Abstract
Purpose
We evaluated the changes of health-related quality of life (HRQOL) during the first 2 years after radical prostatectomy (RP) or external beam radiation therapy (EBRT) in elderly men (70 years old or older) treated for prostate cancer.
Patients and methods
Between 2002 and 2005, a total of 319 elderly men who underwent RP or EBRT participated in our study. Patients completed the general and disease-specific HRQOL with the Short Form-36 (SF-36) and University of California, Los Angeles Prostate Cancer Index, respectively. The participants were asked to complete the questionnaire before and 3, 6, 12, 18 and 24 months after treatment.
Results
The RP group was younger and had more favorable tumor characteristics than the EBRT group. When compared with the EBRT group, the RP group had higher scores for several SF-36 subscales and sexual function, whereas other domains were comparable. In the EBRT group, there were no significant differences in the general HRQOL scores between the baseline and any of the observation periods. The RP group had a significantly worse urinary function and bother scores postoperatively than the EBRT group. The score for sexual function declined over the 24 months, but more so in the RP group. Similarly, sexual bother scores were significantly lower at each postoperative time point for the RP group.
Conclusions
Both aggressive treatments can offer satisfactory functional outcomes from the perspective of HRQOL for selected elderly subjects with localized prostate cancer.
Keywords: Prostate cancer, Quality of life, Radical prostatectomy, External beam radiation, Elderly men
Introduction
Elderly men with localized prostate cancer are offered many curative treatment choices and the process of treatment decision is complex (Krongrad et al. 1998). Other investigators have shown that elderly men with localized cancers were much more likely to die from competing morbidities, even in the absence of treatment (Johansson et al. 2004). In a previous study, Japanese subjects with prostate cancer tended to be older than American subjects (Nakata et al. 2000).
As prostate cancer is increasingly being diagnosed in the early stages and, therefore, with more favorable survival outcomes, the basis on which patients select primary therapy has shifted toward considerations of health-related quality of life (HRQOL) (Mettlin et al. 1997; Davis et al. 2001). Many studies have addressed the effect of treatments for prostate cancer on HRQOL outcomes, but few have focused on HRQOL in men over the age of 70 years with localized prostate cancer. We evaluated the changes of HRQOL during the first 2 years after radical prostatectomy (RP) or external beam radiation therapy (EBRT) in elderly men (70 years or older) treated for localized prostate cancer.
Patients and methods
Patient population and data collection
Between January 2002 and June 2005, a total of 750 patients who underwent RP (n = 575) or EBRT (n = 175) participated in our longitudinal outcomes study at Tohoku University Hospital and its affiliated hospital. Of these patients, 184 RP patients (32%) and 135 RT patients (77%) were 70 years old or older and were included in this analysis. All recruitment and research protocols were approved by the Ethics Committee, Tohoku University School of Medicine. All patients were informed of their cancer diagnosis before being asked to fill out the HRQOL questionnaires. Those who agreed to participate in this study received from their urologist a questionnaire, an informed consent form and a prepaid envelope for returning the questionnaire. The baseline interview was conducted before initiation of either RP or EBRT. Follow-up assessments were completed 3, 6, 12, 18 and 24 months after treatment.
QOL methodology and statistical analysis
We evaluated the general HRQOL with the RAND 36-Item Short Form Health Survey (SF-36) (Hays et al. 1993; Ware and Sherbourne 1992). The general scales cover eight domains, four physical and four emotional. The prostate-specific HRQOL was assessed with the University of California, Los Angeles Prostate Cancer Index (UCLA-PCI) (Litwin et al. 1998). The UCLA-PCI encompasses urinary, bowel, and sexual problems and the extent of bother from problems in each area. The SF-36 and UCLA-PCI quality of life scores for the various domains are shown as mean plus or minus one standard deviation (SD) in scales of 0–100, with a higher score always representing better HRQOL. Both questionnaires have been translated to Japanese and the validity and reliability previously tested (Fukuhara et al. 1998; Kakehi et al. 2002).
Treatment protocols
All of the staff urologists performed RP and used virtually the same technique originally described by Walsh (2002). Every surgeon had considerable experience with the retropubic approach before the beginning of the study. Indications for treatment options depended on pre-treatment factors (the number and Gleason score of the positive biopsies, serum prostate specific antigen and patient preference).
