Abstract
Purpose
Telomere dysfunction is believed to be a significant factor in carcinogenesis. To elucidate the carcinogenesis mechanism in gastric cancer, the expression of telomeric proteins and changes in telomere length were investigated during multistage carcinogenesis of gastric cancer.
Methods
Tissue samples were obtained during surgical operations from the normal gastric mucosa of 10 patients, the precancerous lesions of 15 patients, the gastric cancer tissues (GC) of 20 patients, and of tumors due to gastric cancer with lymph node metastasis (GCLM) from 5 patients. The expression of TRF1, TRF2, and TIN2 proteins was measured by Western blotting, while the expression of TERT, KU70, and BRCA1 proteins was detected using the immunohistochemical method. The mean telomere length was determined by Southern blotting.
Results
Compared with normal gastric mucosa tissues, the expression of TRF1, TRF2, and TIN2 proteins was significantly higher in precancerous lesions, GC, and GCLM (P < 0.01). The expression of TRF1, TRF2, and TIN2 proteins was significantly higher in GC and GCLM than in precancerous lesions (P < 0.01). The expression of TERT and Ku70 proteins in precancerous lesions and GC tissues was significantly higher than that in normal gastric mucosa tissues (P < 0.01). The expression of TERT and Ku70 proteins in GC tissues was significantly higher than in precancerous lesions (P < 0.01). In normal gastric mucosa, the BRCA1 protein was primarily located in the cell nucleus. In precancerous lesions and GC, the expression of the BRCA1 protein was apparent in the cell cytoplasm. The mean telomere length in precancerous lesions, GC, and GCLM was significantly shorter than that in normal gastric mucosa tissues (P < 0.05). The mean telomere length in GC and GCLM was significantly shorter than that in precancerous lesions (P < 0.05). The mean telomere length in all tissue samples was inversely correlated with the level of TRF1, TRF2, TIN2, TERT, and Ku70 proteins.
Conclusions
Our results suggest that the over-expression of telomeric proteins, TRF1, TRF2, TIN2, TERT, and Ku70, and the transposition of the BRCA1 protein may work together to reduce the telomere length in precancerous lesions and gastric cancer, and could contribute to the multistage carcinogenesis of gastric cancer. These findings offer new insight into the mechanism of carcinogenesis in gastric cancer.
Keywords: Telomere, Telomeric protein, Gastric cancer, Carcinogenesis
Introduction
Gastric cancer is a global killer, the burden of which is currently shifting from developed to developing countries, especially China. It has been suggested that the carcinogenesis of gastric cancer is a multistage process that proceeds from normal gastric mucosa to precancerous lesions, to infiltrating carcinoma, and finally, to metastatic carcinoma. Telomeres, which consist of a repetitive G-rich DNA sequence and telomeric proteins, are located in the linear chromosome ends of eukaryotic organisms. The function of telomeres is the prevention of end-to-end chromosome fusion and genomic instability. These are regulated by telomeric proteins through telomere end-capping and length control (Blackburn 1991; Blasco 2005; Chen et al. 2007). A number of human telomeric proteins have been identified, including telomeric repeat binding factors 1 and 2 (TRF1 and TRF2), TRF1-interacting protein 2 (TIN2), telomerase reverse transcriptase (TERT), Ku70, BRCA1, and other factors (Smith et al. 1997; Kim et al. 1999; de Lange et al. 2005; Broccoli et al. 1997). TRF1 and TRF2 directly bind as homodimers to double-stranded telomeric DNA and are involved primarily in telomere length regulation and the protection of telomere end-capping (Smogorzewska et al. 2000; van Steensel et al. 1997). TIN2 interacts directly with TRF1 and is a negative regulator of telomere elongation by telomerase-dependent mechanism (Ye et al. 2004). TERT is a core protein of telomerase that is responsible for the addition of the telomeric repeat sequence (Autexier et al. 2006). Ku70, which serves as the DNA-binding component of a DNA-dependent protein kinase, is thought to be involved in the repair of nonhomologous DNA ends. Ku70 also plays an important role in the regulation and maintenance of telomeres by binding TRF1 and TRF2 (Hsu et al. 1999; Song et al. 2000; Chai et al. 2002; Celli et al. 2006). It has been suggested that BRCA1, which functions as a tumor suppressor in human breast cancer cells, inhibits telomerase enzyme activity in order to control telomere length (Xiong et al. 2003; Li et al. 2002).
Telomere irregulation is a crucial mechanism in genetic instability (Hahn 2003). Many oncogenes, tumor suppressor genes, and apoptosis-related genes are involved in the multistage carcinogenesis of gastric cancer; however, the structure and function of telomeres may also play an important role in the process.
In the current study, the expression of telomeric proteins, TRF1, TRF2, TIN2, TERT, Ku70, and BRCA1, and telomere length were investigated in normal gastric mucosa, precancerous lesions, infiltrating carcinoma, and metastatic carcinoma. The relationship between these factors was analyzed.
