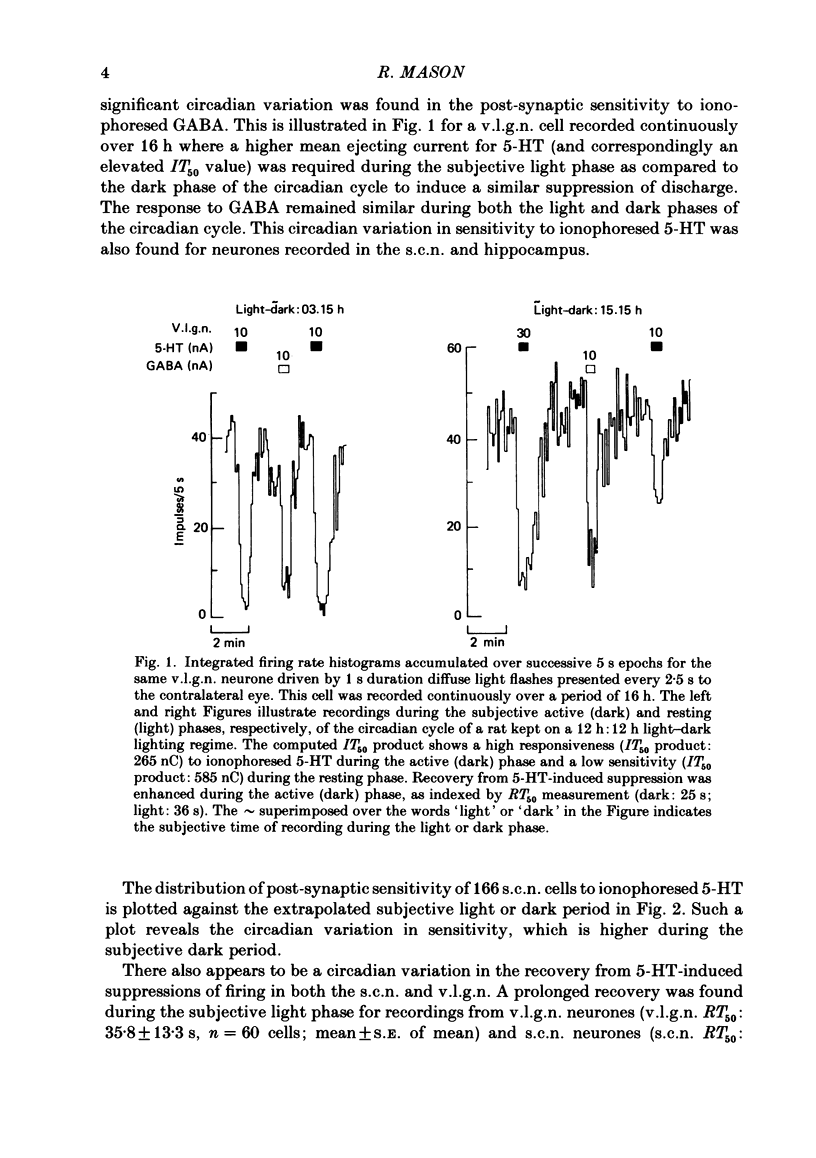
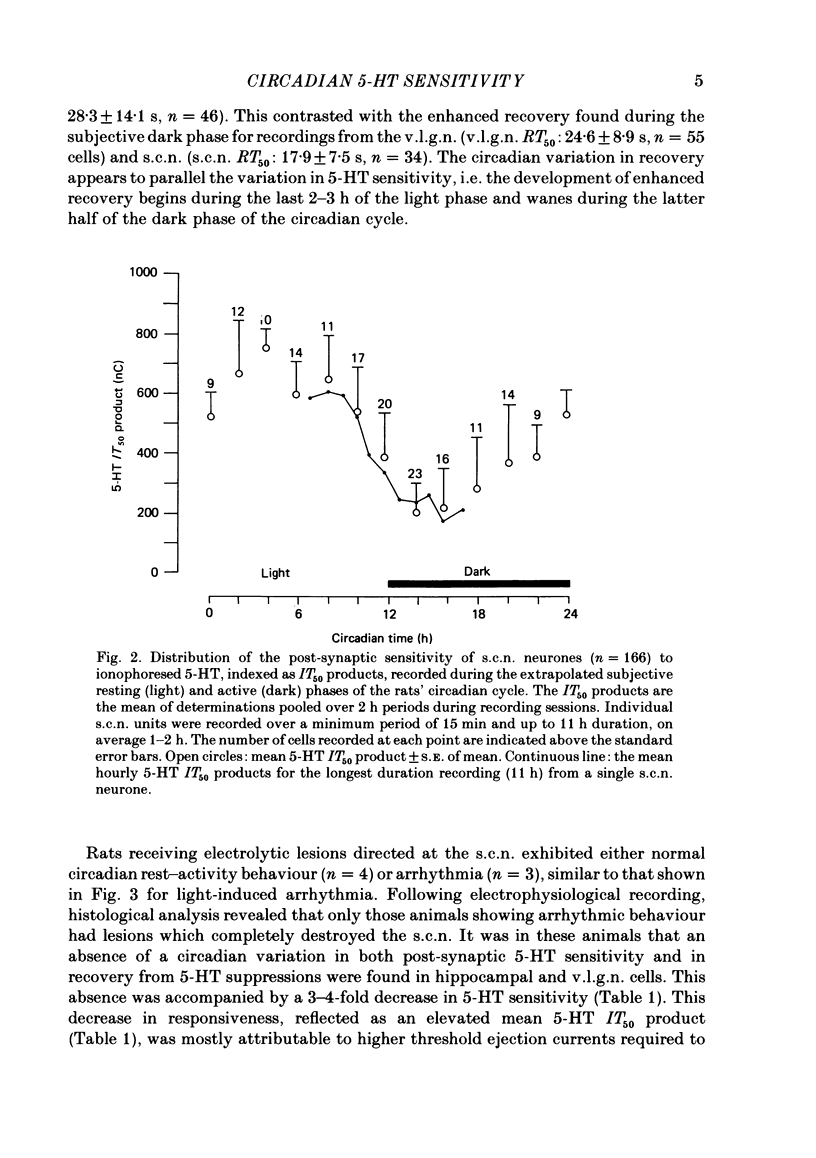
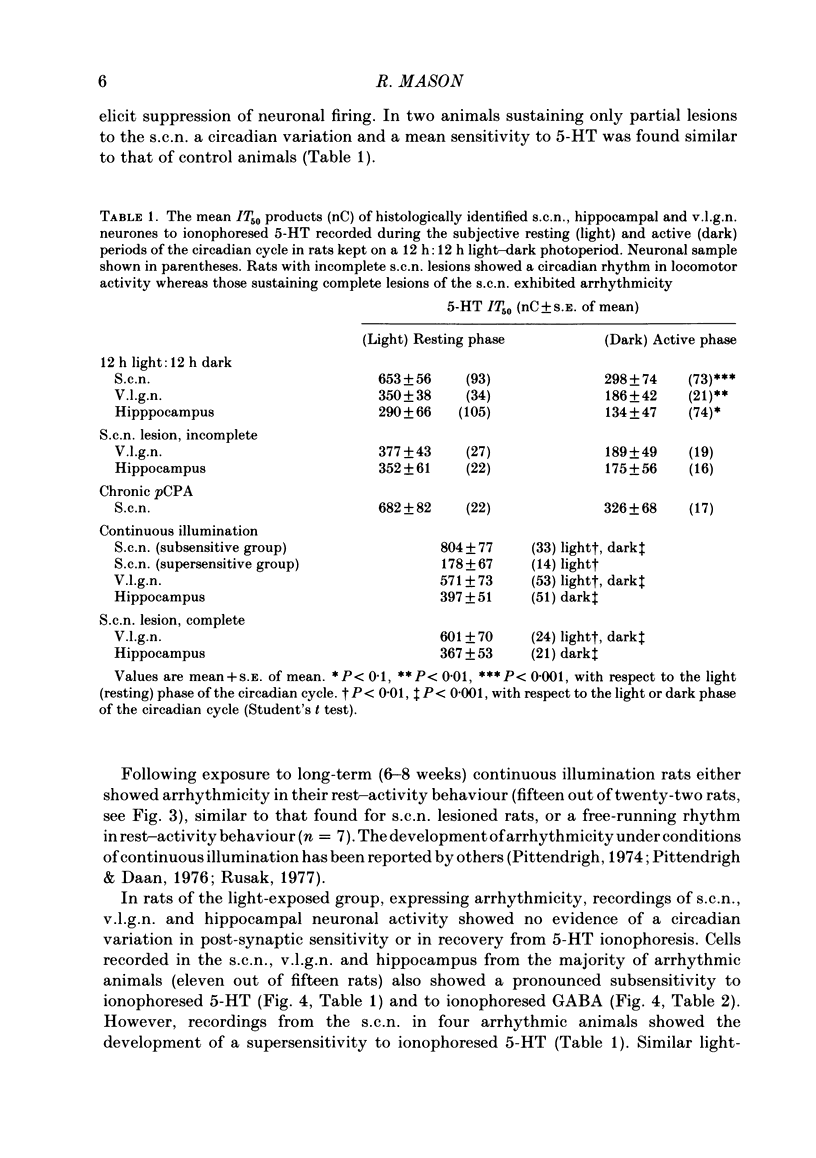
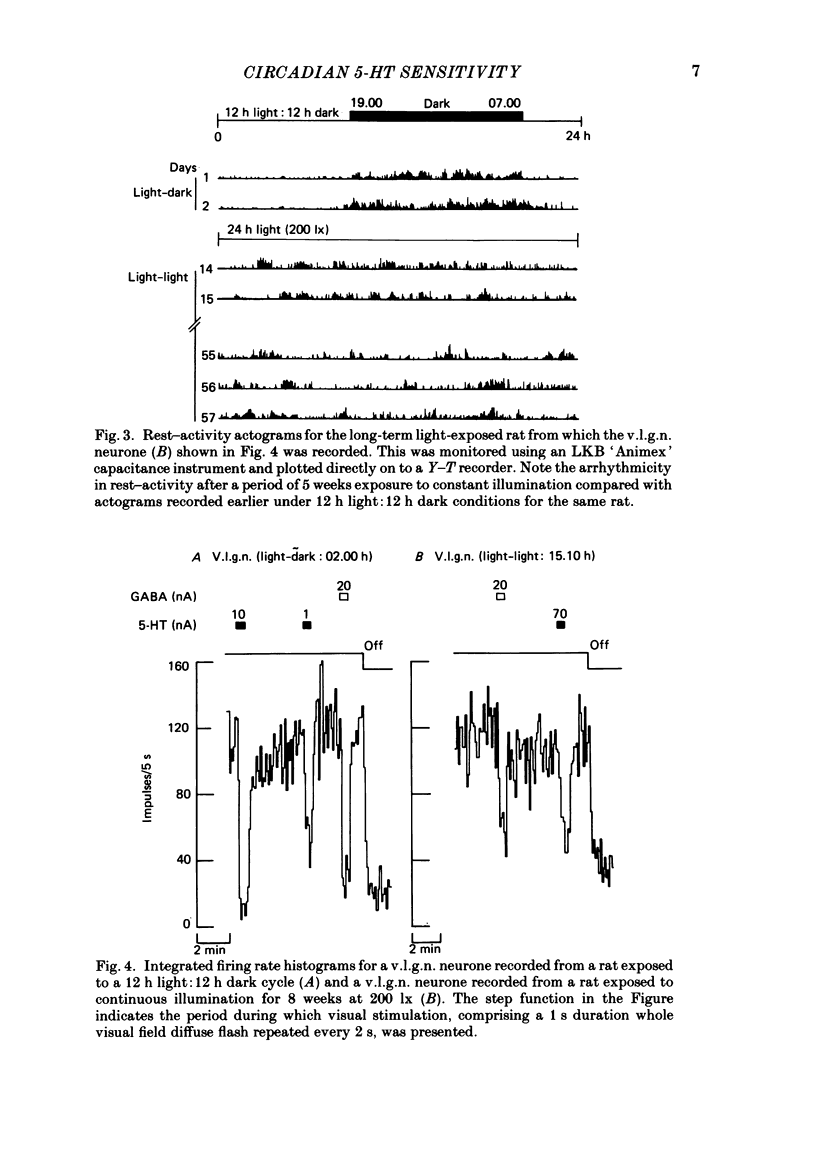
Abstract
Extracellular single-unit recordings were obtained from neurones in the suprachiasmatic nuclei (s.c.n.) of the rat (a putative circadian pace-maker), the ventral lateral geniculate nucleus (v.l.g.n.) and the hippocampus. These areas receive a 5-hydroxytryptamine (5-HT) innervation from the raphe nuclei. Recording of neuronal activity in the s.c.n., v.l.g.n. and the hippocampus revealed a diurnal variation in the response to the ionophoresis of 5-HT. This variation was manifest as a 2-3-fold increase in post-synaptic sensitivity to 5-HT during the subjective dark (active) phase of the circadian cycle. In contrast there was no apparent circadian variation in the sensitivity of s.c.n., v.l.g.n. or hippocampal neurones to ionophoresed gamma-aminobutyric acid (GABA). Neuronal activity recorded in the s.c.n., v.l.g.n. and hippocampus also exhibited a circadian variation in the recovery from 5-HT-induced suppression of firing. This may reflect reuptake processes as recovery can be prolonged by ionophoresis of uptake blockers (imipramine or fluoxetine). Rats (n = 15) expressing circadian arrhythmicity in their rest-activity behaviour induced by long-term continuous illumination (150-200 lx) showed no apparent circadian variation in 5-HT sensitivity. This loss was accompanied by either the development of a 5-6-fold subsensitivity to ionophoresed 5-HT (eleven out of fifteen rats) or