Abstract
Purpose
Focal adhesion kinase (FAK) is a non-receptor protein tyrosine kinase implicated in cancer cell survival, proliferation, and in various steps in the metastatic cascade. In the present study, we took advantage of a cationic liposome as gene carrier and targeted FAK function through both in vitro and in vivo approaches.
Methods
We utilized a plasmid-encoded hairpin RNA targeting the human FAK mRNA (pGensil2-shRNA/FAK), as a means to inhibit FAK expression for evaluating its anti-tumor effect in vitro and in vivo. Human MDA-MB-435S breast cancer cells were transfected with pGensil2-shRNA/FAK and examined for apoptosis by propidium iodide staining, DNA ladder, and flow cytometric analysis. For in vivo study, subcutaneous breast carcinomatosis models in nude mice were established to evaluate the therapeutic potential of pGensil2-shRNA/FAK. Assessments of proliferation (Ki-67), apoptosis (TUNEL) and angiogenesis (CD31) were done using immunohistochemical analysis.
Results
Transcripts expressed from plasmid both in vitro and in vivo were identified by northern blot analysis. pGensil2-shRNA/FAK effectively down-regulated the expression of FAK as demonstrated in vitro by real time RT-PCR and western blot analysis, whereas by real time RT-PCR and IHC staining of MDA-MB-435S tumors growing subcutaneously. Breast cancer cells lacking FAK expression undergo apoptosis in vitro. Systemic delivery of cationic liposome-complexed plasmids targeting FAK, resulted in the diminishment of subcutaneous tumor growth beyond the effects observed with liposomes carrying a non-specific shRNA. This diminishment in growth was associated with elevated levels of apoptosis (TUNEL staining), decreased cell proliferation (Ki-67 staining) and diminished endothelial cell density (CD31 staining).
Conclusion
These results indicate that the systemic delivery of plasmid DNA targeting FAK function using cationic liposome as a gene carrier, represents a promising avenue for breast cancer therapy.
Keywords: FAK, shRNA, CLDC, Gene therapy, Breast cancer, Apoptosis, Proliferation, Antiangiogenesis
Introduction
Metastasis is the ability of tumor cells to disseminate and colonize at distant organs and is the most malignant characteristic of cancer. Despite improvements in surgical techniques and radiochemotherapy, metastasis is a major cause of death in cancer patients and therefore is a major obstacle to the successful treatment of cancer (Patricia 2006). The process of metastasis requires a disseminating cancer cell to survive an environment that actively promotes apoptosis (Frisch and Francis 1994). Thus, for a cancer cell to effectively metastasize and proliferate, it must possess survival signals that suppress apoptosis.
One survival signal that has been shown to modulate apoptotic signaling is the focal adhesion kinase (FAK) (Xu et al. 2000; Damsky et al. 1997). This non-receptor protein-tyrosine kinase localized in focal adhesions—the points of cell contact with the extra-cellular matrix—is recruited to, and activated by, the engagement of trans-membrane integrins by components of the extra-cellular matrix during integrin-mediated cell adhesion (Thomas Parsons 1996; Schlaepfer et al. 1994). Thus far, FAK has been implicated to play an important role in affecting several aspects of cellular processes, including cell spreading and motility (llic et al. 1995; Sieg et al. 2000; Mitra et al. 2005) and cell proliferation (Zhao et al. 1998), as well as cell survival (Xu et al. 2000; Sonoda et al. 2000; Hungerford et al. 1996; Dazin et al. 1998; Frisch et al. 1996). Given that the development of malignancy is often associated with perturbations in these processes, it is not surprising that FAK activity is altered in cancer cells. Numerous impressive evidence suggests that FAK is involved in cancer. The first indication that FAK correlate with tumorigenesis, came from the observation that p125 was one of several highly tyrosine-phosphorylated proteins in oncogenes-transformed fibroblasts (Kanner et al. 1990). In addition, FAK has been shown to be up-regulated compared with normal tissue counterparts in many human tumors. In particular, FAK is elevated in cervical carcinoma cell lines (McCormack et al. 1997), in ovarian carcinoma (Judson et al. 1999), in colorectal tumors (Amy et al. 2003), and in breast tumors (Cance et al. 2000; Owens et al. 1995). Furthermore, several studies have indicated that FAK has a direct role in tumor growth via signaling through the urokinase receptor, RAS, and extra-cellular signal-regulated kinase (ERK)–MAPK (Aguirre Ghiso 2002; Hecker et al. 2002). Moreover, in human tumor cells, FAK inhibition by anti-sense oligonucleotides or dominant-negative FAK, COOH-terminal part of FAK (human analogue of FRNK, FAK-related nonkinase) (Richardson and Parsons 1996), has led to cell rounding, detachment, reduction of invasion, and apoptosis (Xu et al. 2000, 1996, 1998; Dazin et al. 1998; Hauck et al. 2001; Jones et al. 2001). With regards to cell survival, FAK has been shown to suppress not only transformation-associated apoptosis of breast cancer cells (Xu et al. 2000), but also anoikis of human pancreatic adenocarcinoma cells (Duxbury et al. 2004), suggesting that one function of FAK is to promote survival in tumor cells subjected to apoptotic signals. The ability of FAK to protect cells from death also varies between cancer cell lines. Inhibition of FAK function alone only modestly induces some types of cancer cell deaths (Halder et al. 2006; Golubovskaya et al. 2003; Duxbury et al. 2003), whereas some groups have found that inhibition of FAK alone causes apoptosis of breast cancer cells (Xu et al. 2000, 1996; Hauck et al. 2001; Golubovskaya et al. 2002; Beviglia et al. 2003). However, down-regulation of FAK function in normal breast cells had no effect on cell survival, (Xu et al. 2000; Beviglia et al. 2003) raising the intriguing possibility that FAK may be a rational gene-directed target for treating breast cancer.