Materials and methods
Sample collection
The study adhered to Chinese law and to the guidelines of the Ethics Committee of China. All samples were obtained from the First Affiliated Hospital, University of South China. Ten normal gastric mucosa and 15 precancerous lesion tissue samples were obtained from surgical operations performed on gastric ulcer or chronic atrophic gastritis patients. Twenty samples from GC and five from GCLM tissues were obtained from surgical operations performed on gastric cancer patients. The diagnosis of all samples was microscopically confirmed by frozen section. The collected samples were cut into two parts. In those samples with lesions, this division was performed according to the location of the lesions in the frozen sections. For each sample, one part was formalin-fixed and paraffin-embedded for advanced pathologic examination and immunohistochemical study. The other part was stored at −80°C while awaiting Western blotting and TRF analysis. The pathology of each case was reviewed by three pathology professors. Histological classification and tumor staging were performed according to the Lauren classification system and the tumor-node-metastasis classification system.
Western blotting
TRF1, TRF2, and TIN2 protein expression was detected using Western blotting analyses. Protein extraction was carried out using standard methods, and protein concentrations were determined by BCA assay (Beyotime Biotechnology, Haimen, China). Proteins were loaded onto a 15% SDS-polyacrylamide gel for electrophoresis, and then transferred onto nitrocellulose transfer membranes (Osmonics, USA) at 0.8 mA/cm2 for 3 h. Membranes were blocked at room temperature for 2 h with blocking solution (5% skimmed milk in Tris-buffered solution plus Tween-20). The membranes were then incubated overnight at 4°C with goat polyclonal antibody anti-TRF1sc-6165 1:300, anti-TRF2sc-8528 1:300, anti-TIN2sc-13645 1:300 (Santa Cruz Biotechnology, USA), or mouse monoclonal antibody anti-actin 1:1,000 (Zhongshan Goldbridge Biotechnology, China) in blocking solution. After being washed in TBST, membranes were incubated for 1 h at room temperature with a HRP-conjugated anti-goat secondary antibody (Zhongshan Goldbridge Biotechnology, China). Detection was performed by enhanced chemiluminescence (ECL) using a Western blotting luminol reagent (Tiangen Biotechnology, China) according to the manufacturer’s instructions. Film data were analyzed by AlphaImager 2200 soft, compute gray value (see β-actin as a reference).
Immunohistochemical determination
The expression of TERT, Ku70, and BRCA1 proteins in normal gastric mucosa, precancerous lesions, and GC tissues was determined using a standard streptavidin–horseradish peroxidase (S–P) technique (UltraSensitiveTM S–P kit, Fuzhou Maixin Biotechnology Development Co. Ltd., Fushou City, China), according to the manufacturer’s protocol. Phosphate-buffered saline (PBS), substituting for TERT, Ku70, and BRCA1, was used as a negative control. Rabbit polyclonal anti-TERT 1:200 (Biosynthesis Biotechnology, China), anti-Ku70 1:80 (Boster Biotechnology, China), and anti-BRCA1 were separately diluted with PBS and incubated at room temperature for 1 h.
The intensity of protein expression was graded as follows: 0, none; 1, weak; 2, moderate; 3, strong. The percentage of positive cells was evaluated as follows: 0, undetectable; 1, less than 25%; 2, 25–50%; 3, 50–75%; 4, more than 75%. The positive degree was obtained by multiplying the intensity and the area of expression and was classified as undetectable (−) with a score of 0, mild (+) with a score of 1–2, moderate (++) with a score of 3–4, and marked (+++) with a score of 5–7.
Telomere restriction fragment length analysis
The genomic DNA was extracted using the standard method, with proteinase K treatment and phenol/chloroform extraction from 25 to 50 mg of the frozen tissue. The telomere length of tissue DNA was quantitatively determined by Southern blot using Telo TAGGG telomere length assay (Roche Applied Science, Germany) according to the manufacturer’s instructions. Briefly, a 2 µg sample of DNA was digested with HinfI/RsaI, electrophoresed on 0.8% agarose gel, blotted onto a positively charged nylon membrane using an upward capillary transfer, hybridized with a telomeric DNA probe, and then detected with chemiluminescence. The mean telomere length was defined according to the following formula: TRF = Σ(ODi)/Σ(ODi/L i), where ODi is the chemiluminescent signal and L i is the length of the TRF fragment at position.
Statistical analysis
Results were analyzed using the SPSS11.5 statistical software package. All data were expressed as mean ± SD (standard deviation). Comparisons between different groups were made by one-way ANOVA, or chi-square (χ2) and linear regression test. P < 0.05 was taken as being statistically significant.