Subjects who selected EBRT received either 3-dimensional conformal therapy (3DCRT) or intensity-modulated radiation therapy (IMRT). Radiotherapy with neoadjuvant endocrine therapy was generally recommended if the patient had a tumor with a clinical stage of T3a or higher. However, the patient made the final determination of the treatment modality after thorough discussion of the options. The mean dose for the prostate and seminal vesicles was 78 Gy (range 76–80 Gy) for IMRT and 70 Gy (range 66–72 Gy) for 3-dimensional conformal therapy.
Statistical analysis
Demographic and clinical variables were compared with Chi-squared analysis. First, we evaluated the time profiles of SF-36 and UCLA-PCI scores, stratified according to study group, with the use of models containing indicators for each post-treatment time points to assess whether the average scores at each time point differed significantly from that at baseline. The inspection value was shown by using average ± SD, and P < 0.05 was considered as significant. Next, we assessed urinary function and bother, bowel function and bother, and sexual function and bother using the principles of survival analysis with Cox proportional hazard models to characterize recovery trends. We created models based on the occurrence of each subject’s return to his own baseline score. A subject was considered to have returned to baseline if his domain score was at least 90% of his baseline. Once a subject returned to baseline, his time to return was censored.
Results
Background characteristics of the study group
Of these 319 subjects, 4 who died during the 2 years follow-up and 8 who showed clinical recurrence were excluded. There were no treatment-related deaths. Only patients with preoperative HRQOL data and data from at least two later times were included in the analysis, resulting in a final study cohort of 284 who underwent RP (n = 166) or EBRT (n = 118).
Table 1 lists the selected demographic and clinical characteristics of the study sample. The overall mean (SD) age at diagnosis was 73.5 (2.8) years and the RP group was younger than the EBRT group (P < 0.001). The RP group had a lower mean pre-biopsy PSA, earlier clinical T-stage tumors and lower biopsy Gleason scores. Most of the EBRT subjects received neoadjuvant or adjuvant androgen deprivation therapy (P < 0.0001). Neoadjuvant hormonal therapy was administered to 72% of patients given EBRT (n = 85) and 3% of patients given RP (n = 5). Among the RP group, 72 (43%) patients did not undergo nerve preservation, and 94 (57%) patients underwent either unilateral (67 [40%] patients) or bilateral (27 [17%] patients) nerve sparing surgery. Although comorbidity counts were similar between groups (P = 0.19), fewer men in the RP group than the EBRT group reported a history of cardiovascular disease or diabetes (4 vs. 11 and 7 vs. 19%, respectively) (data not shown).
Table 1.
Demographic and clinical characteristics of the study sample (n = 284)
| RP | EBRT | P-value | ||
|---|---|---|---|---|
| No. of patients | 166 (%) | 118 (%) | (RP vs. EBRT) | |
| Age at survey (y.o.) | <0.001 | |||
| Mean ± SD | 72.4 ± 2.0 | 75.0 ± 3.1 | ||
| Median | 72 | 75 | ||
| Range | 70–78 | 70–83 | ||
| PSA at diagnosis (ng/ml) | <0.001 | |||
| Mean ± SD | 11.0 ± 14.7 | 29.4 ± 41.2 | ||
| Median | 7.8 | 15.2 | ||
| Range | 3.8–88 | 2.9–322 | ||
| Clinical tumor stage | <0.001 | |||
| T1 | 87 (52) | 24 (20) | ||
| T2 | 66 (40) | 36 (31) | ||
| T3 | 13 (8) | 58 (49) | ||
| Gleason score | 0.009 | |||
| <7 | 106 (64) | 61 (51) | ||
| 7+ | 60 (36) | 57 (49) | ||
| Hormonal therapy | <0.001 | |||
| Yes | 22 (13) | 103 (87) | ||
| No | 144 (87) | 15 (13) | ||
| Comorbidity counta | 0.190 | |||
| 0 | 60 (36) | 31 (26) | ||
| 1 | 72 (43) | 52 (44) | ||
| 2 | 27 (16) | 26 (22) | ||
| 3+ | 7 (4) | 9 (8) | ||
a Comorbidity checklist included hypertension, stomach, intestinal and gastrointestinal diseases, heart disease, cancer (other than prostate), lung disease, diabetes, stroke and blood disease
RP patients who underwent radical prostatectomy
EBRT patients who undewent external beam radiation therapy
SD standard deviation
HRQOL assessment
The questionnaire submission rates among these patients were 100, 95, 97, 91, 87 and 92% at baseline, 3, 6, 12, 18 and 24 months after treatment, respectively. A comparison of baseline generic and prostate cancer-specific HRQOL between the two treatment groups is presented in Tables 2 and 3, respectively. Of the eight SF-36 domains, the RP group had higher baseline scores on physical function and role limitation due to physical and role emotional problems than the EBRT group (P < 0.05). However, other domains including social functioning, vitality, bodily pain, emotional well-being and general health perception showed no significant differences. For cancer-specific HRQOL, the RP group reported higher score on sexual function (P < 0.01). The EBRT group reported higher scores at baseline for sexual bother, whereas both groups had comparable urinary and bowel HRQOL.