RNAi, a process of sequence-specific posttranscriptional gene silencing mediated by double-stranded RNA, has become a powerful tool for studying gene function. (Mello and Conte 2002; Hannon and Rossi 2004) A major breakthrough in the application of RNAi technology in mammalian cells, came from the observation that RNAi-mediated gene silencing can be efficiently obtained in cultured mammalian cells by the delivery of synthetic siRNA of 21 nt in length that mimic Dicer enzyme cleavage products (Elbashir et al. 2001). Afterwards, intracellular expression of siRNA from plasmid DNA, emerged as another important technical advance for inducing target gene silencing in mammalian cells (Paul et al. 2002; Miyagishi and Taira 2002), thereby holding great potential for the analysis of gene function and for gene-specific therapeutic approaches.
In this paper, we test the therapeutic efficacy of RNAi-based gene therapy directed at the human FAK for breast cancer, using a plasmid expressing hairpin RNA (RNA with a self-complimentary stem loop) that target a specific sequence in the human FAK mRNA(Duxbury et al. 2004). And this plasmid was complexed with cationic liposome to form CLDC for gene transfection. These CLDC proved able to effectively induce apoptosis of 435S breast cancer cells in vitro. Furthermore, i.v. administration of these CLDC showed potent antitumor effects and antiangiogenic effects on the tumor xenografted in nude mice.
Materials and methods
Cell line
The human breast ductal carcinoma cell line MDA-MB-435S was obtained from the American Type Culture Collection (Rockville, MD, USA) and cultured in DMEM tissue culture medium, supplemented with 20% fetal calf serum (FCS), 2 mM l-glutamine and 0.1 mg/ml amikacin. Cells were incubated in a humidified atmosphere containing 5% CO2 at 37°C and passaged every 3 days at a split ratio of 1:4 using trypsin.
Plasmid and liposome preparation
Plasmid were purchased from Gensil Biotechnology Limited Company of Wuhan. The oligonucleotide sequences of FAK-shRNAs and a control HK-shRNA are shown in Table 1. Among them, the sequence designed to target FAK mRNA has been previously shown to down-regulate FAK (Duxbury et al. 2004). The shRNA expression cassette, driven by the U6 promoter, was engineered by inserting the above oligonucleotides into the BamHI and HindIII sites of plasmid pGenesil-2 (Fig. 1), resulting in the following plasmids: pGensil2-shRNA/FAK and pGensil2-shRNA/HK. Briefly, to generate a plasmid for shRNAs targeted to FAK, pGenesil-2 was linearized with BamHI and HindIII restriction enzymes, and purified on 1% agarose gel to remove any undigested circular plasmid. The purified DNA was then diluted to 0.1 μg/μl. The oligonucleotide templates were then incubated in annealing buffer (100 mM potassium acetate, 30 mM Hepes KOH [pH 7.4], and 2 mM magnesium acetate) first at 90°C for 3 min and afterwards at 37°C for 1 h. The annealed oligonucleotides were next ligated, with the linearized pGenesil-2 shRNA expression vector at BamHI–HindIII sites using T4 DNA ligase. All the constructed plasmids were confirmed by DNA sequencing. Colonies of Escherichia coli containing pGensil2-shRNA/FAK or pGensil2-shRNA/HK were cultured in Luria–Bertani broth containing 100 μg of kana/ml. Large-scale plasmid DNA was purified using an EndoFree Plasmid Giga kit (Qiagen, Chatsworth, CA, USA). The DNA was eventually dissolved in sterile endotoxin free water at a concentration of 5.0 mg/ml, stored at −20°C before use.
Table 1.
