Abstract
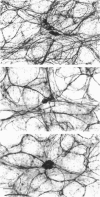
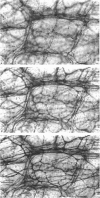
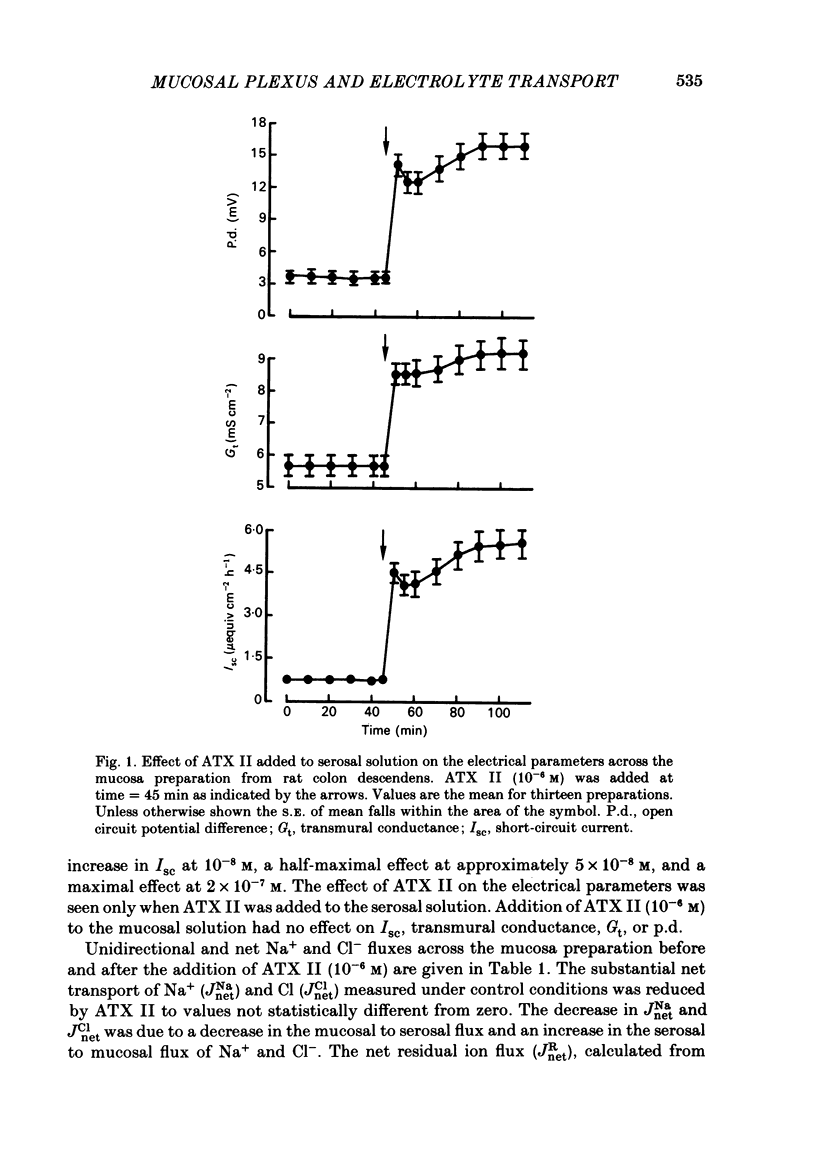
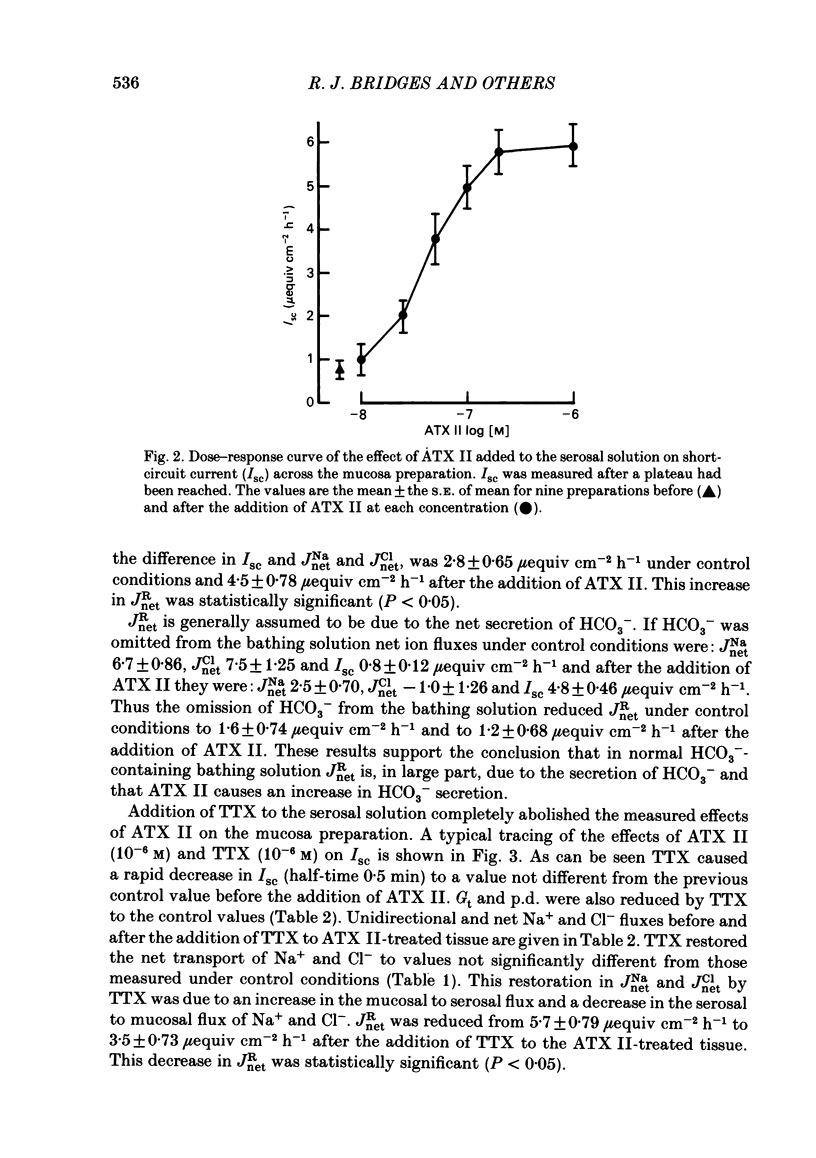
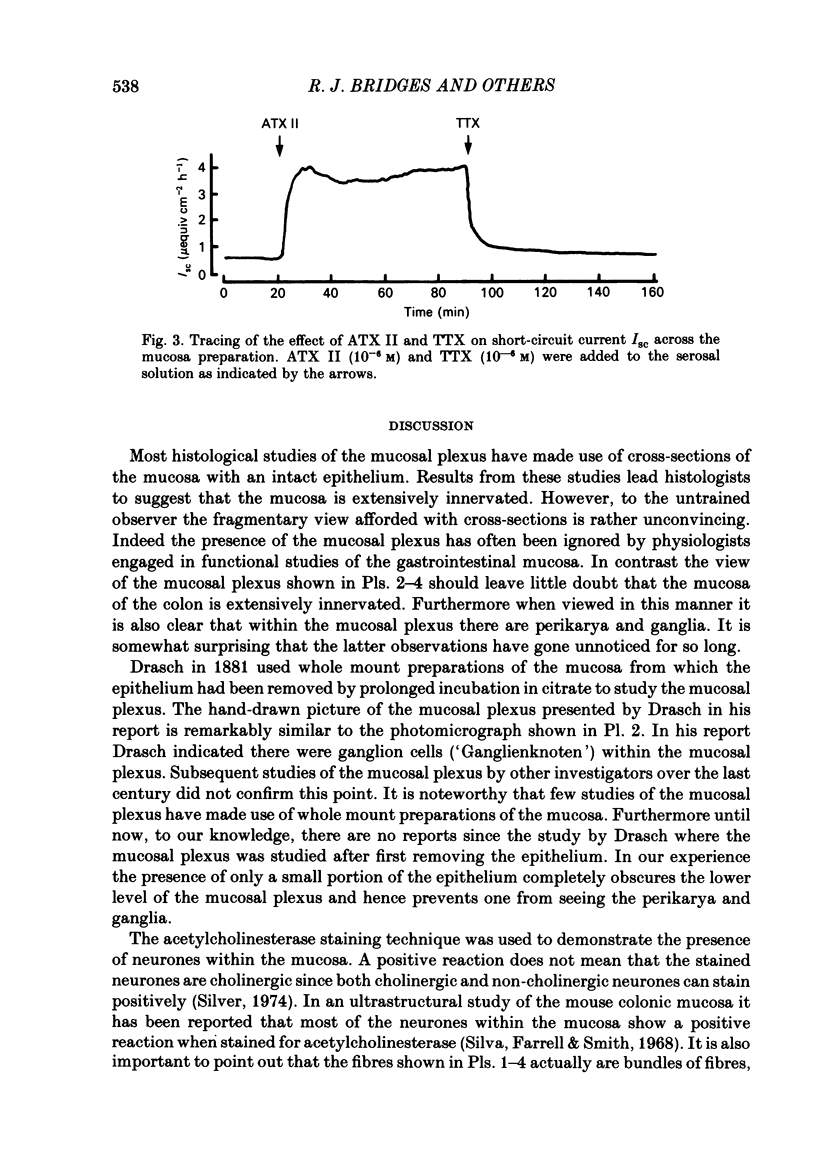
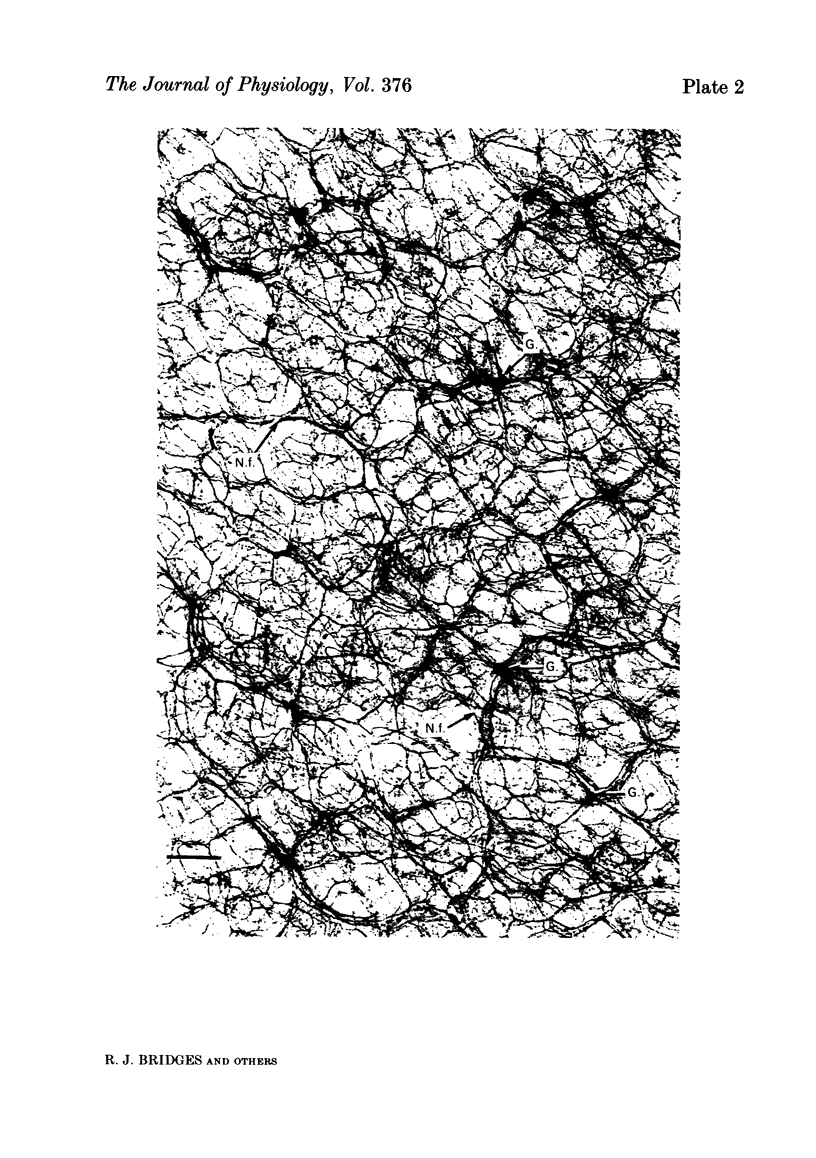
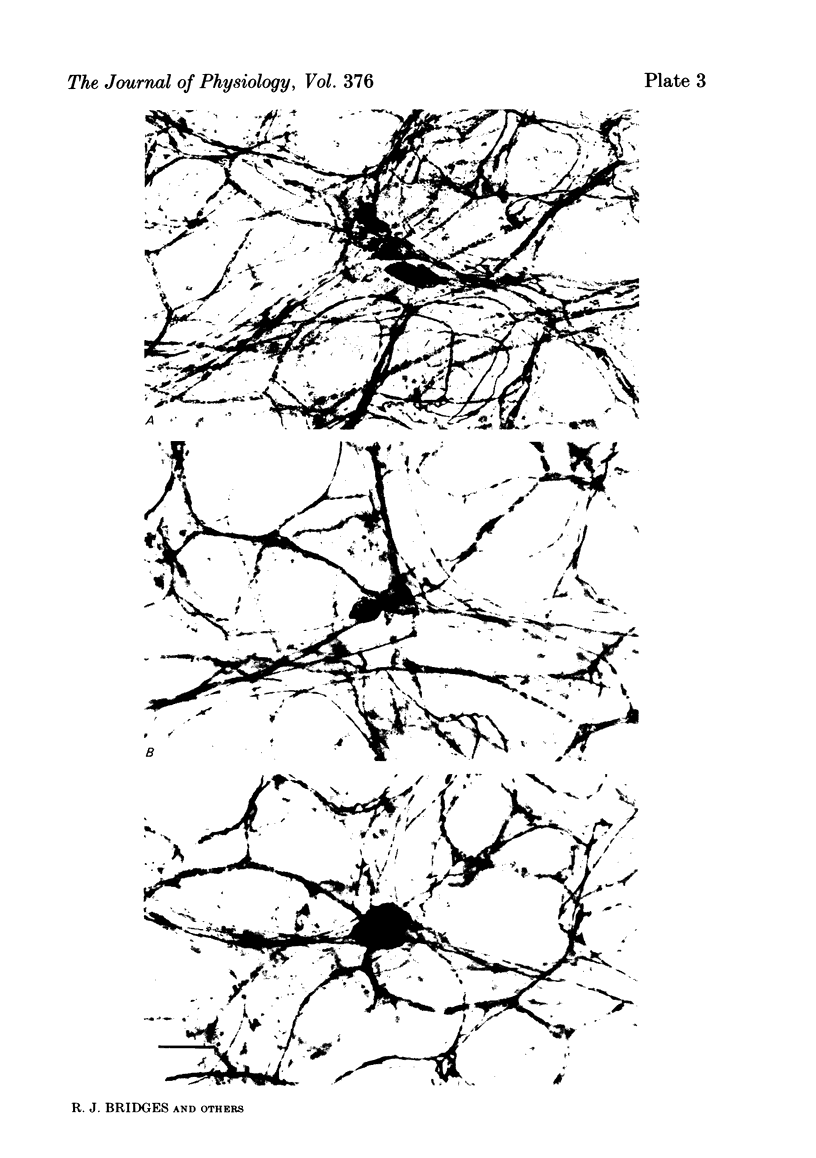
Histological and functional studies were performed on a preparation of rat colonic mucosa from which the myenteric and submucosal plexus were removed. This preparation, referred to as the mucosa preparation, was used to investigate the potential influence of the mucosal plexus on electrolyte transport. Two neuropharmacologically active agents were used: sea anemone toxin (ATX II) to stimulate the fibres of the mucosal plexus and tetrodotoxin (TTX) to block the fibres of the mucosal plexus. The morphology of the neuronal network of the mucosal plexus was visualized after the epithelium was removed and whole mount preparations of the lamina propria and circular muscle layer of muscularis mucosae were stained histochemically