Abstract
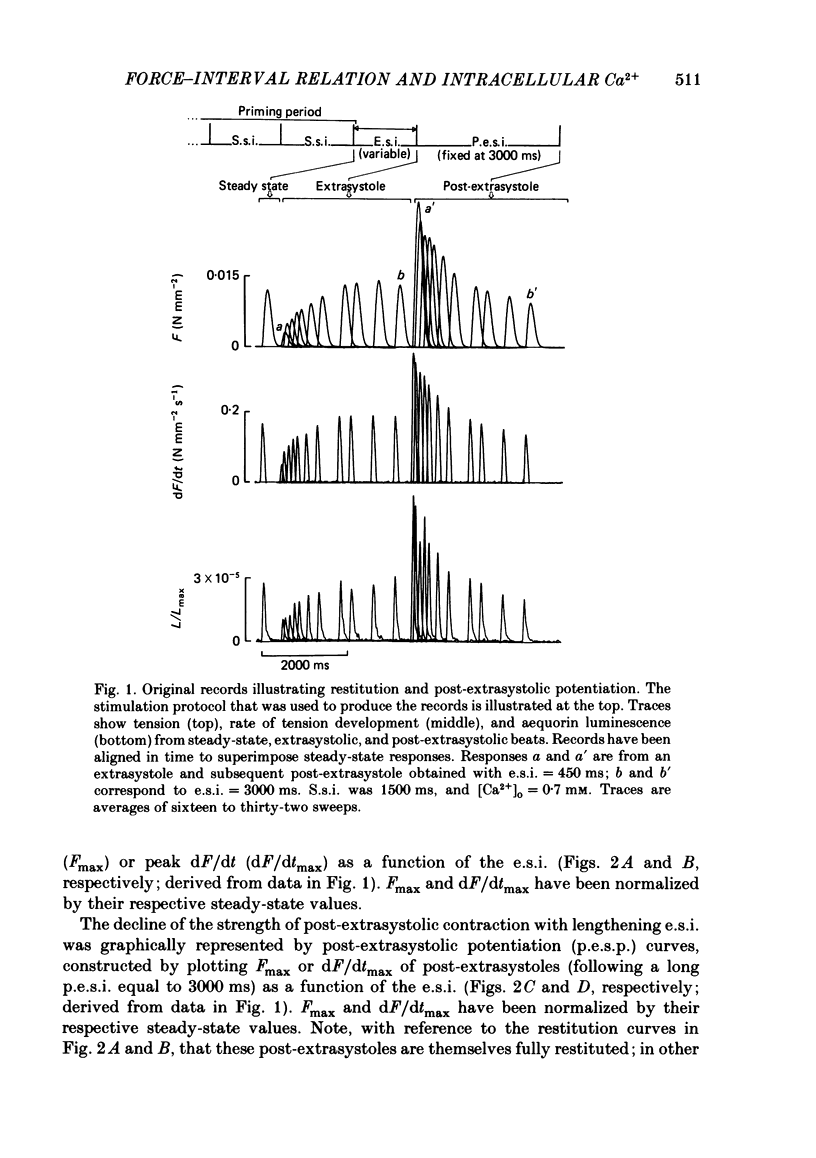
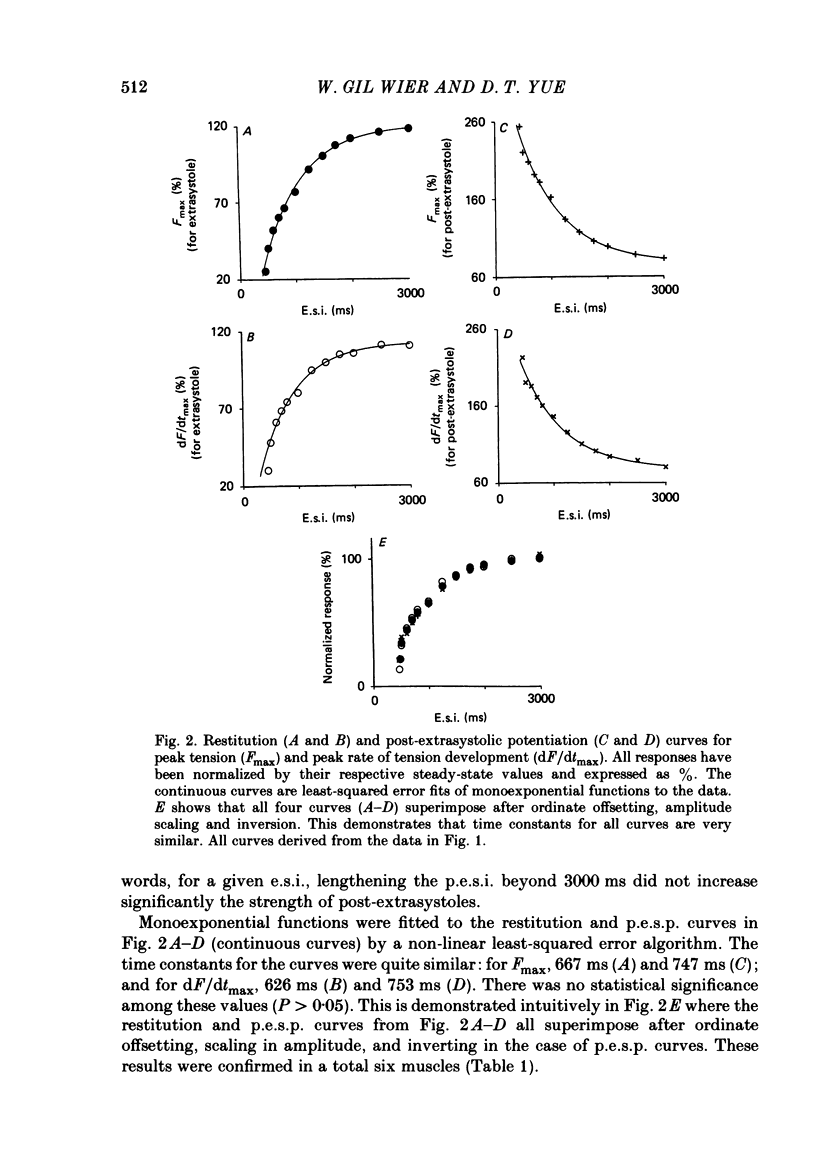
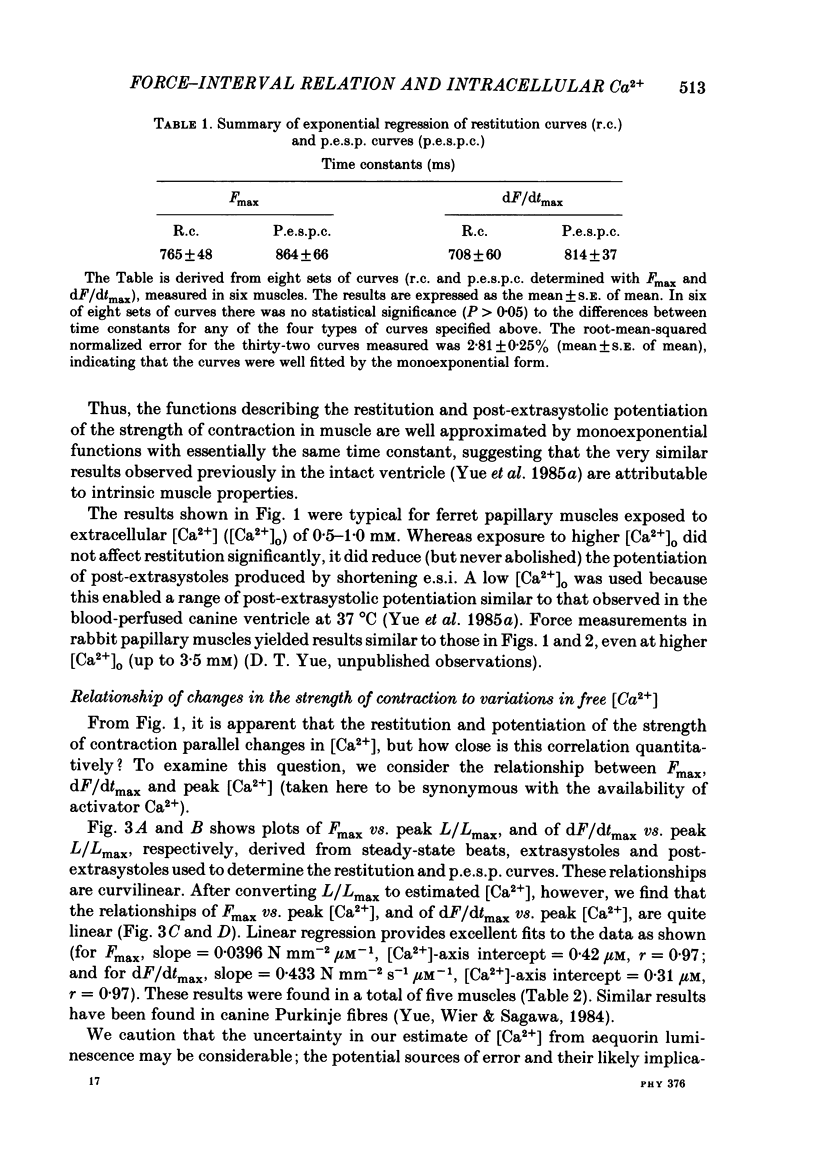
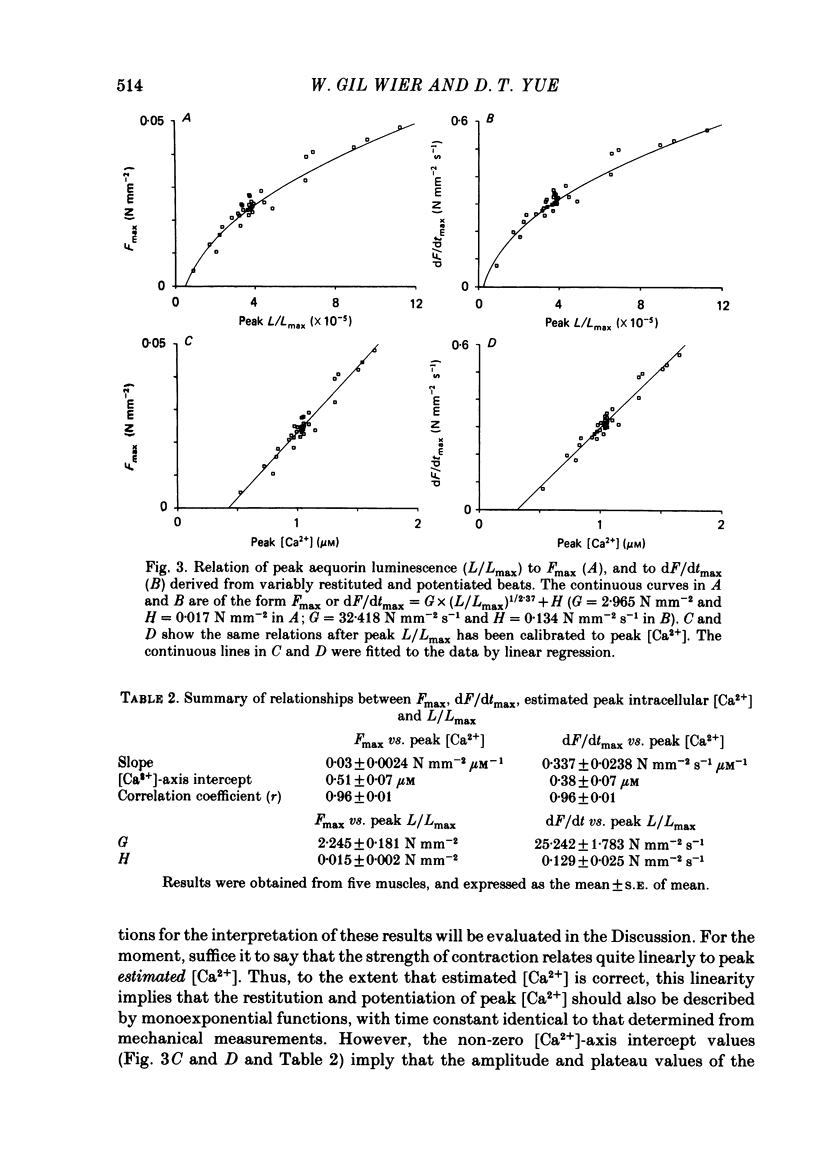
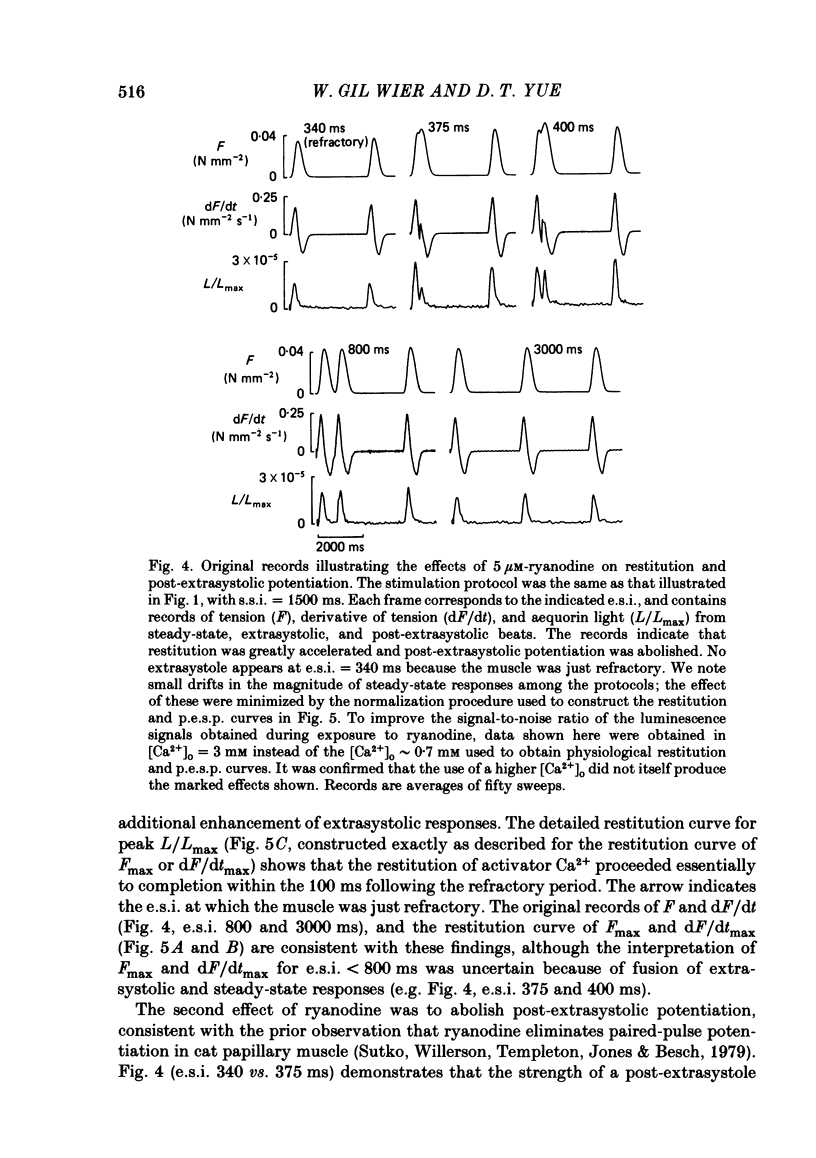
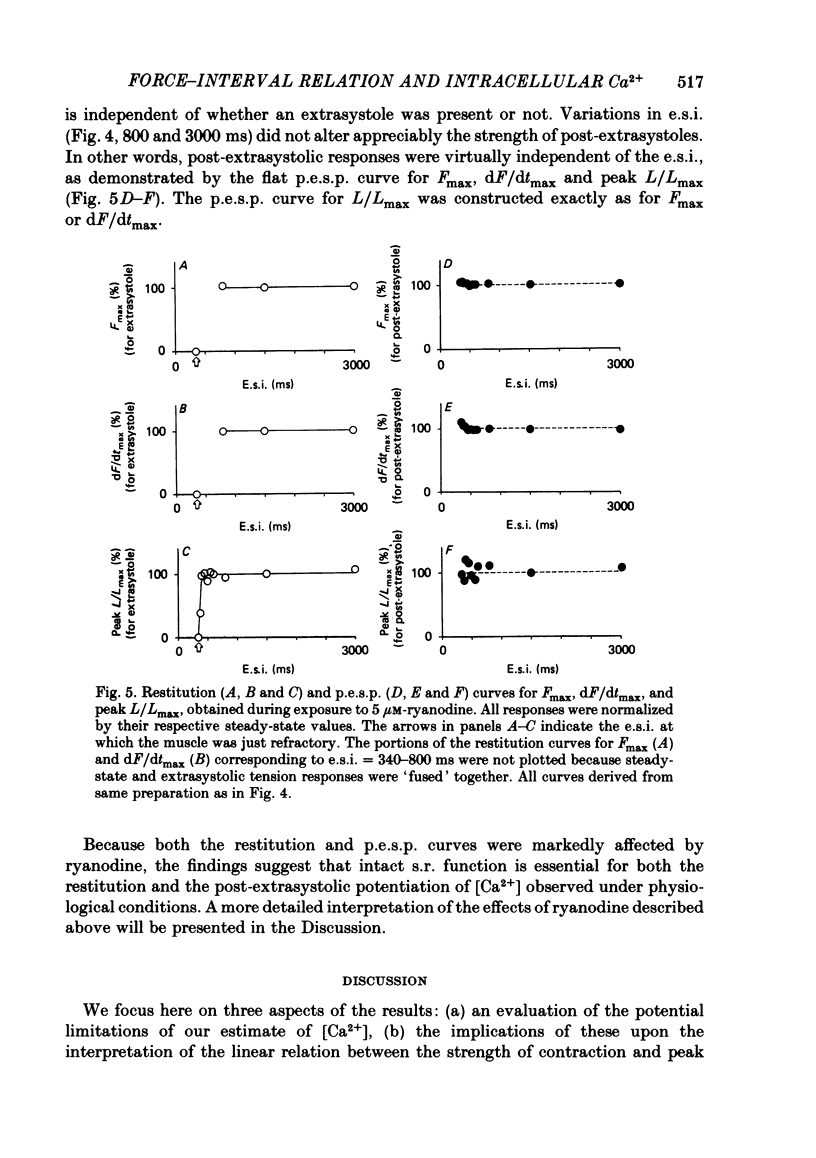
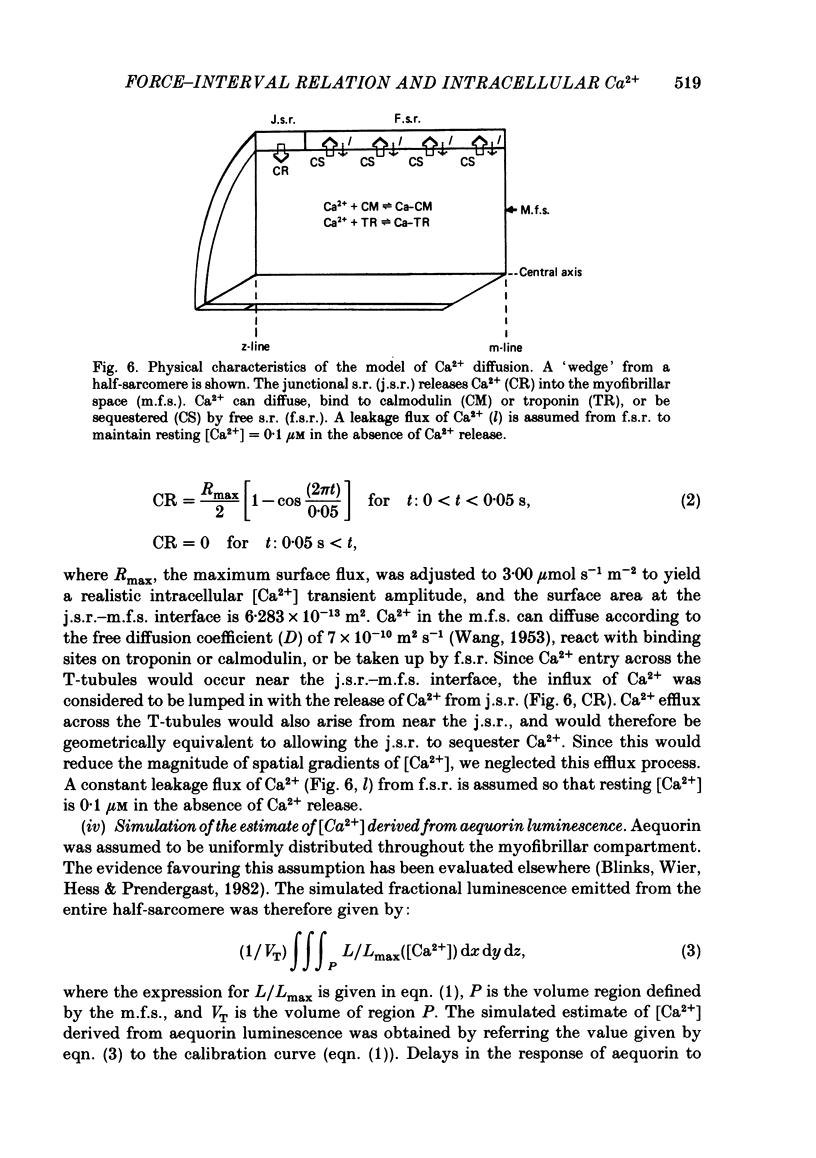
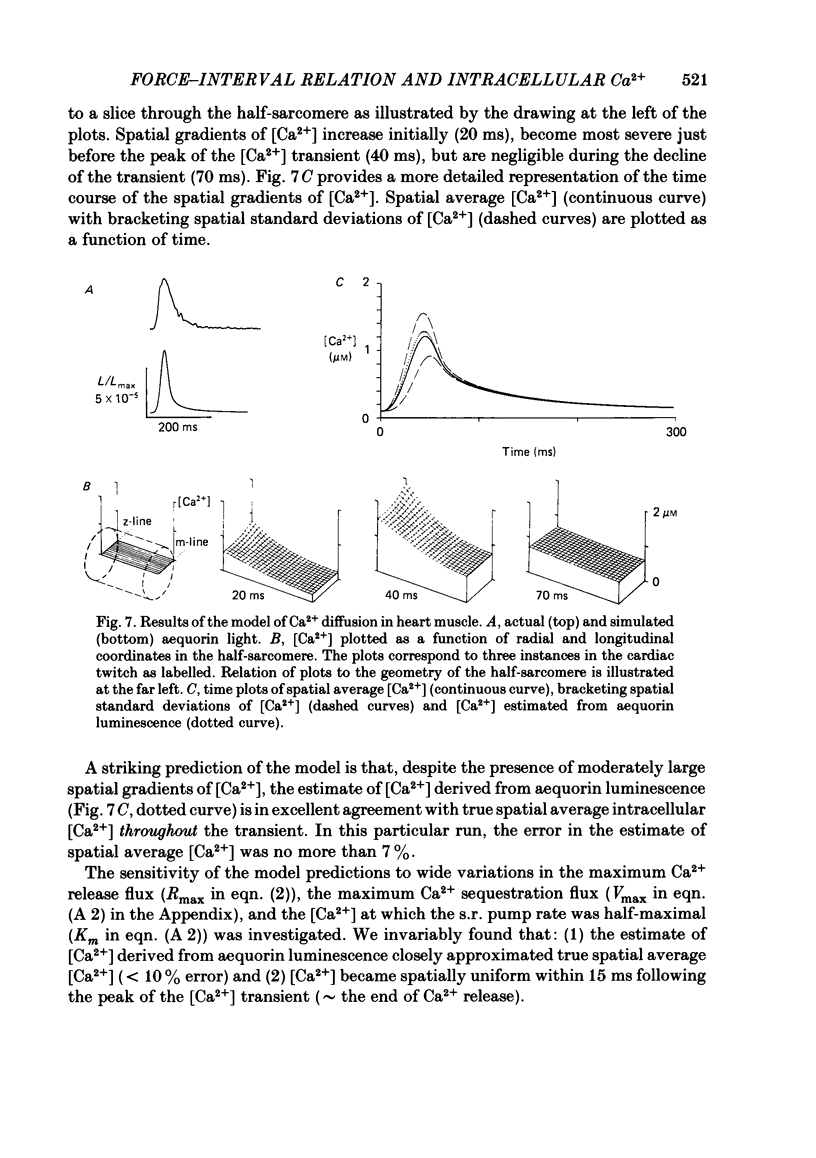