The oligonucleotide sequences of shRNA driven by U6 promoter in pGenesil-2
| shRNA | Oligonucleotide A | Oligonucleotide B |
|---|---|---|
| FAKshRNA |
5′-GATCCGCCACCTGGGCCAGTATTATTTCAAGACG ATAATACTGGCCCAGGTGGTTTTTTGTCGACA-3 |
5-AGCTTGTCGACAAAA AACCACCTGGGCCAGTATTAT CGTCTTGAA ATAATACTGGCCCAGGTGGCG-3′ |
| HKshRNA |
5′-GATCCGACTTCATAAGGCGCATGCTTCAAGACG GCATGCGCCTTATGAAGTCTTTTTTGTCGACA-3′ |
5-AGCTTGTCGACAAAAGACTTCATAAGGCGCATGC CGTCTTGAAGCATGCGCCTTATGAAGTCGGCG-3′ |
Fig. 1.
a pGenesil-2 plasmid DNA encoding the short hairpin RNA (shRNA), which produces the RNA interference (RNAi). The gene encoding the shRNA is driven by the U6 promoter. Oligonucleotide templates are inserted into the BamHI and HindIII sites of plasmid pGenesil-2. b Northern blot analysis of U6 promotor-driven transcripts: total RNA was isolated from in vitro MDA-MB-435S tumor cells at 24 h post transfection (Patricia 2006) and liquid nitrogen snap-frozen tumor tissue (Frisch and Francis 1994) for Northern blot analysis
The lipids DOTAP (dioleyl trimethylammonium propane; Avanti Polar Lipids Inc., AL, USA) and cholesterol (Calbiochem Inc., CA, USA) (1:1 M ratio) were dissolved in chloroform supplemented with methanol (3:1 volume ratio). The resulting mixture was dried in a rotary 100-ml round-bottomed flask, and organic solvent was further removed under vacuum for 2 h. The lipid film was hydrated in the appropriate amount of sterile water to yield a final concentration of 2.5 mg/ml. Finally, the liposome solution was vortexed for 1 min, sonicated for 10 min to form small unilamellar vesicles, and stored at 4°C.
The final null liposome was small multilamellar liposome in a size range of 80–100 nm, with a zeta potential from +50 to +60 mV. When mixed with plasmid at a weight ration of 5:1, the size of CLDC vary from 150 to 170 nm, with zeta potential close to neutral as measured by Malvern Mastersizer.
Transfection
To test the effect of pGensil2-shRNA/FAK in vitro, aliquots of 1 × 105 435S cells were grown in each well of six-well plates in triplicate, and incubated for 72 h to 80% confluence. DNA (pGensil2-shRNA/FAK or pGensil2-shRNA/HK)/liposome complexes were prepared in DMEM medium, which contained 1 μg DNA and 5 μg liposome, and left at room temperature for 30 min. In addition, 5 μg liposome alone and medium alone were also used as control agents. The cells were incubated with the above agents for 6 h, rinsed three times with PBS, after which 1.5 ml of DMEM supplemented with FCS was added to each well with a continued incubation for an additional 48 h for Western blot analysis, and for an additional 72 h for assessment of apoptosis.
Real time RT-PCR
Total RNA was isolated from in vitro MDA-MB-435S tumor cells within 48 h of transfection and liquid nitrogen snap-frozen tumor tissue, respectively, using TRIzol reagent (Invitrogen) and treated for 45 min at 37°C with RQ1 DNase (Promega). One microgram of RNA was reverse transcribed using random primers and the Superscript III-Reverse Transcriptase (Invitrogen Life Technologies) at 50°C for 45 min, according to the manufacturer’s instructions. The Real-time PCR was done in an ABI Prism 7000 Sequence Detection System (Applied Biosystems) using SYBR Green PCR Master mix (Applied Biosystems) and the thermocycler conditions recommended by the manufacturer. Human β-actin was used as reference genes to normalize for differences in the amount of total RNA in each sample. Amplification of human β-actin cDNA was evaluated using the primer sequences 5′-CCTGGCACCCA GCACAAT-3′ (forward primer) and 5′-GCCGATCCACACGGAGTACT-3′ (reverse primer) to exons 5 and 6 of the β-actin gene. Primer pairs for amplification of human FAK cDNA were 5′-CTTCGGACAGCGTGAGAGAGA-3′ (forward primer) and 5′-GACGCATTGTTAAGGCTTCTTGA-3′ (reverse primer) to exons 16 and 17 of the FAK gene. Melting curve analysis showed a single sharp peak with the expected Tm for all samples. mRNA relative quantities were obtained using the 2−∆∆Ct method (Livak and Schmittgen 2001).
Northern blot analysis
Northern blot analysis was performed as described (Miyagishi and Taira 2002). Briefly, total RNA was isolated from in vitro MDA-MB-435S tumor cells at 24 h post transfection and liquid nitrogen snap-frozen tumor tissue, respectively, using TRIzol reagent (Invitrogen) and treated for 45 min at 37°C with RQ1 DNase (Promega). 20 μg total RNA per lane were separated on a 12% polyacrylamide gel containing 7 M urea in 1× TBE and electroblotted to a Hybond N+ membrane (Amersham). Prehybridization was carried out at 42°C in a buffer, containing 30% formamide, 10% dextran sulfate, 2× SSC, 1% SDS, 2× Denhard’s solution, and 0.2 mg/ml salmon sperm DNA. The blot was hybridized overnight with DIG-labeled probes (Dig-5′-ATAATACTGGCCCAGGTGGT-3′) (Invitrogen) at 68°C and washed twice in 2× SSC containing 0.1% SDS at room temperature for 15 min. Then, the membrane was incubated with anti-Dig IgG 1:1,000 (1:1,000) and Strptavdin-HRP (1:500) and was visualized by exposure to cold CCD.
