Abstract
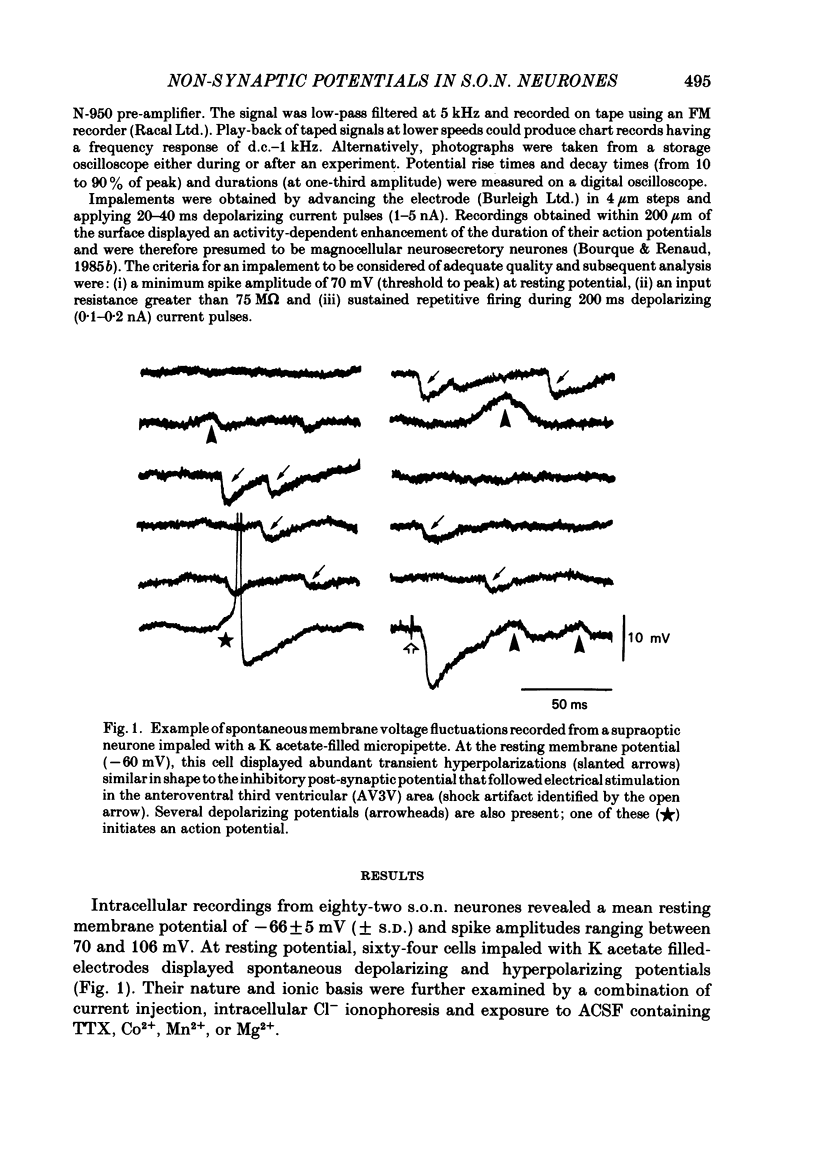
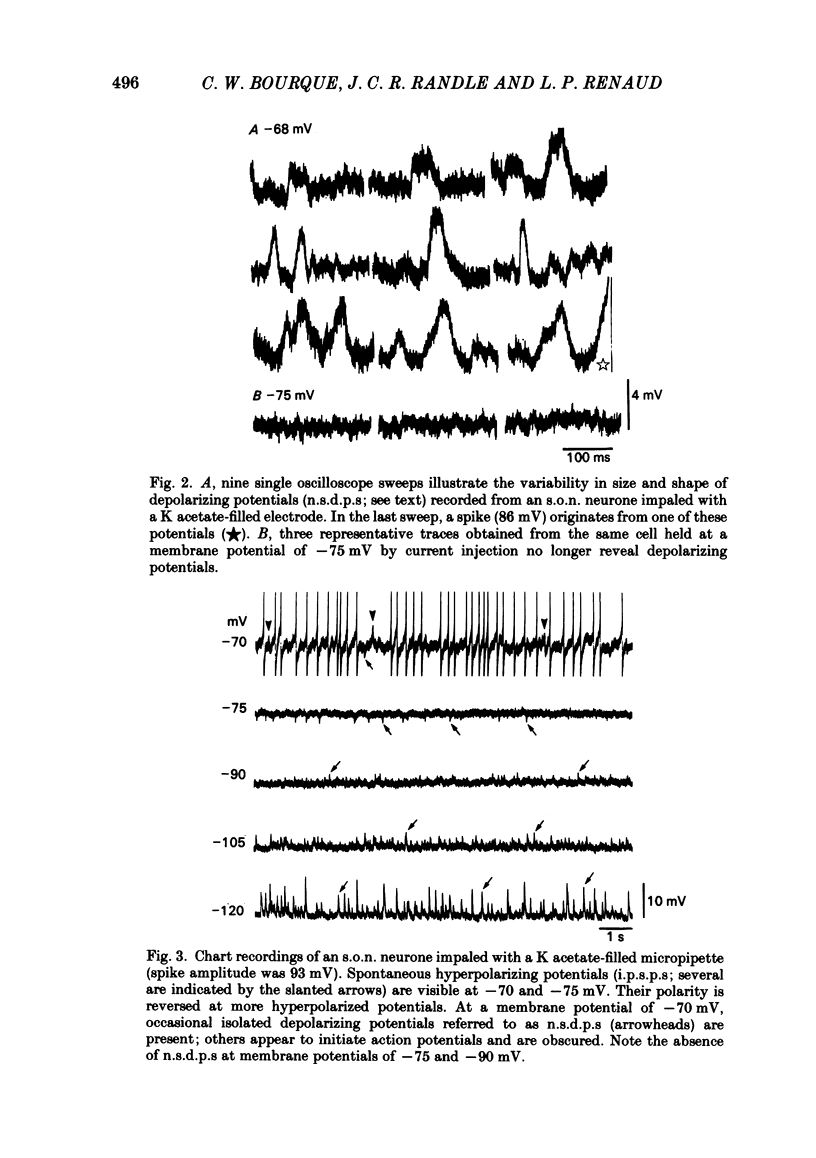
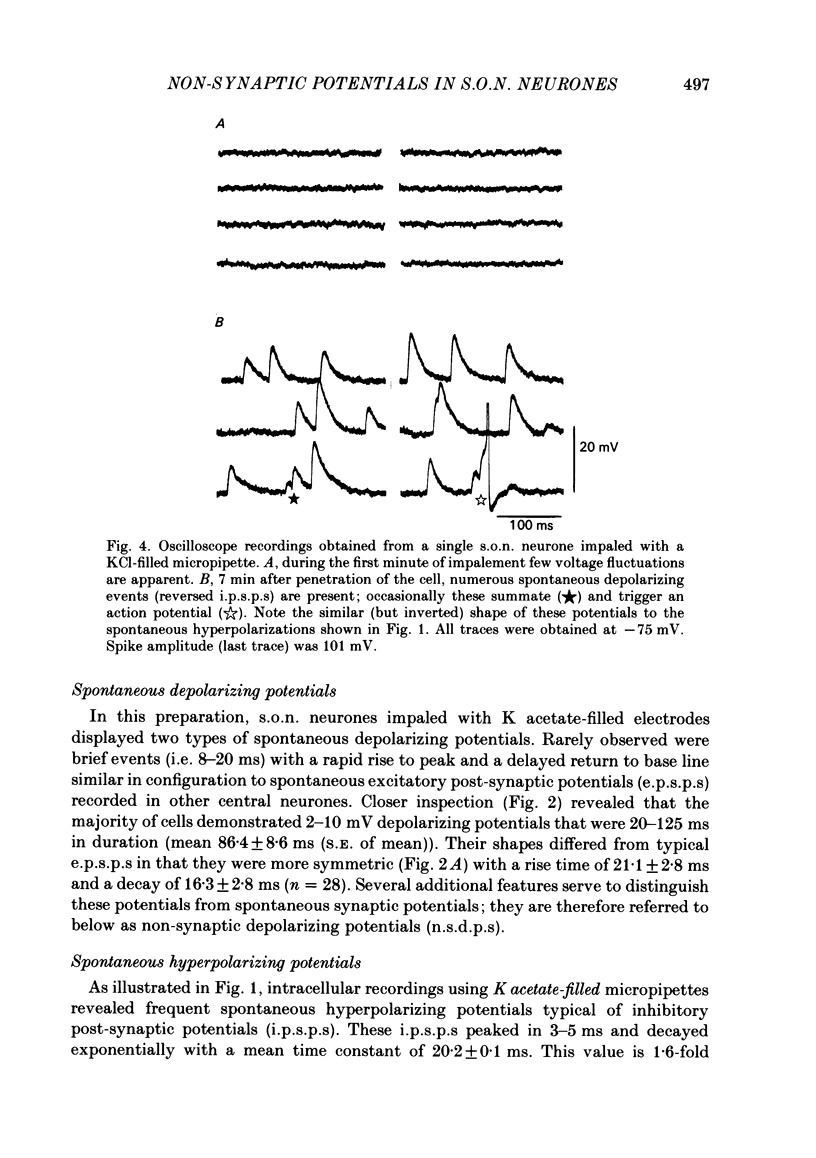
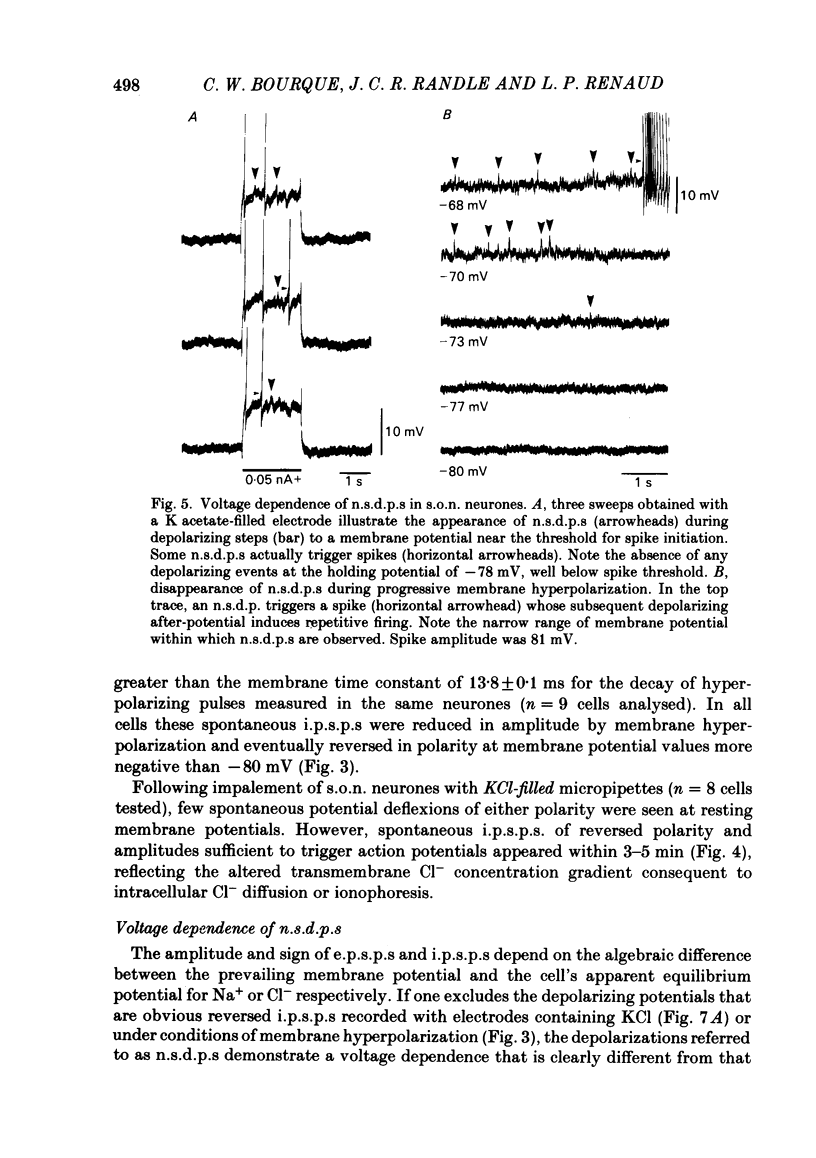
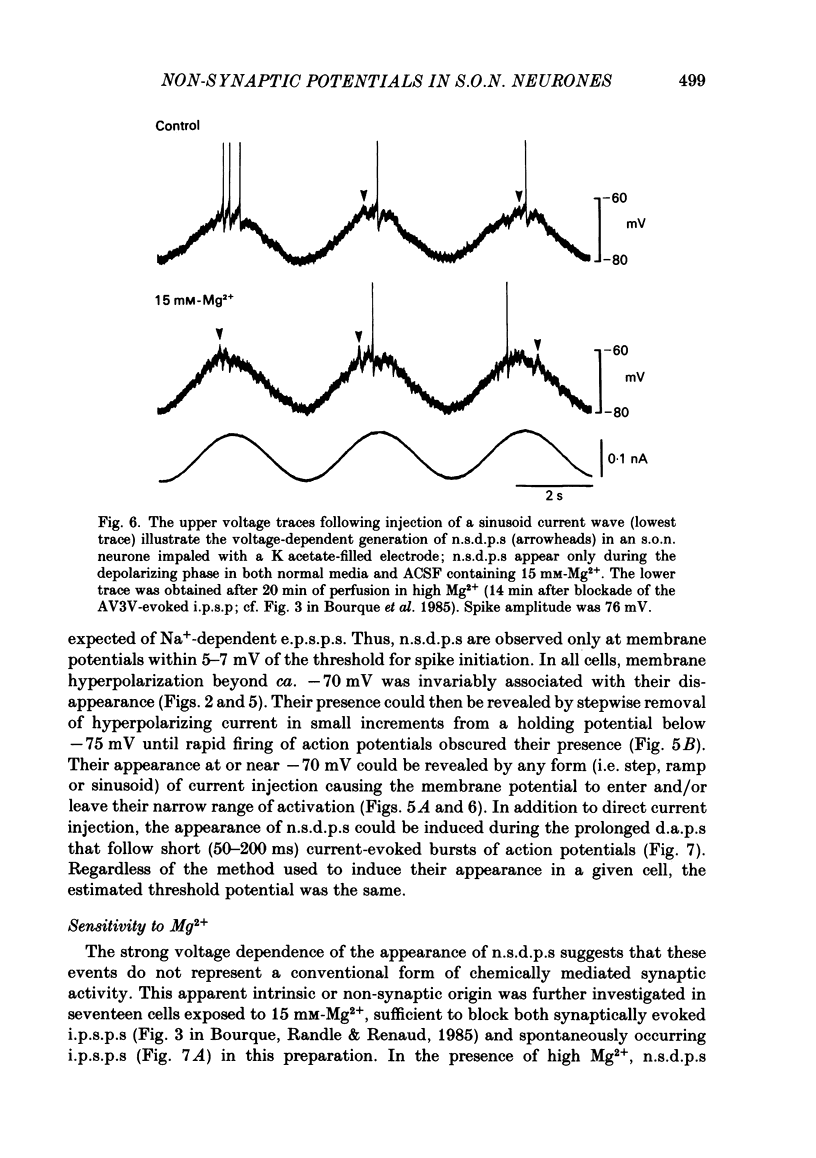
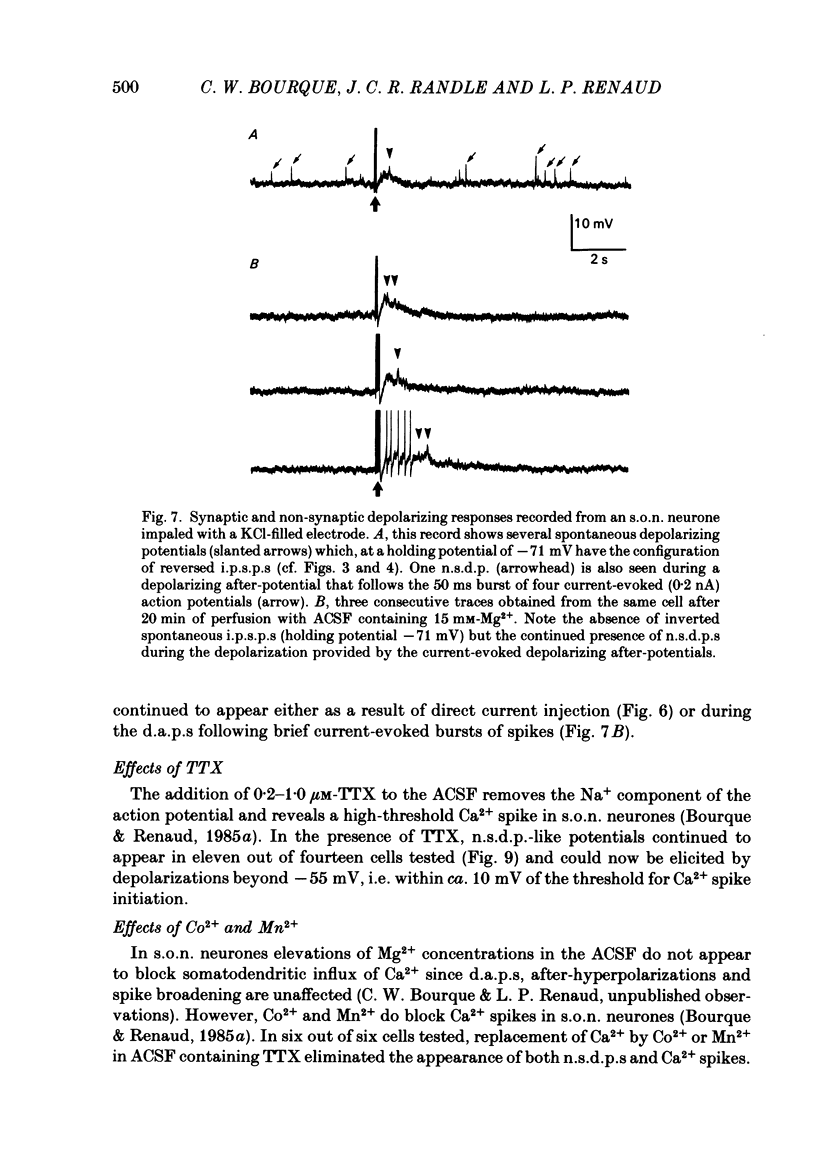
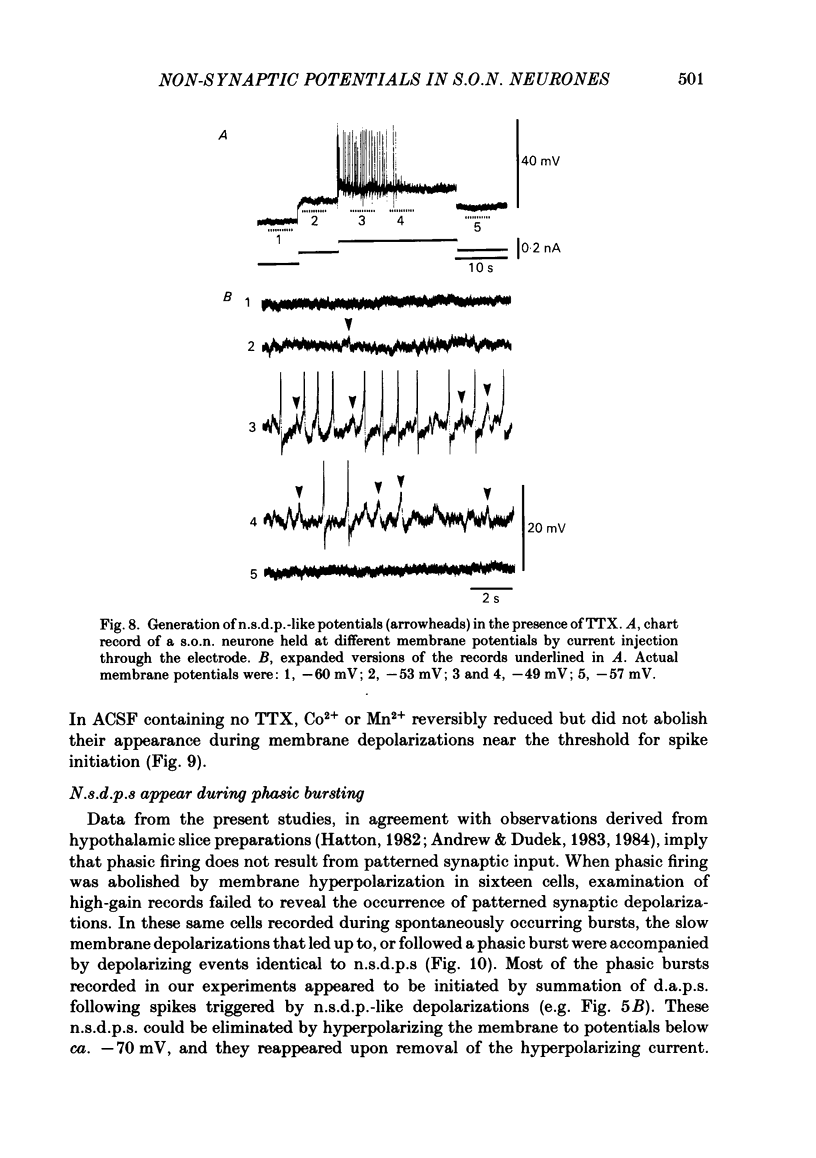
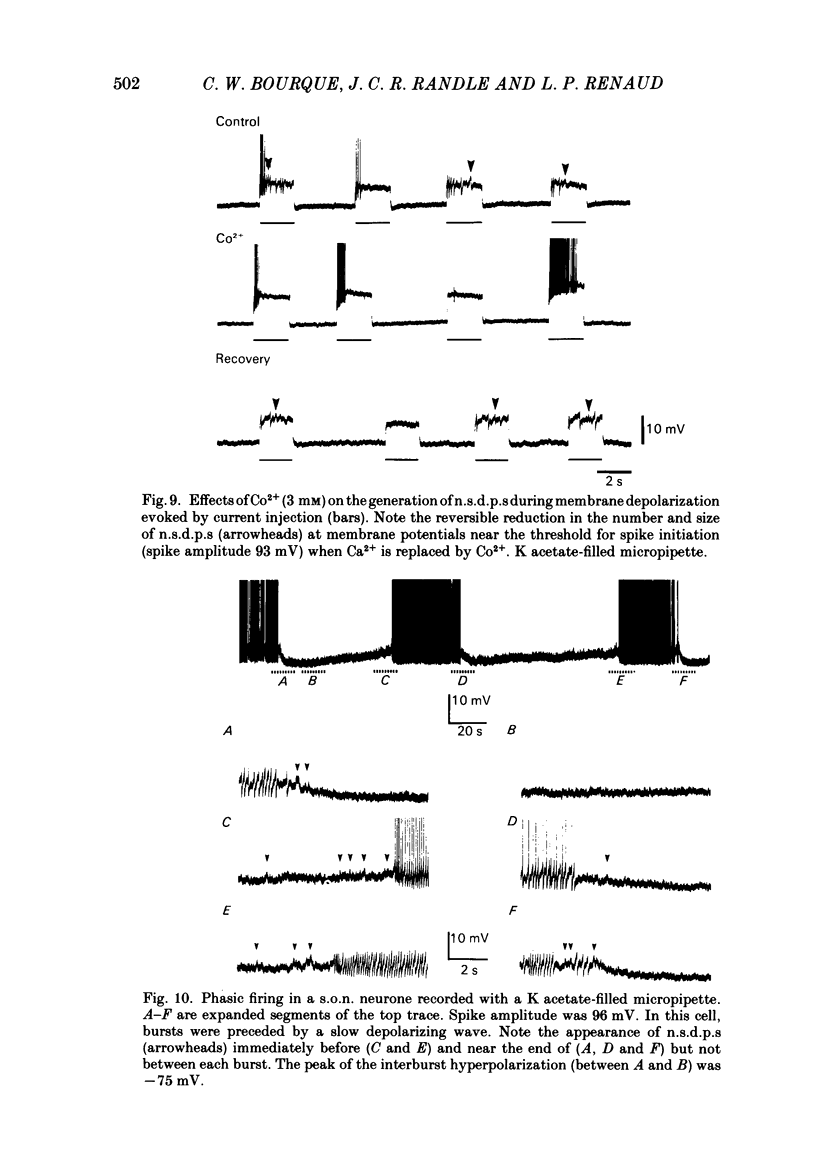
Intracellular recordings obtained from eighty-two supraoptic nucleus neurones in perfused explants of rat hypothalamus revealed a mean resting membrane potential of -66 +/- 5 mV (S.D.) and spike amplitudes of 70-106 mV. When recorded with K acetate-filled micropipettes, non-spike membrane voltage fluctuations included spontaneous depolarizing and hyperpolarizing potentials. Spontaneous hyperpolarizing potentials peaked in 3-5 ms and decayed exponentially with a mean time constant of 20.2 +/- 0.1 ms, 1.6 times the membrane time constant of 13.8 +/- 0.1 ms. These potentials were