The influence of short-term changes in stimulation pattern, both on the strength of contraction and on the amplitude of intracellular free-Ca2+ transients, was investigated in ferret papillary muscles. Intracellular free-Ca2+ concentration ([Ca2+]) was assessed from the luminescence emitted from muscles microinjected with the Ca2+-sensitive photoprotein aequorin. The relationships between the strength of contraction and changes in stimulation pattern lasting 1-2 beats could be described by monoexponential functions, all with very similar time constants (approximately 750 ms at 30 degrees C). Over the entire range that could be obtained, the strength of contraction, quantified by either peak tension or peak rate of tension development, was found to be linearly correlated with peak estimated [Ca2+]. Potential errors in the estimation of [Ca2+] from aequorin luminescence were analysed. To assess the influence of spatial non-homogeneities of [Ca2+] on the estimate of [Ca2+], a model of Ca2+ diffusion in heart muscle was developed. The possible effect of using an inaccurate calibration curve was also examined. The results of these analyses indicate that [Ca2+] estimated from aequorin luminescence should be proportional to, if not equal to, true spatial average [Ca2+] (errors less than 7%). Given the conclusion of the analysis described above, it is inferred from points 2 and 3 that the relationships between peak spatial average [Ca2+] and short-term changes in stimulation pattern are also represented by monoexponential functions, with time constants closely similar to those for the mechanical measurements. Exposure to ryanodine, a substance believed to inhibit the release of Ca2+ from sarcoplasmic reticulum, produced striking alterations in the pattern of variations in [Ca2+] mentioned above. These alterations are consistent with the hypothesis that the functions described above depend essentially upon properties of the sarcoplasmic reticulum.