Western blot analysis
Western blot analysis was performed as previously described (Wei et al. 2001). Briefly, Cells were lysed in modified radioimmunoprecipitation assay buffer (50 mmol/L Tris, 150 mmol/L NaCl, 1% triton, 0.5% deoxycholate plus 25 mg/mL leupeptin, 10 mg/mL aprotinin, 2 mmol/L EDTA, and 1 mmol/L sodium orthovanadate; Sigma Chemical Co., St. Louis, MO, USA). Cells were removed by scraping, and centrifuged at 12,500 rpm for 30 min. The protein concentration of the supernatant was determined using a bicinchoninic acid protein assay reagent kit (Pierce, Rockford, IL, USA), and whole-cell lysates were analyzed by 10% SDS-PAGE. Gels were electroblotted with Sartoblot onto a poly(vinylidene difluoride) membrane. The membrane blots were blocked at 4°C in 5% nonfat dry milk and incubated with rabbit polyclonal antibody, against the carboxyl terminus of FAK (C-903) purchases from Santa Cruz Biotechnology (Santa Cruz, CA, USA) at a dilution of 1:1,000 for 1 h at room temperature. Followed by incubation in 10 mM Tris–HCl pH 7.5, 100 mM NaCl, and 0.1% Tween 20 (TBS-T) containing a horseradish peroxidase-conjugated anti-rabbit IgG at a dilution of 1:10,000 and detected by enhanced chemiluminescence (Amersham Corp.) followed by autoradiography. Equal loading was confirmed by detection of β-actin.
Assessment of apoptosis in vitro
Morphological analysis of apoptosis was performed after Wright-Giemsa staining under a light microscope, and after staining with PI under fluorescence microscopy. The pattern of DNA cleavage was analyzed by agarose gel electrophoresis as previously described (Wei et al. 1994). Briefly, cells (3 × 106) were lysed with 0.5 ml lysis buffer (5 mM Tris–HCL (pH 8), 0.25% Nonidet P40, and 1 mM EDTA), followed by the addition of RNase A (Sigma) at a final concentration of 200 μg/ml, and incubated for 1 h at 37°C. Cells were then treated with 300 μg proteinase K/ml for an additional 1 h at 37°C. After the addition of 4 μl loading buffer, 20 μl samples in each lane were subjected to electrophoresis on a 1.5% agarose, at 50 V for 3 h. DNA was stained with ethidium bromide. Flow cytometric analysis was also performed to identify hypodiploid/apoptotic cells, and to measure the percentage of hypodiploid cells after PI staining in hypotonic buffer as described (Riccardi and Nicoletti 2006). Briefly, cell pellets were suspended in 1 ml hypotonic fluorochrome solution (50 μg/ml PI in 0.1% sodium citrate plus 0.1% Triton X-100; Sigma), and the cells were analyzed by the use of FACScan (Becton and Dickinson, Mountain View, CA, USA) with Cell Fit software. Hypodiploid cells appeared in the cell cycle distribution as cells with DNA content less than G1.
Human tumor xenografts
Female athymic BALB/c mice 5 weeks of age, weighing 20–22 g, and being specific pathogen-free, were obtained from Sichuan University Animal Center [Sichuan, Chengdu, China]. Mice were housed in microisolator cages with autoclaved bedding, in a specific pathogen-free facility with 12 h light–dark cycles. They received water and food ad libitum. Animals were observed for signs of tumor growth, activity, feeding, and pain, in accordance with the guidelines of the Institutional Animal Care and Use Committee. 435S cell suspension (5 × 106 cells in 100 μl of DMEM medium) was severally injected s.c. into the backs of the nude mice. Before i.v. administration, the DNA/liposome complexes were prepared by adding DNA solution to liposome solution at a ratio of 1 μg DNA to 5 μg liposome, and the mixture was gently pipetted up and down twice. Then, the mixture was incubated at room temperature for 30 min. Ten days after s.c. tumor inoculation, 15 of these mice were randomly assigned into one of the following three groups (n = 5): (a) mice treated with 3 μg pGensil2-shRNA/FAK/15 μg liposome complexes, (b) mice treated with 3 μg pGensil2-shRNA/HK/15 μg liposome complexes, and (c) mice treated with 100 μl of 0.9% NaCl solution (NS). They received 15 i.v. administrations via tail vain on a every 2-day basis and were monitored on a daily basis for tumor burden, abdominal distension, cachexia and other abnormalities. The tumors were measured in two dimensions by caliper and the mice were weighed twice weekly. Tumor weight was calculated as a 2 × b × 0.5, where a is the width and b is the length of the tumor. These mice were sacrificed 3 days after the last administration, and then subcutaneous tumors were excised and weighed. All studies involving mice were approved by the West China Hospital Cancer Center’s Animal Care and Use Committee.