for acetylcholinesterase activity. Several levels of organization within the mucosal plexus were seen. Each crypt is encircled by a thin bundle of fibres near the top. These thin fibres connect with thicker bundles of fibres that encircle groups of two to five crypts in a broad band. These bundles of fibres are in turn connected to larger bundles of fibres which lie in a flat plane just below the crypts along the circular muscle layer of muscularis mucosae. In addition perikarya and ganglia were revealed within the mucosal plexus. The base-line net transport of Na+ and Cl- across the mucosa preparation was completely inhibited by ATX II (10(-6) M). This effect of ATX II on net Na+ and Cl- transport was accompanied with an increase in the short-circuit current (Isc), transmural conductance, and open-circuit potential difference across the mucosa preparation. The effect of ATX II on Isc was dose dependent with a half-maximal effective concentration at 5 X 10(-8) M-ATX II and a maximal effective concentration of 10(-7) M. ATX II was effective only when added to the serosal solution. Net Na+ and Cl- transport was restored by TTX (10(-6) M) to base-line values in ATX II-treated tissue. In addition the value of all three electrical parameters rapidly returned to the values measured before the addition of ATX II. TTX was effective in antagonizing the effects of ATX II only when added to the serosal solution. The results suggest that the regulation of electrolyte transport across the epithelium is at least one function of the mucosal plexus. Stimulation of the neurones within the mucosal plexus leads to the inhibition of electrolyte absorption.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andres H., Rock R., Bridges R. J., Rummel W., Schreiner J. Submucosal plexus and electrolyte transport across rat colonic mucosa. J Physiol. 1985 Jul;364:301–312. doi: 10.1113/jphysiol.1985.sp015746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjerknes M., Cheng H. Methods for the isolation of intact epithelium from the mouse intestine. Anat Rec. 1981 Apr;199(4):565–574. doi: 10.1002/ar.1091990412. [DOI] [PubMed] [Google Scholar]