Results
Expression of TRF1, TRF2, TIN2 in normal gastric mucosa, precancerous lesions, and GC
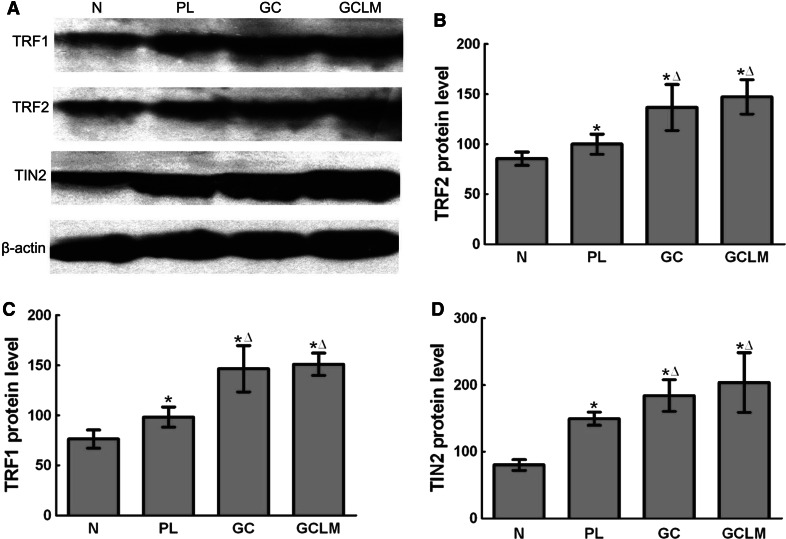
The expression of TRF1, TRF2, and TIN2 protein was evaluated by Western blotting (Fig. 1a). Compared with normal gastric mucosa tissues, the expression of TRF1, TRF2, and TIN2 proteins was significantly higher in precancerous lesions, GC, and GCLM (P < 0.01). The expression of TRF1, TRF2, and TIN2 proteins was significantly higher in GC and GCLM than in precancerous lesions (P < 0.01) (Figs. 1b–d).
Fig. 1.
Expression of TRF1, TRF2, TIN2 in normal gastric mucosa, precancerous lesions, and GC a The expression of TRF1, TRF2, and TIN2 protein was evaluated by Western blotting. Compared with normal gastric mucosa tissues, the expression of TRF1, TRF2, and TIN2 proteins was significantly higher in precancerous lesions, GC, and GCLM. The expression of TRF1, TRF2, and TIN2 proteins was significantly higher in GC and GCLM than in precancerous lesions. b–d Data of TRF1, TRF2 and TIN2 protein levels in various lesions were represented, respectively (mean ± SD). * P < 0.01 vs N. Δ P < 0.01 vs PL. N normal gastric mucosa, PL precancerous lesions, GC gastric cancer, GCLM gastric cancer with lymph node metastasis. The levels of TRF1, TRF2, TIN2 protein = [target protein’s gray density/β-actin’s gray density] × 100
The relationships between the expression of the TRF1, TRF2, and TIN2 proteins and patient clinico-pathological factors in GC are shown in Table 1. Only differentiation of tumors emerged as a significant parameter with the expressions of TRF1, TRF2, and TIN2 proteins (P < 0.05) No significant differences were found between the expression of the protein and age, gender, tumor size, or clinical stage.
Table 1.
The relationships between the expression of the TRF1, TRF2, and TIN2 proteins and patient clinico-pathological factors in GC
| Clinicopathologic factors | TRF1 (mean ± SD) |