a 2-3-fold supersensitivity to ionophoresed 5-HT (four out of fifteen rats). A similar loss of circadian variation and the development of a subsensitivity to ionophoresed 5-HT was also found in three rats sustaining complete electrolytic lesions of the s.c.n. These changes were not found in rats (n = 4) with partial s.c.n. lesions. These results implicate the s.c.n., or fibres passing through it, in the circadian modulation of 5-HT sensitivity in neurones both intrinsic to the s.c.n. circadian pace-maker itself and in the hippocampus and lateral geniculate nucleus (regions remote from the s.c.n.).
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aghajanian G. K., Bloom F. E., Sheard M. H. Electron microscopy of degeneration within the serotonin pathway of rat brain. Brain Res. 1969 Apr;13(2):266–273. doi: 10.1016/0006-8993(69)90286-8. [DOI] [PubMed] [Google Scholar]
- Ajika K., Ochi J. Serotonergic projections to the suprachiasmatic nucleus and the median eminence of the rat: identification by fluorescence and electron microscope. J Anat. 1978 Dec;127(Pt 3):563–576. [PMC free article] [PubMed] [Google Scholar]
- Albers H. E., Ferris C. F. Neuropeptide Y: role in light-dark cycle entrainment of hamster circadian rhythms. Neurosci Lett. 1984 Sep 7;50(1-3):163–168. doi: 10.1016/0304-3940(84)90480-4. [DOI] [PubMed] [Google Scholar]
- Borbély A. A., Huston J. P., Waser P. G. Physiological and behavioral effects of parachlorophenylalanine in the rat. Psychopharmacologia. 1973 Jul 17;31(2):131–142. doi: 10.1007/BF00419813. [DOI] [PubMed] [Google Scholar]