Table 2.
SF-36 scores of patients based on treatment group over time
| RP | EBRT | |
|---|---|---|
| Physical function | ||
| Baseline | 85 ± 21 | 73 ± 17 |
| 3 mos. | 83 ± 20 | 74 ± 15 |
| 6 mos. | 84 ± 20 | 77 ± 15 |
| 12 mos. | 84 ± 23 | 72 ± 18 |
| 18 mos. | 86 ± 21 | 76 ± 20 |
| 24 mos. | 84 ± 22 | 70 ± 17 |
| Role limitation due to physical problems | ||
| Baseline | 79 ± 25 | 71 ± 25 |
| 3 mos. | 72 ± 27* | 71 ± 20 |
| 6 mos. | 78 ± 28 | 70 ± 21 |
| 12 mos. | 79 ± 26 | 72 ± 30 |
| 18 mos. | 81 ± 28 | 73 ± 22 |
| 24 mos. | 83 ± 28 | 67 ± 24 |
| Bodily pain | ||
| Baseline | 84 ± 22 | 79 ± 22 |
| 3 mos. | 78 ± 24* | 74 ± 22 |
| 6 mos. | 81 ± 22 | 79 ± 24 |
| 12 mos. | 82 ± 24 | 77 ± 16 |
| 18 mos. | 82 ± 22 | 80 ± 21 |
| 24 mos. | 80 ± 27 | 75 ± 17 |
| General health perception | ||
| Baseline | 58 ± 16 | 55 ± 18 |
| 3 mos. | 59 ± 16 | 56 ± 14 |
| 6 mos. | 60 ± 18 | 56 ± 15 |
| 12 mos. | 60 ± 18 | 58 ± 15 |
| 18 mos. | 61 ± 18 | 57 ± 17 |
| 24 mos. | 61 ± 19 | 52 ± 19 |
| Emotional well-being | ||
| Baseline | 70 ± 21 | 68 ± 19 |
| 3 mos. | 73 ± 20 | 68 ± 16 |
| 6 mos. | 75 ± 20 | 72 ± 19 |
| 12 mos. | 76 ± 20 | 70 ± 15 |
| 18 mos. | 78 ± 19 | 75 ± 17 |
| 24 mos. | 75 ± 19 | 71 ± 20 |
| Role limitation due to emotional problems | ||
| Baseline | 79 ± 28 | 73 ± 24 |
| 3 mos. | 74 ± 29* | 70 ± 19 |
| 6 mos. | 79 ± 30 | 74 ± 22 |
| 12 mos. | 82 ± 26 | 75 ± 27 |
| 18 mos. | 85 ± 29 | 77 ± 22 |
| 24 mos. | 83 ± 31 | 67 ± 26 |
| Social function | ||
| Baseline | 83 ± 23 | 80 ± 19 |
| 3 mos. | 79 ± 21 | 78 ± 21 |
| 6 mos. | 83 ± 27 | 80 ± 20 |
| 12 mos. | 85 ± 24 | 80 ± 21 |
| 18 mos. | 87 ± 20 | 86 ± 24 |
| 24 mos. | 87 ± 26 | 77 ± 22 |
| Vitality | ||
| Baseline | 69 ± 20 | 64 ± 16 |
| 3 mos. | 65 ± 21 | 61 ± 17 |
| 6 mos. | 69 ± 21 | 60 ± 18 |
| 12 mos. | 69 ± 21 | 61 ± 17 |
| 18 mos. | 72 ± 20 | 62 ± 18 |
| 24 mos. | 70 ± 22 | 61 ± 21 |
RP patients who underwent radical prostatectomy
EBRT patients who underwent external beam radiation therapy
SF-36 the RAND 36-Item Short Form
Data are presented as mean ± SD
Statistically significant changes from baseline are indicated as *(P < 0.05)
Table 3.