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. G., Blinks J. R. Calcium transients in aequorin-injected frog cardiac muscle. Nature. 1978 Jun 15;273(5663):509–513. doi: 10.1038/273509a0. [DOI] [PubMed] [Google Scholar]
- Allen D. G., Kurihara S. Calcium transients in mammalian ventricular muscle. Eur Heart J. 1980;Suppl A:5–15. doi: 10.1093/eurheartj/1.suppl_1.5. [DOI] [PubMed] [Google Scholar]
- BRAVENY P., KRUTA V. Dissociation de deux facteurs: restitution et potentiation dans l'action de l'intervalle sur l'amplitude de la contraction du myocarde. Arch Int Physiol Biochim. 1958 Nov;66(4):633–652. doi: 10.3109/13813455809084239. [DOI] [PubMed] [Google Scholar]
- Baker P. F., Hodgkin A. L., Ridgway E. B. Depolarization and calcium entry in squid giant axons. J Physiol. 1971 Nov;218(3):709–755. doi: 10.1113/jphysiol.1971.sp009641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blinks J. R., Wier W. G., Hess P., Prendergast F. G. Measurement of Ca2+ concentrations in living cells. Prog Biophys Mol Biol. 1982;40(1-2):1–114. doi: 10.1016/0079-6107(82)90011-6. [DOI] [PubMed] [Google Scholar]
- Burkhoff D., Yue D. T., Franz M. R., Hunter W. C., Sagawa K. Mechanical restitution of isolated perfused canine left ventricles. Am J Physiol. 1984 Jan;246(1 Pt 2):H8–16. doi: 10.1152/ajpheart.1984.246.1.H8. [DOI] [PubMed] [Google Scholar]
- Cannell M. B., Allen D. G. Model of calcium movements during activation in the sarcomere of frog skeletal muscle. Biophys J. 1984 May;45(5):913–925. doi: 10.1016/S0006-3495(84)84238-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung W. Y. Calmodulin plays a pivotal role in cellular regulation. Science. 1980 Jan 4;207(4426):19–27. doi: 10.1126/science.6243188. [DOI] [PubMed] [Google Scholar]
- Cohen S. M., Burt C. T. 31P nuclear magnetic relaxation studies of phosphocreatine in intact muscle: determination of intracellular free magnesium. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4271–4275. doi: 10.1073/pnas.74.10.4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson M. J., Gadian D. G., Wilkie D. R. Muscular fatigue investigated by phosphorus nuclear magnetic resonance. Nature. 1978 Aug 31;274(5674):861–866. doi: 10.1038/274861a0. [DOI] [PubMed] [Google Scholar]
- Edman K. A., Jóhannsson M. The contractile state of rabbit papillary muscle in relation to stimulation frequency. J Physiol. 1976 Jan;254(3):565–581. doi: 10.1113/jphysiol.1976.sp011247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am J Physiol. 1983 Jul;245(1):C1–14. doi: 10.1152/ajpcell.1983.245.1.C1. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Myoplasmic free calcium concentration reached during the twitch of an intact isolated cardiac cell and during calcium-induced release of calcium from the sarcoplasmic reticulum of a skinned cardiac cell from the adult rat or rabbit ventricle. J Gen Physiol. 1981 Nov;78(5):457–497. doi: 10.1085/jgp.78.5.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. Rapid ionic modifications during the aequorin-detected calcium transient in a skinned canine cardiac Purkinje cell. J Gen Physiol. 1985 Feb;85(2):189–246. doi: 10.1085/jgp.85.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. Simulated calcium current can both cause calcium loading in and trigger calcium release from the sarcoplasmic reticulum of a skinned canine cardiac Purkinje cell. J Gen Physiol. 1985 Feb;85(2):291–320. doi: 10.1085/jgp.85.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons W. R., Fozzard H. A. Voltage dependence and time dependence of contraction in sheep cardiac Purkinje fibers. Circ Res. 1971 Apr;28(4):446–460. doi: 10.1161/01.res.28.4.446. [DOI] [PubMed] [Google Scholar]
- HOFFMAN B. F., BINDLER E., SUCKLING E. E. Postextrasystolic potentiation of contraction in cardiac muscle. Am J Physiol. 1956 Apr;185(1):95–102. doi: 10.1152/ajplegacy.1956.185.1.95. [DOI] [PubMed] [Google Scholar]
- Hibberd M. G., Jewell B. R. Calcium- and length-dependent force production in rat ventricular muscle. J Physiol. 1982 Aug;329:527–540. doi: 10.1113/jphysiol.1982.sp014317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg G. Ca entry and contraction as studied in isolated bovine ventricular myocytes. Z Naturforsch C. 1982 May-Jun;37(5-6):502–512. doi: 10.1515/znc-1982-5-623. [DOI] [PubMed] [Google Scholar]
- Kass R. S., Sanguinetti M. C. Inactivation of calcium channel current in the calf cardiac Purkinje fiber. Evidence for voltage- and calcium-mediated mechanisms. J Gen Physiol. 1984 Nov;84(5):705–726. doi: 10.1085/jgp.84.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marban E., Wier W. G. Ryanodine as a tool to determine the contributions of calcium entry and calcium release to the calcium transient and contraction of cardiac Purkinje fibers. Circ Res. 1985 Jan;56(1):133–138. doi: 10.1161/01.res.56.1.133. [DOI] [PubMed] [Google Scholar]
