation and spread of S-potentials in fish (Cyprinidae). J Physiol. 1967 Sep;192(2):437–461. doi: 10.1113/jphysiol.1967.sp008308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley B., 2nd, Green D. G. Correlation of light-induced changes in retinal extracellular potassium concentration with c-wave of the electroretinogram. J Neurophysiol. 1976 Sep;39(5):1117–1133. doi: 10.1152/jn.1976.39.5.1117. [DOI] [PubMed] [Google Scholar]
- Owen W. G., Torre V. High-pass filtering of small signals by retinal rods. Ionic studies. Biophys J. 1983 Mar;41(3):325–339. doi: 10.1016/S0006-3495(83)84444-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccolino M., Gerschenfeld H. M. Characteristics and ionic processes involved in feedback spikes of turtle cones. Proc R Soc Lond B Biol Sci. 1980 Jan 17;206(1165):439–463. doi: 10.1098/rspb.1980.0007. [DOI] [PubMed] [Google Scholar]
- Piccolino M., Neyton J., Gerschenfeld H. M. Decrease of gap junction permeability induced by dopamine and cyclic adenosine 3':5'-monophosphate in horizontal cells of turtle retina. J Neurosci. 1984 Oct;4(10):2477–2488. doi: 10.1523/JNEUROSCI.04-10-02477.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzan T., Gatti G., Dozio N., Vicentini L. M., Meldolesi J. Ca2+-dependent and -independent release of neurotransmitters from PC12 cells: a role for protein kinase C activation? J Cell Biol. 1984 Aug;99(2):628–638. doi: 10.1083/jcb.99.2.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebecchi M. J., Gerry R. H., Gershengorn M. C. Thyrotropin-releasing hormone causes loss of cellular calcium without calcium uptake by rat pituitary cells in culture. Studies using arsenazo III for direct measurement of calcium. J Biol Chem. 1982 Mar 25;257(6):2751–2753. [PubMed] [Google Scholar]
- Rink T. J., Sanchez A., Hallam T. J. Diacylglycerol and phorbol ester stimulate secretion without raising cytoplasmic free calcium in human platelets. Nature. 1983 Sep 22;305(5932):317–319. doi: 10.1038/305317a0. [DOI] [PubMed] [Google Scholar]
- Schwartz E. A. Calcium-independent release of GABA from isolated horizontal cells of the toad retina. J Physiol. 1982 Feb;323:211–227. doi: 10.1113/jphysiol.1982.sp014069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz E. A. Electrical properties of the rod syncytium in the retina of the turtle. J Physiol. 1976 May;257(2):379–406. doi: 10.1113/jphysiol.1976.sp011374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg R. H., Oakley B., 2nd, Niemeyer G. Light-evoked changes in [K+]0 in retina of intact cat eye. J Neurophysiol. 1980 Nov;44(5):897–921. doi: 10.1152/jn.1980.44.5.897. [DOI] [PubMed] [Google Scholar]
- Stewart W. W. Functional connections between cells as revealed by dye-coupling with a highly fluorescent naphthalimide tracer. Cell. 1978 Jul;14(3):741–759. doi: 10.1016/0092-8674(78)90256-8. [DOI] [PubMed] [Google Scholar]
- Streb H., Irvine R. F., Berridge M. J., Schulz I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature. 1983 Nov 3;306(5938):67–69. doi: 10.1038/306067a0. [DOI] [PubMed] [Google Scholar]
- Teranishi T., Negishi K., Kato S. Regulatory effect of dopamine on spatial properties of horizontal cells in carp retina. J Neurosci. 1984 May;4(5):1271–1280. doi: 10.1523/JNEUROSCI.04-05-01271.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. Y., Pozzan T., Rink T. J. Calcium homeostasis in intact lymphocytes: cytoplasmic free calcium monitored with a new, intracellularly trapped fluorescent indicator. J Cell Biol. 1982 Aug;94(2):325–334. doi: 10.1083/jcb.94.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazulla S., Kleinschmidt J. Carrier-mediated release of GABA from retinal horizontal cells. Brain Res. 1983 Mar 14;263(1):63–75. doi: 10.1016/0006-8993(83)91201-5. [DOI] [PubMed] [Google Scholar]