UCLA-PCI scores of patients based on treatment group over time
| RP | EBRT | |
|---|---|---|
| Urinary function | ||
| Baseline | 92 ± 21 | 87 ± 17 |
| 3 mos. | 63 ± 17* | 91 ± 16 |
| 6 mos. | 72 ± 22* | 89 ± 17 |
| 12 mos. | 75 ± 20* | 89 ± 17 |
| 18 mos. | 78 ± 18* | 89 ± 17 |
| 24 mos. | 78 ± 24* | 85 ± 13 |
| Urinary bother | ||
| Baseline | 87 ± 24 | 85 ± 27 |
| 3 mos. | 71 ± 20* | 86 ± 16 |
| 6 mos. | 79 ± 25* | 86 ± 25 |
| 12 mos. | 81 ± 23 | 88 ± 22 |
| 18 mos. | 82 ± 21 | 88 ± 18 |
| 24 mos. | 84 ± 24 | 84 ± 24 |
| Bowel function | ||
| Baseline | 88 ± 16 | 85 ± 18 |
| 3 mos. | 82 ± 16 | 84 ± 11 |
| 6 mos. | 85 ± 18 | 82 ± 17 |
| 12 mos. | 83 ± 15 | 85 ± 16 |
| 18 mos. | 86 ± 14 | 86 ± 16 |
| 24 mos. | 87 ± 15 | 83 ± 18 |
| Bowel bother | ||
| Baseline | 91 ± 21 | 90 ± 22 |
| 3 mos. | 87 ± 21 | 86 ± 9 |
| 6 mos. | 88 ± 24 | 88 ± 14 |
| 12 mos. | 87 ± 17 | 89 ± 19 |
| 18 mos. | 90 ± 18 | 88 ± 20 |
| 24 mos. | 89 ± 26 | 84 ± 19 |
| Sexual function | ||
| Baseline | 24 ± 20 | 14 ± 18 |
| 3 mos. | 6 ± 12* | 8 ± 12* |
| 6 mos. | 6 ± 11* | 6 ± 11* |
| 12 mos. | 7 ± 12* | 6 ± 12* |
| 18 mos. | 9 ± 11* | 8 ± 12* |
| 24 mos. | 9 ± 11* | 8 ± 10* |
| Sexual bother | ||
| Baseline | 68 ± 31 | 77 ± 27 |
| 3 mos. | 60 ± 29* | 80 ± 30 |
| 6 mos. | 58 ± 31* | 79 ± 28 |
| 12 mos. | 60 ± 32* | 80 ± 28 |
| 18 mos. | 58 ± 36* | 73 ± 32 |
| 24 mos. | 57 ± 34* | 77 ± 36 |
RP patients who underwent radical prostatectomy
EBRT patients who underwent external beam radiation therapy
UCLA-PCI University of California, Los Angeles Prostate Cancer Index
Data are presented as mean ± SD
Statistically significant changes from baseline are indicated as *(P < 0.05)
A longitudinal assessment on general and cancer-specific HRQOL scores is also shown in Tables 2 and 3, respectively. The RP group reported an initial decline at 3 months with regard to role limitations due to physical problems, and bodily pain and role limitations due to emotional problems, but had values at 24 months similar to baseline. With regard to the EBRT group, there were no significant differences in the general HRQOL scores between the baseline and any of the observation periods. Moreover, no significant differences among either type of radiation therapy (3DCRT and IMRT) were observed throughout the follow-up period.