- Mitchell M. R., Powell T., Terrar D. A., Twist V. W. Ryanodine prolongs Ca-currents while suppressing contraction in rat ventricular muscle cells. Br J Pharmacol. 1984 Jan;81(1):13–15. doi: 10.1111/j.1476-5381.1984.tb10735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page E. Quantitative ultrastructural analysis in cardiac membrane physiology. Am J Physiol. 1978 Nov;235(5):C147–C158. doi: 10.1152/ajpcell.1978.235.5.C147. [DOI] [PubMed] [Google Scholar]
- Polimeni P. I. Extracellular space and ionic distribution in rat ventricle. Am J Physiol. 1974 Sep;227(3):676–683. doi: 10.1152/ajplegacy.1974.227.3.676. [DOI] [PubMed] [Google Scholar]
- Robertson S. P., Johnson J. D., Potter J. D. The time-course of Ca2+ exchange with calmodulin, troponin, parvalbumin, and myosin in response to transient increases in Ca2+. Biophys J. 1981 Jun;34(3):559–569. doi: 10.1016/S0006-3495(81)84868-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigekawa M., Finegan J. A., Katz A. M. Calcium transport ATPase of canine cardiac sarcoplasmic reticulum. A comparison with that of rabbit fast skeletal muscle sarcoplasmic reticulum. J Biol Chem. 1976 Nov 25;251(22):6894–6900. [PubMed] [Google Scholar]
- Solaro R. J., Wise R. M., Shiner J. S., Briggs F. N. Calcium requirements for cardiac myofibrillar activation. Circ Res. 1974 Apr;34(4):525–530. doi: 10.1161/01.res.34.4.525. [DOI] [PubMed] [Google Scholar]
- Sutko J. L., Kenyon J. L. Ryanodine modification of cardiac muscle responses to potassium-free solutions. Evidence for inhibition of sarcoplasmic reticulum calcium release. J Gen Physiol. 1983 Sep;82(3):385–404. doi: 10.1085/jgp.82.3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutko J. L., Willerson J. T., Templeton G. H., Jones L. R., Besch H. R., Jr Ryanodine: its alterations of cat papillary muscle contractile state and responsiveness to inotropic interventions and a suggested mechanism of action. J Pharmacol Exp Ther. 1979 Apr;209(1):37–47. [PubMed] [Google Scholar]
- Tritthart H., Kaufmann R., Volkmer H. P., Bayer R., Krause H. Ca-movement controlling myocardial contractility. I. Voltage-, current- and time-dependence of mechanical activity under voltage clamp conditions (cat papillary muscles and trabeculae). Pflugers Arch. 1973 Feb 6;338(3):207–231. doi: 10.1007/BF00587388. [DOI] [PubMed] [Google Scholar]
- Wier W. G. Calcium transients during excitation-contraction coupling in mammalian heart: aequorin signals of canine Purkinje fibers. Science. 1980 Mar 7;207(4435):1085–1087. doi: 10.1126/science.7355274. [DOI] [PubMed] [Google Scholar]
- Wier W. G., Hess P. Excitation-contraction coupling in cardiac Purkinje fibers. Effects of cardiotonic steroids on the intracellular [Ca2+] transient, membrane potential, and contraction. J Gen Physiol. 1984 Mar;83(3):395–415. doi: 10.1085/jgp.83.3.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wier W. G., Kort A. A., Stern M. D., Lakatta E. G., Marban E. Cellular calcium fluctuations in mammalian heart: direct evidence from noise analysis of aequorin signals in Purkinje fibers. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7367–7371. doi: 10.1073/pnas.80.23.7367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wier W. G., Yue D. T., Marban E. Effects of ryanodine on intracellular Ca2+ transients in mammalian cardiac muscle. Fed Proc. 1985 Dec;44(15):2989–2993. [PubMed] [Google Scholar]
- Wohlfart B. Relationships between peak force, action potential duration and stimulus interval in rabbit myocardium. Acta Physiol Scand. 1979 Aug;106(4):395–409. doi: 10.1111/j.1748-1716.1979.tb06419.x. [DOI] [PubMed] [Google Scholar]
- Wood E. H., Heppner R. L., Weidmann S. Inotropic effects of electric currents. I. Positive and negative effects of constant electric currents or current pulses applied during cardiac action potentials. II. Hypotheses: calcium movements, excitation-contraction coupling and inotropic effects. Circ Res. 1969 Mar;24(3):409–445. doi: 10.1161/01.res.24.3.409. [DOI] [PubMed] [Google Scholar]
- Yue D. T., Burkhoff D., Franz M. R., Hunter W. C., Sagawa K. Postextrasystolic potentiation of the isolated canine left ventricle. Relationship to mechanical restitution. Circ Res. 1985 Mar;56(3):340–350. doi: 10.1161/01.res.56.3.340. [DOI] [PubMed] [Google Scholar]
- Yue D. T., Marban E., Wier W. G. Relationship between force and intracellular [Ca2+] in tetanized mammalian heart muscle. J Gen Physiol. 1986 Feb;87(2):223–242. doi: 10.1085/jgp.87.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue D. T., Wier W. G. Estimation of intracellular [Ca2+] by nonlinear indicators. A quantitative analysis. Biophys J. 1985 Sep;48(3):533–537. doi: 10.1016/S0006-3495(85)83810-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