identified as spontaneous inhibitory post-synaptic potentials, and all demonstrated a common reversal potential near -80 mV, a depolarizing shift of this reversal potential during intracellular Cl- accumulation, and reversible blockade by raising [Mg2+] to 15 mM in the perfusate. Depolarizing potentials with features typical of spontaneous excitatory post-synaptic potentials i.e. brief (8-20 ms) depolarizing transients, were rarely recorded with K acetate-filled micropipettes. Instead, most neurones demonstrated what are termed non-synaptic depolarizing potentials (n.s.d.p.s) lasting 20-125 ms (mean 86.4 +/- 8.6 ms (S.E. of mean)) with a rise time 21.1 +/- 2.8 ms and a decay time of 16.3 +/- 2.8 ms (n = 28 measured). Unlike typical spontaneous post-synaptic potentials, these events could sustain a constant peak amplitude for most of their duration. These n.s.d.p.s displayed a strong voltage-dependent behaviour and were detected only at membrane potentials within 5-7 mV of the threshold for spike initiation. Spontaneous slow depolarizing membrane shifts preceding or following phasic bursts, or any manipulation (e.g. current step, sinusoid, depolarizing after-potential) causing the membrane potential to enter this range of activation, prompted their appearance. N.s.d.p.s were completely insensitive to the presence of 15 mM-Mg2+ but they were reduced in size and frequency when Ca2+ were replaced with Co2+ or Mn2+. They were detected at a more positive membrane potential when Na+-dependent action potentials were blocked with tetrodotoxin. The size, voltage-dependent and non-synaptic nature of these depolarizing potentials raises the possibility that they reflect the activity of individual (or small clusters of) ionic channels carrying inward current. Their ability to serve as prepotentials to trigger spikes is deemed to be particularly important for promoting the onset of phasic bursts in supraoptic neurosecretory neurones.