According to UCLA-PCI scores, which represent disease-specific HRQOL, the urinary function substantially declined at 3 months and continued to recover at 6, 12, 18, 24 months, but scored lower than the baseline for the RP group (P < 0.01). Urinary bother had a significantly worse score at 3 and 6 months than that at baseline (P < 0.01). At 12 months after surgery, however, it returned to baseline. In the domain of bowel function and bother, no significant difference was observed in the RP group between the baseline and any of the post-treatment time. For the EBRT group, both domains tended to decline over the 24 months, but not significantly. For both treatment groups, the score for sexual function declined over the 24 months, but more so in the RP group. Similarly, sexual bother scored significantly lower at each postoperative time point for the RP group (P < 0.01). With regard to the RP group, bilateral nerve sparing made a significant contribution to the recovery of sexual function compared with non-nerve sparing (12 vs. 4, P = 0.011), whereas there were no significant differences in sexual bother scores (54 vs. 59, P = 0.616). In the EBRT group, however, there were no significant differences of sexual bother scores throughout the follow-up period.
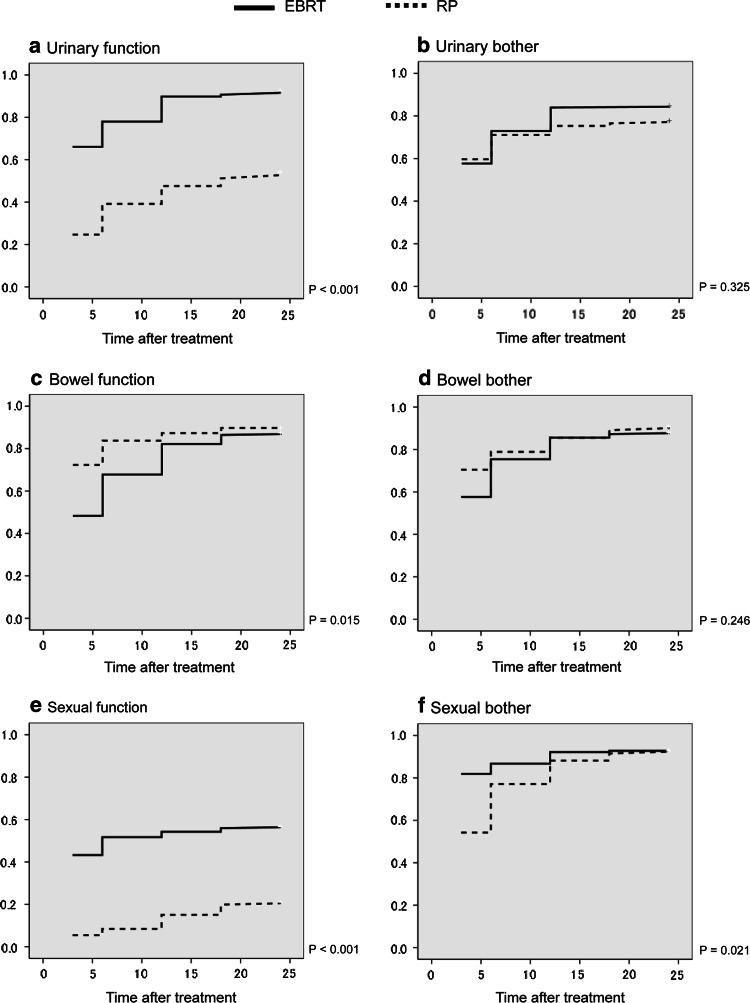
Figure 1a–f shows Kaplan–Meier curves representing return to baseline HRQOL score with regard to the prostate cancer-specific HRQOL controlling for age. Urinary function, bowel function, sexual function and sexual bother had significant differences in hazard ratios between the two groups. The results of backward stepwise log-linear regression model for analyzing the predictor of 24-month HRQOL indicated that EBRT was associated with higher scores on urinary function (OR = 3.31, CI = 1.06–10.2) and sexual bother (OR = 5.99, CI = 2.58–13.9).
Fig. 1.