Histologic analysis
Subcutaneous tumors were fixed in 4% paraformaldehyde in PBS, embedded in paraffin, and cut into 3–5 μm sections. The sections were then stained with hematoxylin and eosin (H&E). Immunohistochemical analysis of FAK expression and proliferation marker Ki-67 was done on any excised tumors as described below. The antiangiogenesis effects of FAK silencing were determined by CD31 immunostaining. Paraffin-embedded tumor sections were probed with a monoclonal rat anti-mouse CD31 antibody (1:400) at 4°C overnight, followed by incubation with biotinylated polyclonal goat anti-rat antibody (1:200), in a humidified chamber for 1 h, and were then immersed in 0.3% H2O2 in absolute methanol for 15 min to block endogenous peroxidase.
Positive reaction was visualized using 3,3-diaminobenzidine as chromagen (DAB substrate kit). Sections were counterstained with hematoxylin and mounted with glass coverslips. Then, tissue sections were visualized in an Olympus microscope at 400× magnification to determine microvessel density (MVD) as previously described (Wei et al. 2001). Terminal deoxynucleotidyl transferase-mediated dUTPnick-end labeling (TUNEL) staining, was performed with an in situ apoptotic cell detection kit according to the manufacturer’s directions (Promega), Images of the representative sections were taken using the ZEISS AXIOVERT 400 microscope and Axio Cam MRm camera. Five equal-sized fields were randomly chosen and analyzed. Density was evaluated in each field, yielding the density of apoptotic cells (apoptosis index). For Ki-67 labeling index, the number of cancer cell nuclei that were strongly Ki-67 positive were counted and divided by the total number of cells. Five fields per slide and at least three slides per group were counted at a magnification of 400×.
Statistical analysis
All statistical tests were performed using the SPSS 13.0 software. Statistical comparisons were made with one-factor analysis of variance (ANOVA) with the Student–Newmann–Keuls (SNK) test used for post hoc comparisons. Values of P < 0.05 were considered significant. Results are presented as means ± SD. Experiments were performed at least in duplicate.
Result
Detection of RNA transcripts in vitro and in vivo
Transcripts expressed from plasmid both in vitro and in vivo were identified by northern blot analysis. Bands of expected length were clearly discernible when RNAs were extracted from cells that were transfected with plasmid (Fig. 1b.1) and tumors 72 h after last injection (Fig. 1b.2). These data demonstrated that U6 promotor-driven siRNAs can be produced both in cells and tumors.
Down-regulation of focal adhesion kinase by pGensil2-shRNA/FAK treating
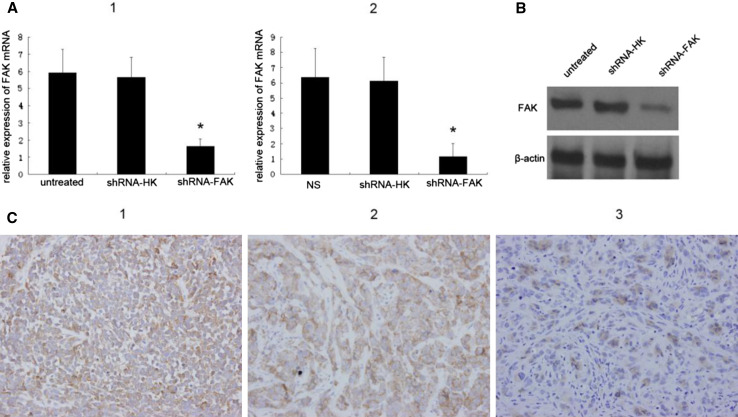
To specifically silence the FAK gene in vitro, MDA-MB-435S cells were transfected with pGensil2-shRNA/FAK. As control for specificity of RNAi, MDA-MB-435S cells were transfected with pGensil2-shRNA/HK carrying a sequence with no homology to any human mRNA or with no treating. The ability of pGensil2-shRNA/FAK to suppress FAK expression at mRNA level and protein level was confirmed by real time RT-PCR and Western blot analyses, respectively. Real-time RT-PCR using total RNA isolated from each group confirmed that FAK mRNA level was indeed down-regulated by pGensil2-shRNA/FAK treatment (Fig. 2a.1). Significant suppression of FAK protein expression was observed within 48 h of transfection. β-actin expression was unaffected by either controls or pGensil2-shRNA/FAK treatment, indicating that non-specific down-regulation of protein expression does not occur (Fig. 2b). Similarly, FAK mRNA level was also down-regulated in tumor tissues harvested from nude mices 3 days after the last administration (Fig. 2a.2). Subsequently, In order to confirm inhibition of FAK expression at protein level by pGensil2-shRNA/FAK treatment in vivo, tumors were harvested after treatment for immunohistochemistry for assessing level of FAK expression. Immunohistochemistry revealed that tumor collected 1 month following i.v. administration of pGensil2-shRNA/FAK had significantly decreased FAK expression, compared with treatment with a non-specific RNAi or NaCl solution (Fig. 2c.1, 2 and 3).
Fig. 2.