P | TRF2 (mean ± SD) |
P | TIN2 (mean ± SD) |
P | Telomere length (kb) (mean ± SD) | P |
|---|---|---|---|---|---|---|---|---|
| Age (years) | 0.774 | 0.930 | 0.264 | 0.968 | ||||
| ≤50 (n = 7) | 148.7 ± 12.36 | 135.9 ± 12.60 | 175.8 ± 35.04 | 7.50 ± 0.821 | ||||
| >50 (n = 13) | 145.4 ± 27.68 | 136.9 ± 27.59 | 188.5 ± 14.63 | 7.74 ± 2.148 | ||||
| Gender | 0.695 | 0.443 | 0.168 | 0.175 | ||||
| Male (n = 10) | 144.5 ± 17.78 | 140.7 ± 29.03 | 191.5 ± 14.63 | 7.11 ± 1.798 | ||||
| Female (n = 10) | 148.7 ± 28.33 | 132.5 ± 15.54 | 176.7 ± 29.15 | 8.20 ± 1.653 | ||||
| Tumor length (cm) | 0.294 | 0.326 | 0.143 | 0.441 | ||||
| <4 (n = 10) | 141.0 ± 19.16 | 129.8 ± 11.23 | 176.2 ± 28.18 | 7.97 ± 2.289 | ||||
| >4 (n = 10) | 152.1 ± 26.32 | 143.4 ± 29.87 | 191.9 ± 15.95 | 7.34 ± 1.081 | ||||
| Differentiation | 0.002 | 0.012 | 0.014 | 0.011 | ||||
|
Well, moderate (n = 8) |
128.7 ± 20.09 | 121.5 ± 11.47 | 168.7 ± 29.53 | 8.68 ± 2.313 | ||||
| Poorly (n = 12) | 158.5 ± 16.75 | 146.7 ± 23.59 | 194.3 ± 11.42 | 6.98 ± 0.870 | ||||
| Clinical stage | 0.085 | 0.316 | 0.064 | 0.487 | ||||
| 1–2 (n = 8) | 135.7 ± 17.90 | 137.0 ± 34.69 | 177.19 ± 37.54 | 8.11 ± 2.670 | ||||
| 3–4 (n = 12) | 153.9 ± 23.99 | 136.3 ± 12.31 | 188.7 ± 3.98 | 7.35 ± 0.795 |
The expression of TERT, Ku70, and BRCA1 proteins in normal gastric mucosa, precancerous lesions, and GC
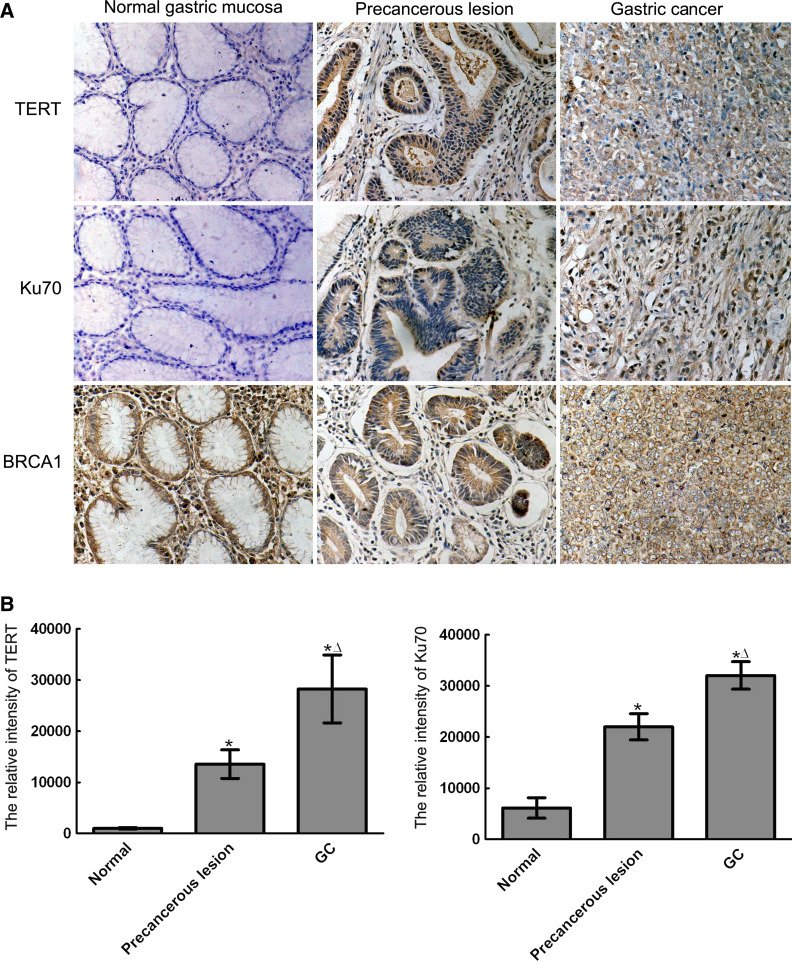
Using the S–P immunohistochemical method, positive results appeared as brown-stained particles in the cells. The expression of the TERT and Ku70 proteins was significantly up-regulated in precancerous lesion and in GC tissues. The expression of TERT and Ku70 proteins in GC tissues was significantly higher than in precancerous lesions (P < 0.01). In normal gastric mucosa, the BRCA1 protein was primarily located in the cell nucleus. In precancerous lesions and GC, expression of the BRCA1 protein was apparent in the cell cytoplasm (Fig. 2).
Fig. 2.