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe H., Ogata N. Ionic mechanism for the osmotically-induced depolarization in neurones of the guinea-pig supraoptic nucleus in vitro. J Physiol. 1982 Jun;327:157–171. doi: 10.1113/jphysiol.1982.sp014225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew R. D., Dudek F. E. Analysis of intracellularly recorded phasic bursting by mammalian neuroendocrine cells. J Neurophysiol. 1984 Mar;51(3):552–566. doi: 10.1152/jn.1984.51.3.552. [DOI] [PubMed] [Google Scholar]
- Andrew R. D., Dudek F. E. Burst discharge in mammalian neuroendocrine cells involves an intrinsic regenerative mechanism. Science. 1983 Sep 9;221(4615):1050–1052. doi: 10.1126/science.6879204. [DOI] [PubMed] [Google Scholar]
- Andrew R. D., MacVicar B. A., Dudek F. E., Hatton G. I. Dye transfer through gap junctions between neuroendocrine cells of rat hypothalamus. Science. 1981 Mar 13;211(4487):1187–1189. doi: 10.1126/science.7466393. [DOI] [PubMed] [Google Scholar]
- Atwater I., Dawson C. M., Eddlestone G. T., Rojas E. Voltage noise measurements across the pancreatic beta-cell membrane: calcium channel characteristics. J Physiol. 1981 May;314:195–212. doi: 10.1113/jphysiol.1981.sp013701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourque C. W., Randle J. C., Renaud L. P. Calcium-dependent potassium conductance in rat supraoptic nucleus neurosecretory neurons. J Neurophysiol. 1985 Dec;54(6):1375–1382. doi: 10.1152/jn.1985.54.6.1375. [DOI] [PubMed] [Google Scholar]
- Bourque C. W., Renaud L. P. A perfused in vitro preparation of hypothalamus for electrophysiological studies on neurosecretory neurons. J Neurosci Methods. 1983 Mar;7(3):203–214. doi: 10.1016/0165-0270(83)90002-x. [DOI] [PubMed] [Google Scholar]