- Card J. P., Brecha N., Moore R. Y. Immunohistochemical localization of avian pancreatic polypeptide-like immunoreactivity in the rat hypothalamus. J Comp Neurol. 1983 Jun 20;217(2):123–136. doi: 10.1002/cne.902170202. [DOI] [PubMed] [Google Scholar]
- Card J. P., Moore R. Y. Ventral lateral geniculate nucleus efferents to the rat suprachiasmatic nucleus exhibit avian pancreatic polypeptide-like immunoreactivity. J Comp Neurol. 1982 Apr 20;206(4):390–396. doi: 10.1002/cne.902060407. [DOI] [PubMed] [Google Scholar]
- Faradji H., Cespuglio R., Jouvet M. Voltammetric measurements of 5-hydroxyindole compounds in the suprachiasmatic nuclei: circadian fluctuations. Brain Res. 1983 Nov 21;279(1-2):111–119. doi: 10.1016/0006-8993(83)90168-3. [DOI] [PubMed] [Google Scholar]
- Ferron A., Reader T. A., Descarries L. Responsiveness of cortical neurons to serotonin after 5,7-DHT denervation or PCPA depletion. J Physiol (Paris) 1981;77(2-3):381–384. [PubMed] [Google Scholar]
- Green D. J., Gillette R. Circadian rhythm of firing rate recorded from single cells in the rat suprachiasmatic brain slice. Brain Res. 1982 Aug 5;245(1):198–200. doi: 10.1016/0006-8993(82)90361-4. [DOI] [PubMed] [Google Scholar]
- Groos G., Hendriks J. Circadian rhythms in electrical discharge of rat suprachiasmatic neurones recorded in vitro. Neurosci Lett. 1982 Dec 31;34(3):283–288. doi: 10.1016/0304-3940(82)90189-6. [DOI] [PubMed] [Google Scholar]
- Groos G., Mason R., Meijer J. Electrical and pharmacological properties of the suprachiasmatic nuclei. Fed Proc. 1983 Aug;42(11):2790–2795. [PubMed] [Google Scholar]