Down-regulation of FAK by pGensil2-shRNA/FAK. a Real-time RT-PCR was carried out with FAK, β-actin-specific primers. Columns, ratio of FAK mRNA relative to β-actin in MDA-MB-435S cells culture collected 48 h after transfection (Patricia 2006) and tumors 72 h after last administration (Frisch and Francis 1994); bars SD of each sample measured in triplicate(*P < 0.05). b A representative Western blot of three independent experiments. Lysate from MDA-MB-435S cells culture was collected 48 h after a single administration of pGensil2-shRNA/HK or pGensil2-shRNA/FAK. pGensil2-shRNA/HK did not significantly affect FAK expression compared to untreated cells, whereas FAK-specific pGensil2-shRNA/FAK induced marked suppression of FAK expression. β-actin expression was unaffected by either RNAi treatment. c Immunohistochemical staining for FAK expression after long-term therapy in the 435S model (original magnification, 400×). After treatment with NaCl solution (NS), the typical overexpressed intracytoplasmic protein is noted (Patricia 2006). Long-term treatment with pGensil2-shRNA/HK is shown and had no detectable affect on FAK expression (Frisch and Francis 1994). Long-term treatment of FAK-targeting pGensil2-shRNA/FAK effectively down-regulated FAK expression (Xu et al. 2000)
Induction of apoptosis in vitro by FAK silencing
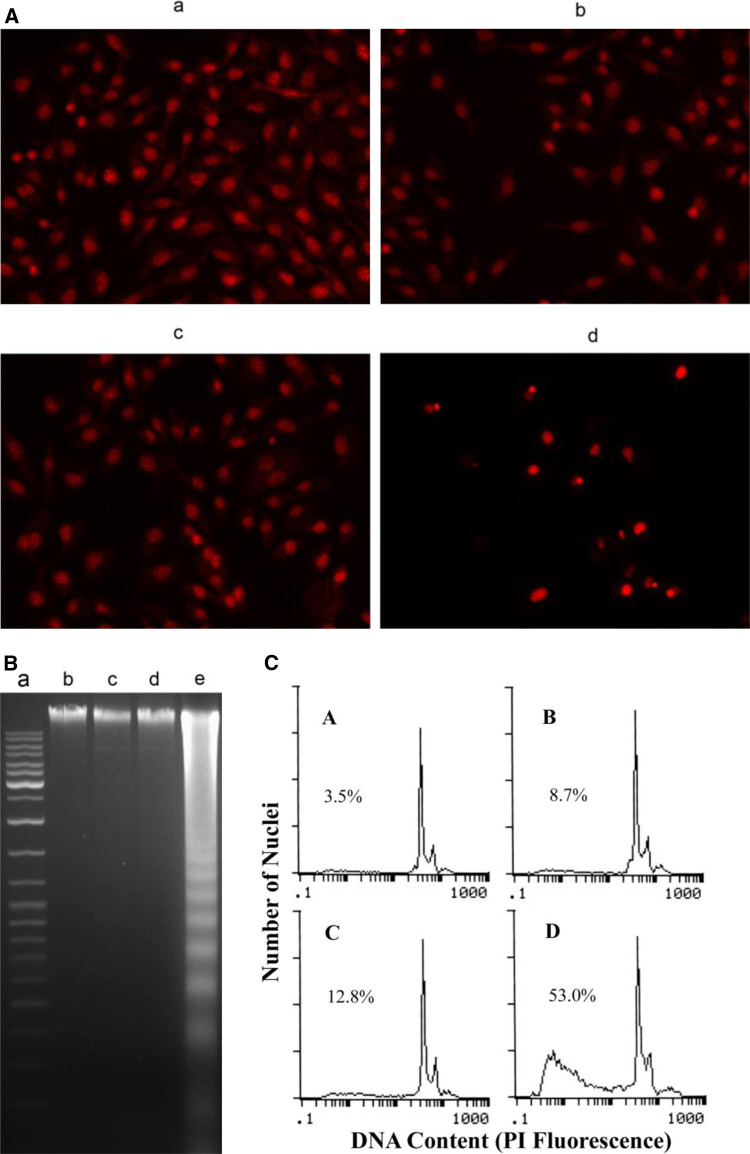
Treatment with pGensil2-shRNA/FAK of tumor cells resulted in morphological changes characteristic for apoptosis: a brightly red-fluorescent condensed nuclei (intact or fragmented) by fluorescence microscopy of PI-stained nuclei, blebbing, reduction of cell volume, condensation of nuclear chromatin, nuclear fragmentation, and apoptotic bodies (Fig. 3a). Agarose gel electrophoresis of pGensil2-shRNA/FAK-treated cells demonstrated a ladder- like pattern of DNA fragments consisting of multiples of approximately 180–200 base pairs, (Fig. 3b) consistent with internucleosomal DNA fragmentation. By the use of flow cytometry, we could unequivocally assess the number of hypodiploid cells (apoptotic cells) and cells with diploid DNA content (nonapoptotic cells). Results obtained in flow cytometry strongly correlated with those in classical DNA ladder assays, and cell counting with PI-staining fluorescence microscopy. The pGensil2-shRNA/FAK treatment resulted in 53% apoptosis compared with 3.5% in non-treated group, 8.7% in liposome alone group and 12.8% in HK group (Fig. 3c). Therefore, the quantitative assessment of hypodiploid cells by flow cytometry was used to estimate the number of apoptotic cells.