Kaplan–Meier analysis of the proportion of subjects returning to baseline score over time. RP broken line, EBRT unbroken line. a Urinary function, b urinary bother, c bowel function, d bowel bother, e sexual function, f sexual bother
Discussion
The current study has four important findings. First, in our cohort, the RP subjects were younger, healthier and had more favorable tumor characteristics than those who received radiation therapy comprising an elderly population. The results of the current study confirmed the findings of earlier studies, which showed that healthy and elderly subjects with no or mild comorbidity tended to undergo RP. Radiation oncologists may also have an age threshold below which patients are considered appropriate candidates for EBRT, regardless of comorbidity (Harlan et al. 2001). Age has strong influences on treatment pattern; younger men prefer RP, middle-aged men prefer EBRT and older men prefer either no treatment or hormonal therapy (Alibhai et al. 2004). Krahn et al. (2002) found that the odds for management of a moderately differentiated tumor of a 75-year-old man being offered RP were only 0.003 times the odds of a 55-year-old man being offered the same treatment. Suzuki et al. (2002) reported that elderly Japanese men with prostate cancer present with similar histologic grade and disease stage compared with younger men. In addition, aggressively treated older men with localized prostate cancer were healthier at baseline than those who were managed conservatively (Hoffman et al. 2006). A recent decision analysis suggested that healthy older men with localized prostate cancer might gain quality adjusted life years with aggressive treatment (Alibhai et al. 2003).
Second, there was overall stability in the general HRQOL in both the RP and EBRT groups. Specifically, those who underwent EBRT reported no significant changes with regard to general or disease-specific HRQOL between pre- and post-treatment scores, except for the sexual function domain, whereas the RP group was younger and had less comorbidity. These findings were consistent with those of previous investigators who reported that the general HRQOL was relatively good after RP or EBRT (Litwin et al. 2007). Selection of the treatment depends on the health status, such as comorbidities, stage of disease or age. Both aggressive treatments can offer satisfactory functional outcomes from the perspective of HRQOL for selected elderly subjects with localized prostate cancer.
Consistent with previous analyses, we identified significant decline in urinary function among patients treated with surgery. It is important to emphasize that urinary incontinence after RP increases with age (Stanford et al. 2000). Our previous survey revealed that 88% younger men (younger than 60) reached baseline urinary function at 24 months postoperatively using the same methods (Namiki et al. 2009). In the current study, however, only 54% of patients returned to baseline values, which was less than that observed in younger RP subjects.
Third, using a self-reported questionnaire, elderly subjects in both groups reported low sexual function after treatment. The most dramatic decline in sexual function was in the RP group, leading to a comparable score with the EBRT group post-treatment. Moreover, despite lower sexual function scores initially and greater sexual bother scores seen in the EBRT group, both sets of scores were maintained throughout the follow-up periods. This observation may result from the use of hormonal therapy before and/or after EBRT, because combination therapy with hormonal therapy and EBRT has led to improved, disease-free, progression-free and overall survival in specific patient groups (D’Amico et al. 2004). Other authors report that the majority of prostate cancer patients in Japan receive EBRT plus hormonal therapy, which is a distinctively different trend compared to Europe and North America (Akaza et al. 2006). The differences in outcomes between these reports and our findings may result from several factors, including different patient characteristics such as age at analysis, periods of follow-up and duration of hormonal therapy. Another explanation is that sexual distress of Japanese men who received EBRT showed no alteration before and after treatment (Yoshimura et al. 2007). Japanese men reported less sexual activity than American men, but Japanese men were less likely to be bothered (Namiki et al. 2008). In Japan, most men take no action, while in America men may seek help from the partner, family members or other sources of social support (Nicolosi et al. 2005). Among those who did not seek treatment, older men resisted seeking treatment because they may have felt that erectile dysfunction was a natural part of aging. Given the small subset of patients on which the results were based, future confirmatory studies will be necessary to assess the impact of hormonal therapy on sexual related HRQOL.
Fourth, bowel function and bother were comparable between treatment groups at baseline and at the follow-up. This finding is inconsistent with other reports in which bowel function differs substantially in men treated with RP or EBRT for localized prostate cancer (Litwin et al. 2004). In this paper, the EBRT group also improved markedly during the first 3 months, which may also be due to lack of 1-month data in our study. Another possible explanation lies in the finding that 19% (n = 29) of the Japanese men received IMRT and several studies have reported that IMRT produces little impairment in HRQOL (Zelefsky et al. 2002; Namiki et al. 2006).
Our findings are limited by several methodological considerations. First, because there was no randomization of treatment, the results might not be representative of all elderly patients receiving either RP or EBRT, and there is potential for inherited treatment bias. Second, the vast majority of EBRT patients underwent androgen deprivation therapy, in contrast to few men after RP. Androgen deprivation therapy is known to impact on HRQOL in multiple domains including sexual function, fatigue and physical function. Third, the sample was relatively small and may not be representative of the general elderly population. In particular, there may be variability between clinicians with regard to the completeness with which comorbidity was recorded. Fourth, we did not distinguish between those who used erectile aids such as type 5 phosphodiesterase inhibitors or vacuum devices, and there were no documentations of the use of alpha-blockers or anticholinergics. These factors may be significant predictors of urinary or sexual function recovery.