Expression of TERT, Ku70, and BRCA1 proteins in normal gastric mucosa, precancerous lesions, and GC (the S–P immunohistochemical method. original magnification 200×). a Positive results appeared as brown-stained particles in the cells. The expression of TERT and Ku70 protein was significantly up-regulated in precancerous lesion and in GC tissues. In normal gastric mucosa, the BRCA1 protein was primarily located in the cell nucleus. In precancerous lesions and GC, expression of the BRCA1 protein was apparent in the cell cytoplasm. b Data of TERT and Ku70 protein were represented, respectively (mean ± SD). * P < 0.01 vs N. Δ P < 0.01 vs PL
Telomere shortening during multistage carcinogenesis of GC
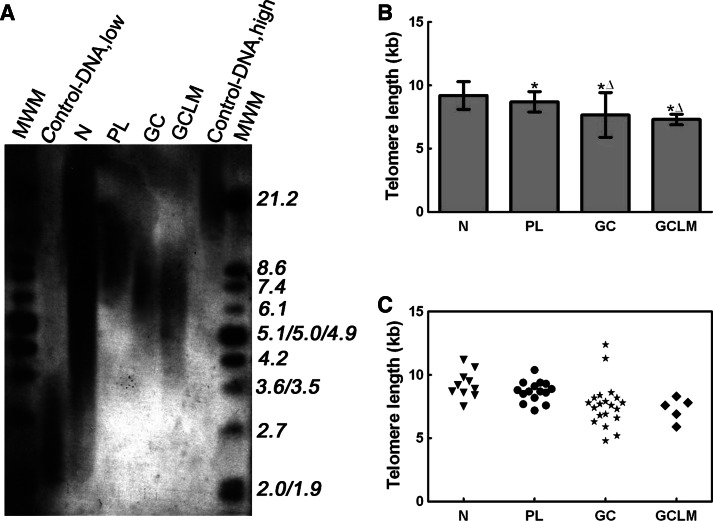
The Southern blot analysis of TRF length is shown in Fig. 3a. The mean telomere length in normal gastric mucosa, precancerous lesions, GC, and GCLM was 9.2 ± 1.09, 8.7 ± 0.81, 7.7 ± 1.77, and 7.3 ± 0.42 kb, respectively. The mean telomere length in precancerous lesions, GC, and GCLM was significantly shorter than that of normal gastric mucosa tissues (P < 0.01; Fig. 3b). The mean telomere length of GC and GCLM was significantly shorter than that of precancerous lesions (P < 0.01; Fig. 3b). The telomere length of the GC had a wide range, from 4.8 to 12.4 kb. The telomere length in two of the GC cases were >11 kb and thus significantly longer than that found in normal gastric mucosa tissues (Fig. 3c). Telomere length negatively correlated with patient age in normal gastric mucosa (R 2 = 0.851, P < 0.001), but no such linear correlation was found between telomere length and patient age in the GC samples (P > 0.05). The telomere length of the GC was not significantly associated with age, gender, tumor size, differentiation, or clinical stage (P > 0.05; Table 1).
Fig. 3.
Telomere restriction fragment length (TRF) in normal gastric mucosa, precancerous lesion and gastric cancer was analyzed. a The TRF length was examined by Southern blotting. b Data were represented by mean ± SD. The mean telomere length in precancerous lesions, GC, and GCLM was significantly shorter than that of normal gastric mucosa tissues. The mean telomere length of GC and GCLM was significantly shorter than that of precancerous lesions. c The TRF length in each sample. * P < 0.01 versus N. Δ P < 0.01 versus PL. N normal gastric mucosa, PL precancerous lesions, GC gastric cancer, GCLM gastric cancer with lymph node metastasis. The size markers and control-DNA are indicated on the side
TRF1, TRF2, TIN2, TERT, and Ku70 associated with telomere length regulation
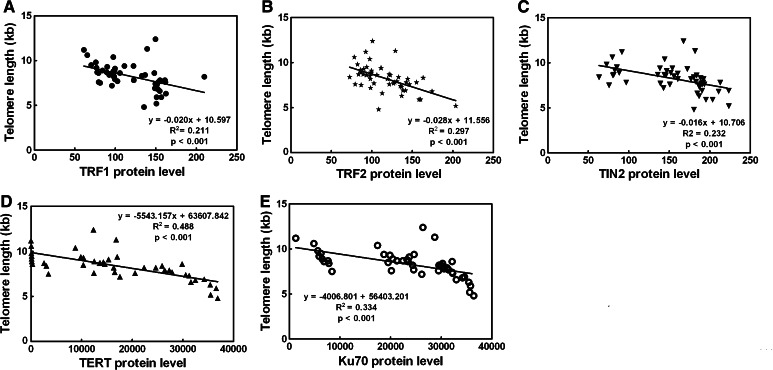
The levels of TRF1, TRF2, TIN2, TERT, and Ku70 proteins were analyzed for correlations with telomere length (Fig. 4). The results indicated that telomere length was significantly negatively correlated with TRF1, TRF2, TIN2, TERT, and Ku70 (R 2 = 0.211, P < 0.001; R 2 = 0.297, P < 0.001; R 2 = 0.232, P < 0.001; R 2 = 0.488, P < 0.001; R 2 = 0.334, P < 0.001, respectively).
Fig. 4.
TRF1, TRF2, TIN2, TERT, and Ku70 associated with telomere length regulation. The levels of TRF1, TRF2, TIN2, TERT, and Ku70 proteins were analyzed for correlations with telomere length and shown in a–e, respectively
Discussion
Gastric cancer is the fourth most frequent cancer type worldwide and the second most common cause of cancer-related death (Chen et al. 2004). The carcinogenesis of gastric cancer is thought to result from a combination of environmental factors and the accumulation of specific genetic alterations due to increasing genetic instability. Telomeres serve multiple functions in the preservation of chromosome stability. They protect the ends of chromosomes from degradation and prevent end-to-end chromosome fusion. The maintenance of telomere structure and function depends on the interaction between telomere length and telomeric proteins.