Fig. 3.
Assessment of apoptosis induced by FAK silencing in vitro. a Fluorescence microscopic appearance of PI-stained nuclei of FAK silencing-treated tumor cells. 435S cells were treated with DNA (pGensil2-shRNA/FAK or pGensil2-shRNA/HK) and liposome complexes (d), (c), with liposome alone (b) or untreated (a) for 72 h, stained with PI, and examined under a fluorescence microscope (×200). b Agarose gel electrophoretic patterns of DNA isolated from FAK silencing(72 h)-treated and untreated tumor cells. Lane a marker. Lane b untreated 435S cells. Lane c liposome alone. Lane d pGensil2-shRNA/HK-treated 435S cells. Lane e pGensil2-shRNA/FAK-treated 435S cells. c Representative flow cytometry histograms for 435S cancer cells exposed to untreated (a), liposome alone (b), pGensil2-shRNA/HK (c) and pGensil2-shRNA/FAK (d) for 72 h. The cells in sub-G1 phase were considered as apoptotic cells. The apoptosis rates in nontreated and drug-treated cells were 3.5% (a), 8.7% (b), 12.8% (c), 53.0% (d) as assessed by flow cytometry
In vivo efficacy of pGensil2-shRNA/FAK of breast cancer
The in vivo efficacy and specificity of the i.v injection of pGensil2-shRNA/FAK were tested using nude mice bearing human 435S breast carcinoma xenografts. After 30 days of therapy, we euthanized the mice and compared the tumor growth curve and tumor weight. pGensil2-shRNA/FAK (15 every 2-days i.v. administration) treatment resulted in a significant inhibition of tumor growth to extent by 97.8 and 96.8%, as compared to the NS control group (p < 0.001) and HK control group (p < 0.005) respectively (Fig. 4a). In pGensil2-shRNA/FAK-treated animals, tumor weight was reduced by 97.6 and 96.5%, as compared to the NS control (p < 0.001) and HK control (p < 0.005) respectively (Fig. 4b). Interestingly, tumor suppression effect was also observed in the HK group, compared with no treatment group, though this effect was clearly weaker than that of FAK silencing. Histopathological analysis of control mice showed a typical hypercellular solid carcinoma invading the dermis and subcutaneum, and tumor cells at a high nuclear grade with frequent mitosis. In contrast, treated mice showed a marked reduction in tumor volume, partial encapsulation by fibrous connective tissue and no significant invasion into the surrounding skin tissue. Tumor cells in treated mice had a lower nuclear grade and focal glandular differentiation, considerable apoptosis, and no frequent mitosis or necrosis (Fig. 4d). No apparent toxicity was observed in the livers or kidneys in pGensil2-shRNA/FAK-treated mice, nor were there any significant changes in body weight gain compared with control mice (data not shown). These results indicate that the i.v. administration of pGensil2-shRNA/FAK is effective in reducing growth of 435S tumors in mice.
Fig. 4.
In vivo efficacy and specificity of FAK silencing in breast cancer growth. Mice were i.v. administered with respectively 0.1 ml NS, 3 μg pGensil2-shRNA/HK/15 μg liposome complexes, 3 μg pGensil2-shRNA/FAK/15 μg liposome complexes every 2 days. a Tumor growth curves. b Three days after the last treatment, mice were sacrificed and subcutaneous tumors were weighed. Values are means ± SD; n = 5 mice/group. *p < 0.005; **p < 0.001. c H&E staining of subcutaneous tumors 3 days after the last administration. Top panel magnification ×5, bottom panel magnification ×20
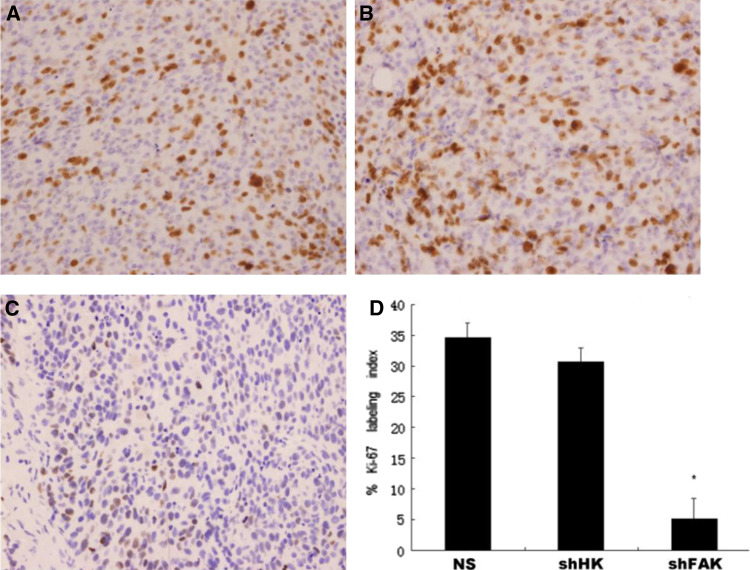
FAK silencing correlated with increased apoptosis and decreased cell proliferation