- Güldner F. H., Ingham C. A. Plasticity in synaptic appositions of optic nerve afferents under different lighting conditions. Neurosci Lett. 1979 Oct;14(2-3):235–240. doi: 10.1016/0304-3940(79)96154-8. [DOI] [PubMed] [Google Scholar]
- Hale P. T., Sefton A. J. A comparison of the visual and electrical response properties of cells in the dorsal and ventral lateral geniculate nuclei. Brain Res. 1978 Sep 29;153(3):591–595. doi: 10.1016/0006-8993(78)90343-8. [DOI] [PubMed] [Google Scholar]
- Ibuka N., Inouye S. I., Kawamura H. Analysis of sleep-wakefulness rhythms in male rats after suprachiasmatic nucleus lesions and ocular enucleation. Brain Res. 1977 Feb 11;122(1):33–47. doi: 10.1016/0006-8993(77)90660-6. [DOI] [PubMed] [Google Scholar]
- Meyer D. C., Quay W. B. Hypothalamic and suprachiasmatic uptake of serotonin in vitro: twenty-four-hour changes in male and proestrous female rats. Endocrinology. 1976 May;98(5):1160–1165. doi: 10.1210/endo-98-5-1160. [DOI] [PubMed] [Google Scholar]
- Murakami N., Takahashi K. Circadian rhythm of adenosine-3',5'-monophosphate content in suprachiasmatic nucleus (SCN) and ventromedial hypothalamus (VMH) in the rat. Brain Res. 1983 Oct 16;276(2):297–304. doi: 10.1016/0006-8993(83)90737-0. [DOI] [PubMed] [Google Scholar]
- O'Steen W. K., Anderson K. V. Photically evoked responses in the visual system of rats exposed to continuous light. Exp Neurol. 1971 Mar;30(3):525–534. doi: 10.1016/0014-4886(71)90152-x. [DOI] [PubMed] [Google Scholar]