The alteration of telomere length in tumors has been reported in several previous studies. Kim et al. (2002) found that the mean telomere length was significantly reduced in colorectal cancers, while Oh et al. (2005) found a gradual shortening of telomeres during human multi-step hepatocarcinogenesis. Other studies have demonstrated a lengthening of telomeres in renal cell carcinomas (Dahse et al. 1999) and basal cell carcinomas of the skin (Wainwright et al. 1995). Our results have shown a significant reduction in mean telomere length in precancerous lesions, GC, and GCLM compared to that found in normal gastric mucosa tissues. The mean telomere lengths in GC and GCLM were significantly shorter than that in precancerous lesions. In normal gastric mucosa, mean telomere length negatively correlated with patient age but no such correlation was found in GC. Telomere shortening might be a symptom of an early stage in the carcinogenesis of gastric cancer. Telomere shortening could lead to the genetic instability that promotes carcinogenesis and the development of gastric cancer. Furthermore, our study found that two (10%) of the GC samples had telomeres >11 kb. This was significantly longer than those in normal gastric mucosa. There are two possible explanations for this. First, a mechanism of alternative lengthening of the telomere (ALT) may maintain telomere length (Reddel et al. 2001). Second, higher telomerase activity may occur in earlier stages of GC to compensate for later telomere shortening.
A number of previous studies have demonstrated that mRNA levels of TRF1, TRF2, and TIN2 are increased in gastric cancer (Miyachi et al. 2002), liver cancer (Oh et al. 2005), adult T-cell leukemia (Bellon et al. 2006), and colorectal carcinoma (Garcia-Aranda et al. 2006). Other studies have demonstrated that mRNA levels of TRF1, TRF2, and TIN2 are decreased in gastric cancer (Yamada et al. 2002), non-small cell lung cancer (Hu et al. 2006), and B chronic lymphocytic leukemia (Poncet et al. 2008). In our study, the expression of TRF1, TRF2, and TIN2 proteins was significantly higher in precancerous lesions, GC, and GCLM than in normal gastric mucosa. The expression of TRF1, TRF2, and TIN2 proteins was significantly higher in GC and GCLM than in precancerous lesions. TRF1, TRF2, and TIN2 proteins might be involved in the carcinogenesis and development of gastric cancer. Our results found the expression of TRF1, TRF2, and TIN2 proteins to be were higher in poorly differentiated GC than in well differentiated and moderately differentiated GC. TRF1, TRF2, and TIN2 protein over-expression could induce loss of differentiation, increased cell growth, and a more aggressive tumor phenotype.
TRF1 and TRF2 bind directly to double-stranded telomeric DNA at the telomere terminus, and to telomeric DNA in a complex with other proteins. TRF1 has been found in association with TIN2 (Kim et al. 1999), tankyrase 1 and 2 (Cook et al. 2002), and PINX1 (Zhou and Lu 2001), while TRF2 has been shown to interact with hRap1 (Li et al. 2000) and the Mre11 (Zhu et al. 2000) complex. TIN2 connects TRF1 to TRF2 and this link contributes to the stabilization of TRF2 on telomeres. The TRF1 protein inhibits telomerase-mediated lengthening of telomeres. TRF2 seems to be important in the formation of telomere loops (Smogorzewska et al. 2000), while over-expression of dominant-negative forms of TRF2 results in dramatic telomere ‘uncapping’ (Stansel et al. 2001). TIN2 modulates the activity of TRF1 by inhibiting the action of tankyrase 1, which ADP ribosylates TRF1, causing its release from the telomere.
The expression of TERT and Ku70 proteins was significantly up-regulated in both precancerous lesion and GC tissues and was significantly higher in GC tissues than that in precancerous lesions. In normal gastric mucosa, the BRCA1 protein was primarily located in the cell nucleus. In precancerous lesions and GC, the expression of the BRCA1 protein was apparent in the cell cytoplasm.
The core of the mammalian telomerase holoenzyme is the catalytic subunit. TERT is a reverse transcriptase and adds hexameric DNA repeats (TTAGGG). The principal role of Ku proteins is the mediation of DNA repair. However, they have been implicated in other cellular processes, including telomere maintenance, anti-apoptosis, tumor suppression, and regulation of specific gene transcription. Ku70 may have a role in the regulation and maintenance of telomeres through its interaction with TRF2 (Song et al. 2000). BRCA1 appears to function as a classic tumor suppressor, since BRCA1 mutation is associated with cancers and has exhibited loss of the wild-type allele in tumor cells (Cornelis et al. 1995; Neuhausen and Marshall 1994). Over-expression of the BRCA1 gene inhibits TERT expression and telomerase enzyme activity in various cell types and causes telomere shortening (Xiong et al. 2003; Li et al. 2002). Our study demonstrated a negative correlation between the telomere length and the expression of TRF1, TRF2, TIN2, TERT, and Ku70 proteins.