Despite these limitations, the findings of our survey may aid in the counseling of elderly patients regarding therapy decisions for those with newly diagnosed, localized prostate cancer. These differences highlight the need for pretreatment discussions with patients and families about quality of life expectations post-treatment. Additionally, more attention should be given to design and implementation of support services and family counseling during the post-treatment periods.
Conclusion
Elderly men often have a wide variation of comorbid conditions and functional limitations that may affect their treatment pattern and outcomes. Both aggressive treatments can offer satisfactory functional outcomes from the perspective of HRQOL for selected elderly subject with localized prostate cancer. Thus, managing prostate cancer in this group requires a comprehensive assessment and multidisciplinary approach to maximize their HRQOL.
Acknowledgments
We thank Emiko Izutsu, Tohoku University Hospital, who assisted with data collection and management. The study was supported in part by a Grant-in-Aid for Health Research from the Pfizer Health Research Foundation.
References
- Akaza H, Hinotsu S, Usami M et al (2006) The case for androgen deprivation as primary therapy for early stage disease: results from J-CaP and CaPSURE. J Urol 176:S47–S49 [DOI] [PubMed] [Google Scholar]
- Alibhai SM, Naglie G, Nam R, Trachtenberg J, Krahn MD (2003) Do older men benefit from curative therapy of localized prostate cancer? J Clin Oncol 21:3318–3327 [DOI] [PubMed] [Google Scholar]
- Alibhai SM, Krahn MD, Cohen MM, Fleshner NE, Tomlinson GA, Naglie G (2004) Is there age bias in the treatment of localized prostate carcinoma? Cancer 100:72–81 [DOI] [PubMed] [Google Scholar]
- D’Amico AV, Manola J, Loffredo M, Renshaw AA, DellaCroce A, Kantoff PW (2004) 6-month androgen suppression plus radiation therapy versus radiation therapy alone for patients with clinically localized prostate cancer: a randomized controlled trial. JAMA 292:821–827 [DOI] [PubMed] [Google Scholar]
- Davis JW, Kuban DA, Lynch DF, Schellhammer PF (2001) Quality of life after treatment for localized prostate cancer: differences based on treatment modality. J Urol 166:947–952 [PubMed] [Google Scholar]
- Fukuhara S, Je Ware, Koshinski M (1998) Psychometric and clinical tests of validity of the Japanese SF-36 health survey. J Clin Epidemiol 51:1045–1053 [DOI] [PubMed] [Google Scholar]
- Harlan LC, Potosky A, Gilliland FD et al (2001) Factors associated with initial therapy for clinically localized prostate cancer: Prostate Cancer Outcomes Study. J Natl Cancer Inst 93:1864–1871 [DOI] [PubMed] [Google Scholar]
- Hays RD, Sherbourne CD, Mazel RM (1993) The RAND 36-item health survey 1.0. Health Econ 2:217–227 [DOI] [PubMed] [Google Scholar]
- Hoffman RM, Barry MJ, Stanford JL, Hamilton AS, Hunt WC, Collins MM (2006) Health outcomes in older men with localized prostate cancer: results from the Prostate Cancer Outcomes Study. Am J Med 119:418–425 [DOI] [PubMed] [Google Scholar]
- Johansson JE, Andrén O, Andersson SO, Dickman PW, Holmberg L, Magnuson A, Adami HO (2004) Naural history of early, localized prostate cancer. JAMA 291:2713–2719 [DOI] [PubMed] [Google Scholar]
- Kakehi Y, Kamoto T, Osamu O, Arai Y, Litwin MS, Fukuhara S (2002) Development of Japanese version of the UCLA Prostate Cancer Index: a pilot validation study. Int J Clin Oncol 7:306–311 [DOI] [PubMed] [Google Scholar]
- Krahn MD, Bremner KE, Asaria J, Alibhai SM, Nam R, Tomlinson G, Jewett MA, Warde P, Naglie G (2002) The ten-year rule revisited: accuracy of clinicians’ estimates of life expectancy in patients with localized prostate cancer. Urology 60:258–263 [DOI] [PubMed] [Google Scholar]