- Ogren S. O., Fuxe K., Agnati L. F., Celani M. F. Effects of antidepressant drugs on cerebral serotonin receptor mechanisms. Acta Pharmacol Toxicol (Copenh) 1985;56 (Suppl 1):105–127. doi: 10.1111/j.1600-0773.1985.tb02503.x. [DOI] [PubMed] [Google Scholar]
- Pickard G. E. Bifurcating axons of retinal ganglion cells terminate in the hypothalamic suprachiasmatic nucleus and the intergeniculate leaflet of the thalamus. Neurosci Lett. 1985 Apr 9;55(2):211–217. doi: 10.1016/0304-3940(85)90022-9. [DOI] [PubMed] [Google Scholar]
- Pickard G. E. The afferent connections of the suprachiasmatic nucleus of the golden hamster with emphasis on the retinohypothalamic projection. J Comp Neurol. 1982 Oct 10;211(1):65–83. doi: 10.1002/cne.902110107. [DOI] [PubMed] [Google Scholar]
- Rusak B., Zucker I. Neural regulation of circadian rhythms. Physiol Rev. 1979 Jul;59(3):449–526. doi: 10.1152/physrev.1979.59.3.449. [DOI] [PubMed] [Google Scholar]
- Schwartz W. J., Davidsen L. C., Smith C. B. In vivo metabolic activity of a putative circadian oscillator, the rat suprachiasmatic nucleus. J Comp Neurol. 1980 Jan 1;189(1):157–167. doi: 10.1002/cne.901890109. [DOI] [PubMed] [Google Scholar]
- Shibata S., Liou S., Ueki S., Oomura Y. Influence of environmental light-dark cycle and enucleation on activity of suprachiasmatic neurons in slice preparations. Brain Res. 1984 Jun 4;302(1):75–81. doi: 10.1016/0006-8993(84)91286-1. [DOI] [PubMed] [Google Scholar]
- Shibata S., Oomura Y., Kita H., Hattori K. Circadian rhythmic changes of neuronal activity in the suprachiasmatic nucleus of the rat hypothalamic slice. Brain Res. 1982 Sep 9;247(1):154–158. doi: 10.1016/0006-8993(82)91041-1. [DOI] [PubMed] [Google Scholar]