The above findings indicate that there is an interaction between telomeric proteins and telomere length during the carcinogenesis of gastric cancer. We infer from this that, because telomere shortening leads to genetic instability in the early stage of GC, the DNA repair-associated protein Ku70 was up-regulated and telomerase was activated. The up-regulation of the TERT protein prevents telomere shortening. Meanwhile, the over-expression of TRF1, TRF2, and TIN2 proteins counteracts the effects of the TERT protein and lead to further telomere shortening. These contribute to the multistage carcinogenesis of gastric cancer.
Conclusion
Our results suggest that the over-expression of telomeric proteins, TRF1, TRF2, TIN2, TERT, and Ku70 and the transposition of the BRCA1 protein may work together to reduce telomere length in precancerous lesions and gastric cancer, and could contribute to multistage carcinogenesis of gastric cancer. These findings provide new insight into the mechanism of carcinogenesis in gastric cancer.
Acknowledgments
This work was supported by the construct program of the key discipline in Hunan Province.
References
- Autexier C, Lue NF (2006) The structure and function of telomerase reverse transcriptase. Annu Rev Biochem 75:493–517. doi:10.1146/annurev.biochem.75.103004.142412 [DOI] [PubMed] [Google Scholar]
- Bellon M, Datta A, Brown M et al (2006) Increased expression of telomere length regulating factors TRF1, TRF2 and TIN2 in patients with adult T-cell leukemia. Int J Cancer 119(9):2090–2097. doi:10.1002/ijc.22026 [DOI] [PubMed] [Google Scholar]
- Blackburn EH (1991) Structure and function of telomeres. Nature 350(6319):569–573. doi:10.1038/350569a0 [DOI] [PubMed] [Google Scholar]
- Blasco MA (2005) Telomeres and human disease: ageing, cancer and beyond. Nat Rev Genet 6(8):611–622. doi:10.1038/nrg1656 [DOI] [PubMed] [Google Scholar]
- Broccoli D, Smogorzewska A, Chong L et al (1997) Human telomeres contain two distinct Myb-related proteins, TRF1 and TRF2. Nat Genet 17(2):231–235. doi:10.1038/ng1097-231 [DOI] [PubMed] [Google Scholar]
- Celli GB, Denchi EL, de Lange T et al (2006) Ku70 stimulates fusion of dysfunctional telomeres yet protects chromosome ends from homologous recombination. Nat Cell Biol 8(8):885–890. doi:10.1038/ncb1444 [DOI] [PubMed] [Google Scholar]
- Chai W, Ford LP, Lenertz L et al (2002) Human Ku70/80 associates physically with telomerase through interaction with Htert. J Biol Chem 277(49):47242–47247. doi:10.1074/jbc.M208542200 [DOI] [PubMed] [Google Scholar]
- Chen J, Rocken C, Malfertheiner P et al (2004) Recent advances in molecular diagnosis and therapy of gastric cancer. Dig Dis 22:380–385. doi:10.1159/000083602 [DOI] [PubMed] [Google Scholar]
- Chen LY, Liu D, Songyang Z et al (2007) Telomere Maintenance through Spatial Control of Telomeric Proteins. Mol Cell Biol 27(16):5898–5909. doi:10.1128/MCB.00603-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook BD, Dynek JN, Chang W et al (2002) Role for the related poly(ADP-ribose) polymerases tankyrase 1 and 2 at human telomeres. Mol Cell Biol 22:332–342. doi:10.1128/MCB.22.1.332-342.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelis RS, Neuhausen SL, Johannson O et al (1995) High allele loss rates at 17q12–q21 in breast and ovarian tumors from BRCA1-linked families. the Breast Cancer Linkage Consortium. Genes Chromosom Cancer 13:203–210. doi:10.1002/gcc.2870130310 [DOI] [PubMed] [Google Scholar]
- Dahse R, Fiedler W, Junker K et al (1999) Telomerase activity and telomere lengths: alterations in renal cell carcinomas. Kidney Int 56(4):1289–1290. doi:10.1046/j.1523-1755.1999.00688.x [DOI] [PubMed] [Google Scholar]
- de Lange T (2005) Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev 19(18):2100–2110. doi:10.1101/gad.1346005 [DOI] [PubMed] [Google Scholar]
- Garcia-Aranda C, de Juan C, Diaz-Lopez A et al (2006) Correlations of telomere length, telomerase activity, and telomeric-repeat binding factor 1 expression in colorectal carcinoma. Cancer 106(3):541–551. doi:10.1002/cncr.21625 [DOI] [PubMed] [Google Scholar]
- Hahn WC (2003) Role of telomeres and telomerase in the pathogenesis of human cancer. J Clin Oncol 21(10):2034–2043. doi:10.1200/JCO.2003.06.018 [DOI] [PubMed] [Google Scholar]