- Bock R., Mühlen K aus der Beiträge zur funktionellen Morphologie der Neurohypophyse. I. Uber ein "gomoripostitive" Substanz in der Zona extrtna infundibuli beidseitig adrenalektomierter weisser Mäuse. Z Zellforsch Mikrosk Anat. 1968;92(1):130–148. [PubMed] [Google Scholar]
- Béress L., Béress R. Purification of three polypeptides with neuro- and cardiotoxic activity from the sea anemone Anemonia sulcata. Toxicon. 1975 Nov;13(5):359–367. doi: 10.1016/0041-0101(75)90196-8. [DOI] [PubMed] [Google Scholar]
- Béress L., Béress R., Wunderer G. Isolation and characterisation of three polypeptides with neurotoxic activity from Anemonia sulcata. FEBS Lett. 1975 Feb 15;50(3):311–314. doi: 10.1016/0014-5793(75)80517-5. [DOI] [PubMed] [Google Scholar]
- Catterall W. A. Neurotoxins that act on voltage-sensitive sodium channels in excitable membranes. Annu Rev Pharmacol Toxicol. 1980;20:15–43. doi: 10.1146/annurev.pa.20.040180.000311. [DOI] [PubMed] [Google Scholar]
- Hubel K. A. Intestinal nerves and ion transport: stimuli, reflexes, and responses. Am J Physiol. 1985 Mar;248(3 Pt 1):G261–G271. doi: 10.1152/ajpgi.1985.248.3.G261. [DOI] [PubMed] [Google Scholar]
- KARNOVSKY M. J., ROOTS L. A "DIRECT-COLORING" THIOCHOLINE METHOD FOR CHOLINESTERASES. J Histochem Cytochem. 1964 Mar;12:219–221. doi: 10.1177/12.3.219. [DOI] [PubMed] [Google Scholar]
- Narahashi T. Chemicals as tools in the study of excitable membranes. Physiol Rev. 1974 Oct;54(4):813–889. doi: 10.1152/physrev.1974.54.4.813. [DOI] [PubMed] [Google Scholar]
- Silva D. G., Farrell K. E., Smith G. C. Ultrastructural and histochemical studies on the innervation of the mucous membrane of the mouse colon. Anat Rec. 1968 Oct;162(2):157–176. doi: 10.1002/ar.1091620204. [DOI] [PubMed] [Google Scholar]
- Tapper E. J. Local modulation of intestinal ion transport by enteric neurons. Am J Physiol. 1983 May;244(5):G457–G468. doi: 10.1152/ajpgi.1983.244.5.G457. [DOI] [PubMed] [Google Scholar]