- Trulson M. E., Jacobs B. L. Raphe unit activity in freely moving cats: lack of diurnal variation. Neurosci Lett. 1983 Apr 29;36(3):285–290. doi: 10.1016/0304-3940(83)90014-9. [DOI] [PubMed] [Google Scholar]
- Wang R. Y., de Montigny C., Gold B. I., Roth R. H., Aghajanian G. K. Denervation supersensitivity to serotonin in rat forebrain: single cell studies. Brain Res. 1979 Dec 14;178(2-3):479–497. doi: 10.1016/0006-8993(79)90708-x. [DOI] [PubMed] [Google Scholar]
- Wehr T. A., Sack D., Rosenthal N., Duncan W., Gillin J. C. Circadian rhythm disturbances in manic-depressive illness. Fed Proc. 1983 Aug;42(11):2809–2814. [PubMed] [Google Scholar]
- Wirz-Justice A., Kräuchi K., Morimasa T., Willener R., Feer H. Circadian rhythm of [3H]imipramine binding in the rat suprachiasmatic nuclei. Eur J Pharmacol. 1983 Feb 18;87(2-3):331–333. doi: 10.1016/0014-2999(83)90348-5. [DOI] [PubMed] [Google Scholar]
- de Montigny C., Aghajanian G. K. Tricyclic antidepressants: long-term treatment increases responsivity of rat forebrain neurons to serotonin. Science. 1978 Dec 22;202(4374):1303–1306. doi: 10.1126/science.725608. [DOI] [PubMed] [Google Scholar]
- van de Kar L. D., Lorens S. A. Differential serotonergic innervation of individual hypothalamic nuclei and other forebrain regions by the dorsal and median midbrain raphe nuclei. Brain Res. 1979 Feb 16;162(1):45–54. doi: 10.1016/0006-8993(79)90754-6. [DOI] [PubMed] [Google Scholar]