- Hsu HL, Gilley D, Blackburn EH et al (1999) Ku is associated with the telomere in mammals. Proc Natl Acad Sci USA 96(22):12454–12458. doi:10.1073/pnas.96.22.12454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Sun L, Zhang C et al (2006) Expression of telomeric repeat binding factor 1 in non-small cell lung cancer. J Surg Oncol 93(1):62–67. doi:10.1002/jso.20421 [DOI] [PubMed] [Google Scholar]
- Kim SH, Kaminker P, Campisi J et al (1999) TIN2, a new regulator of telomere length in human cells. Nat Genet 23(4):405–412. doi:10.1038/70508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HR, Kim YJ, Kim HJ et al (2002) Telomere length changes in colorectal cancers and polyps. J Korean Med Sci 17(3):360–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Oestreich S, de Lange T et al (2000) Identification of human Rap1: implications for telomere evolution. Cell 101:471–483. doi:10.1016/S0092-8674(00)80858-2 [DOI] [PubMed] [Google Scholar]
- Li H, Lee TH, Avraham H et al (2002) A novel tricomplex of BRCA1, Nmi, and c-Myc inhibits c-Myc-induced human telomerase reverse transcriptase gene (hTERT) promoter activity in breast cancer. J Biol Chem 277(23):20965–20973. doi:10.1074/jbc.M112231200 [DOI] [PubMed] [Google Scholar]
- Miyachi K, Fujita M, Tanaka N et al (2002) Correlation between telomerase activity and telomeric-repeat binding factors in gastric cancer. J Exp Clin Cancer Res 21(2):269–275 [PubMed] [Google Scholar]
- Neuhausen SL, Marshall CJ (1994) Loss of heterozygosity in familial tumors from three BRCA1-linked kindreds. Cancer Res 54:6069–6072 [PubMed] [Google Scholar]
- Oh BK, Kim YJ, Park C et al (2005) Up-regulation of telomere-binding proteins, TRF1, TRF2, and TIN2 is related to telomere shortening during human multistep hepatocarcinogenesis. Am J Pathol 166(1):73–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poncet D, Belleville A, t’kint de Roodenbeke C et al (2008) Changes in the expression of telomere maintenance genes suggest global telomere dysfunction in B-chronic lymphocytic leukemia. Blood 111(4):2388–2391. doi:10.1182/blood-2007-09-111245 [DOI] [PubMed] [Google Scholar]
- Reddel RR, Bryan TM, Colgin LM et al (2001) Alternative lengthening of telomeres in human cells. Radiat Res 155(1 Pt 2):194–200. doi:10.1667/0033-7587(2001)155[0194:ALOTIH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Smith S, de Lange T (1997) TRF1, a mammalian telomeric protein. Trends Genet 13(1):21–26. doi:10.1016/S0168-9525(96)10052-4 [DOI] [PubMed] [Google Scholar]
- Smogorzewska A, van Steensel B, Bianchi A et al (2000) Control of human telomere length by TRF1 and TRF2. Mol Cell Biol 20(5):1659–1668. doi:10.1128/MCB.20.5.1659-1668.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song K, Jung D, Jung Y et al (2000) Interaction of human Ku70 with TRF2. FEBS Lett 481(1):81–85. doi:10.1016/S0014-5793(00)01958-X [DOI] [PubMed] [Google Scholar]
- Stansel RM, de Lange T, Griffith JD (2001) T-loop assembly in vitro involves binding of TRF2 near the 3′ telomeric overhang. EMBO J 20:5532–5540. doi:10.1093/emboj/20.19.5532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Steensel Band de Lange T (1997) Control of telomere length by the human telomeric protein TRF1. Nature 385(6618):740–743. doi:10.1038/385740a0 [DOI] [PubMed] [Google Scholar]
- Wainwright LJ, Middleton PG, Rees JL et al (1995) Changes in mean telomere length in basal cell carcinomas of the skin. Genes Chromosom Cancer 12(1):45–49. doi:10.1002/gcc.2870120108 [DOI] [PubMed] [Google Scholar]
- Xiong J, Fan S, Meng Q et al (2003) BRCA1 inhibition of telomerase activity in cultured cells. Mol Cell Biol 23(23):8668–8690. doi:10.1128/MCB.23.23.8668-8690.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Tsuji N, Nakamura M et al (2002) Down-regulation of TRF1, TRF2 and TIN2 genes is important to maintain telomeric DNA for gastric cancers. Anticancer Res 22(6A):3303–3307 [PubMed] [Google Scholar]
- Ye JZ, Donigian JR, van Overbeek M et al (2004) TIN2 binds TRF1 and TRF2 simultaneously and stabilizes the TRF2 complex on telomeres. J Biol Chem 279(45):47264–47271. doi:10.1074/jbc.M409047200 [DOI] [PubMed] [Google Scholar]
- Zhou XZ, Lu KP (2001) The Pin2/TRF1 interacting protein PinX1 is a potent telomerase inhibitor. Cell 107:347–359. doi:10.1016/S0092-8674(01)00538-4 [DOI] [PubMed] [Google Scholar]
- Zhu XD, Kuster B, Mann M et al (2000) Cellcycle-regulated association of Rad50/MRE11/NBS1 with TRF2 and human telomeres. Nat Genet 25:347–352. doi:10.1038/77139 [DOI] [PubMed] [Google Scholar]