- Bourque C. W., Renaud L. P. Activity dependence of action potential duration in rat supraoptic neurosecretory neurones recorded in vitro. J Physiol. 1985 Jun;363:429–439. doi: 10.1113/jphysiol.1985.sp015720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourque C. W., Renaud L. P. Calcium-dependent action potentials in rat supraoptic neurosecretory neurones recorded in vitro. J Physiol. 1985 Jun;363:419–428. doi: 10.1113/jphysiol.1985.sp015719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek F. E., Hatton G. I., Macvicar B. A. Intracellular recordings from the paraventricular nucleus in slices of rat hypothalamus. J Physiol. 1980 Apr;301:101–114. doi: 10.1113/jphysiol.1980.sp013192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. A patch-clamp study of bovine chromaffin cells and of their sensitivity to acetylcholine. J Physiol. 1982 Oct;331:577–597. doi: 10.1113/jphysiol.1982.sp014393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getting P. A. Modification of neuron properties by electrotonic synapses. I. Input resistance, time constant, and integration. J Neurophysiol. 1974 Sep;37(5):846–857. doi: 10.1152/jn.1974.37.5.846. [DOI] [PubMed] [Google Scholar]
- Hatton G. I. Phasic bursting activity of rat paraventricular neurones in the absence of synaptic transmission. J Physiol. 1982 Jun;327:273–284. doi: 10.1113/jphysiol.1982.sp014231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas R., Nicholson C. Electrophysiological properties of dendrites and somata in alligator Purkinje cells. J Neurophysiol. 1971 Jul;34(4):532–551. doi: 10.1152/jn.1971.34.4.532. [DOI] [PubMed] [Google Scholar]
- Maruyama Y., Peterson O. H. Single-channel currents in isolated patches of plasma membrane from basal surface of pancreatic acini. Nature. 1982 Sep 9;299(5879):159–161. doi: 10.1038/299159a0. [DOI] [PubMed] [Google Scholar]
- Mason W. T. Electrical properties of neurons recorded from the rat supraoptic nucleus in vitro. Proc R Soc Lond B Biol Sci. 1983 Jan 22;217(1207):141–161. doi: 10.1098/rspb.1983.0003. [DOI] [PubMed] [Google Scholar]
- Mason W. T. Supraoptic neurones of rat hypothalamus are osmosensitive. Nature. 1980 Sep 11;287(5778):154–157. doi: 10.1038/287154a0. [DOI] [PubMed] [Google Scholar]
- Mason W. T., Waring D. W. Electrophysiological recordings from gonadotrophs. Evidence for Ca2+ channels mediated by gonadotrophin-releasing hormone. Neuroendocrinology. 1985 Sep;41(3):258–268. doi: 10.1159/000124186. [DOI] [PubMed] [Google Scholar]
- Randle J. C., Bourque C. W., Renaud L. P. Serial reconstruction of Lucifer yellow-labeled supraoptic nucleus neurons in perfused rat hypothalamic explants. Neuroscience. 1986 Feb;17(2):453–467. doi: 10.1016/0306-4522(86)90259-9. [DOI] [PubMed] [Google Scholar]