- Krongrad A, Litwin MS, Lai H, Lai S (1998) Dimensions of quality of life in prostate cancer. J Urol 160:807–810 [DOI] [PubMed] [Google Scholar]
- Litwin MS, Hays RD, Fink A, Ganz PA, Leake B, Brook RH (1998) The UCLA Prostate Cancer Index: development, reliability, and validity of a health-related quality of life measure. Med Care 36:1002–1012 [DOI] [PubMed] [Google Scholar]
- Litwin MS, Sadetsky N, Pasta DJ, Lubeck DP (2004) Bowel function and bother after treatment for early stage prostate cancer: a longitudinal quality of life analysis from CaPSURE. J Urol 172:515–519 [DOI] [PubMed] [Google Scholar]
- Litwin MS, Gore JL, Kwan L, Brandeis JM, Lee SP, Withers HR, Reiter RE (2007) Quality of life after surgery, external beam irradiation, or brachytherapy for early-stage prostate cancer. Cancer 109:2239–2247 [DOI] [PubMed] [Google Scholar]
- Mettlin CJ, Murphy GP, Babaian RJ et al (1997) Observations on the early detection of prostate cancer from the American Cancer Society National Prostate Cancer Detection Project. Cancer 80:1814–1817 [DOI] [PubMed] [Google Scholar]
- Nakata S, Takahashi H, Ohtake N, Takei T, Yamanaka H (2000) Trends and characteristics in prostate cancer mortality in Japan. Int J Urol 7:254–257 [DOI] [PubMed] [Google Scholar]
- Namiki S, Ishidoya S, Tochigi T et al (2006) Health-related quality of life after intensity modulated radiation therapy for localized prostate cancer: comparison with conventional and conformal radiotherapy. Jpn J Clin Oncol 36:224–230 [DOI] [PubMed] [Google Scholar]
- Namiki S, Kwan L, Kagawa-Singer M et al (2008) Sexual function reported by Japanese and American men. J Urol 179:245–249 [DOI] [PubMed] [Google Scholar]
- Namiki S, Ishidoya S, Ito A et al (2009) Quality of life after radical prostatectomy in Japanese men: a 5-year follow-up study. Int J Urol 16:75–81 [DOI] [PubMed] [Google Scholar]
- Nicolosi A, Glasser DB, Kim SC, Marumo K, Laumann EO, GSSAB Investigators’ Group (2005) Sexual behaviour and dysfunction and help-seeking patterns in adults aged 40–80 years in the urban population of Asian countries. BJU Int 95:609–614 [DOI] [PubMed] [Google Scholar]
- Stanford JL, Feng Z, Hamilton AS et al (2000) Urinary and sexual function after radical prostatectomy for clinically localized prostate cancer: the Prostate Cancer Outcomes Study. JAMA 283:354–360 [DOI] [PubMed] [Google Scholar]
- Suzuki H, Akakura K, Ueda T et al (2002) Clinical characteristics of prostate cancer in elderly Japanese patients 80 years of age or older. Eur Urol 41:172–177 [DOI] [PubMed] [Google Scholar]
- Walsh PC (2002) Anatomical radical retropubic prostatectomy. In: Walsh PC, Retik AB, Vaughan AD, Wein AJ (eds) Campbell’s urology, 8th edn. WB Saunders, Philadelphia, pp 3107–3129 [Google Scholar]
- Ware JE, Sherbourne CD (1992) The MOS 36-Item Short-Form Health Survey (SF-36) conceptual framework and item selection. Med Care 30:473–483 [PubMed] [Google Scholar]
- Yoshimura K, Kamoto T, Nakamura E et al (2007) Health-related quality-of-life after external beam radiation therapy for localized prostate cancer: intensity-modulated radiation therapy versus conformal radiation therapy. Prostate Cancer Prostatic Dis 10:288–292 [DOI] [PubMed] [Google Scholar]
- Zelefsky MJ, Fuks Z, Hunt M et al (2002) High-dose intensity-modulated radiation therapy for prostate cancer: early toxicity and biochemical outcome in 772 patients. Int J Radiat Oncol Biol Phys 53:1111–1116 [DOI] [PubMed] [Google Scholar]