Non-specific DNA/liposome complex have been reported to induce potent antitumor activity when administered systemically (Dow et al. 1999). This is in agreement with the result of HK control group in our work. In the pGensil2-shRNA/FAK group, however, the tumor suppression was much stronger than the HK control group; this was difficult to be explained in the DNA itself, but not the suppressed FAK activity, is assumed to cause tumor suppression. Therefore, we performed the TUNEL assay to detect apoptotic cells and immunohistochemistry for Ki-67, to detect cell proliferation level in the tumors 72 h after the last injection. Immunofluorescence microscopy of TUNEL staining revealed many strongly positive nuclei in pGensil2-shRNA/FAK-treated tumor tissues, whereas such nuclei were rare in tumor tissues of control groups (Fig. 5a–c). It showed that pGensil2-shRNA/FAK induced a significant enhancement of apoptosis cells, with the apoptosis index of 13.6 ± 2.4% compared with 1.3 ± 0.6% (NS), 1.7 ± 0.7% (HK), respectively (p < 0.05, Fig. 5b). Simultaneously, we examined the effects of FAK-targeted therapy on tumor cell proliferation by using Ki-67 staining (Fig. 6a–c). Proliferation as measured by Ki-67 labeling index was significantly (P < 0.05, Fig. 6d) slower in MDA-MB-435S breast cancer treated by pGensil2-shRNA/FA compared with both controls, demonstrating the role for pGensil2-shRNA/FAK in inhibiting tumor cells proliferation.
Fig. 5.
Apoptosis assays of tumor tissues in different groups. a–c TUNEL staining of tumors revealed that pGensil2-shRNA/FAK induced a significant enhancement of apoptotic cells in contrast to control therapies; ×400. Apoptosis index of pGensil2-shRNA/FAK-treated tumor was much higher than that of control groups. Values are means ± SD.*p < 0.05 (d)
Fig. 6.
Tumor sections from each group were stained for Ki-67. Representative sections from each group. ×400. a–c The number of cancer cell nuclei that were strongly Ki-67 positive were counted and divided by the total number of cells. Columns, mean percentage of Ki-67-positive cells; bars SD. *p < 0.05 (d)
Effects of FAK silencing therapy on vascular density
Growing evidence has shown that FAK is related to angiogenesis by inhibiting VEGF secretion of the tumor itself (Mitra et al. 2006; Sheta et al. 2000). To assess the possible antiangiogenic effects of FAK down-regulation, paraffin-embedded tumors obtained at the conclusion of long-term therapy trials, were subjected to immunohistochemistry for CD31, and MVD was calculated for each group. Representative sections are shown in Fig. 7a–c. The MVD in mice who were treated with NS, pGensil2-shRNA/HK, and pGensil2-shRNA/FAK, was respectively 48.60 ± 3.07, 47.60 ± 2.18, 13.10 ± 2.12 (Fig. 7d) (P < 0.05). These observations suggest that the FAK silencing may inhibit tumor angiogenesis to a significant extent.
Fig. 7.
Tumor angiogenesis was assessed by immunohistochemical staining with anti-CD31 antibody on paraffin-embedded tumor sections. Microvessel counting was performed at ×400. Significantly reduced numbers of blood vessels in tumors treated with pGensil2-shRNA/FAK (c) in comparison with NS (a) or pGensil2-shRNA/HK (b). d The influence of FAK silencing therapy on MVD. Data represent the mean ± SD of microvessels per high-power field. *p < 0.05
Discussion
Based on the previous information demonstrating that the interruption of FAK caused loss of adhesion and increase of apoptosis in breast cancer cell lines (Xu et al. 2000, 1996, 1998; Golubovskaya et al. 2002; Beviglia et al. 2003), in this study, we sought to influence the development of breast tumor by using RNAi targeting FAK, and we have shown that the suppression of FAK expression by RNA interference alone induces apoptosis of the breast tumor in vitro, as demonstrated by classical features of apoptotic cell death (DNA ladder, morphological analysis). In contrast to the methods for the suppression of FAK activity in prior studies, the suppression of FAK by RNAi suppresses the expression of the entire FAK molecule at the posttranscriptional level, which avoids potentially complicating effects from dominant-negative FAK (FRNK) (Mitra et al. 2006), and offers greater efficacy and duration of action than antisense techniques (Bertrand et al. 2002). Promising results from our and other in vitro studies prompted us to examine the therapeutic efficacy of FAK silencing in vivo, and to the best of our knowledge, our study is the first to use a clinically relevant delivery system for FAK RNAi in an subcutaneous model of breast cancer. In addition, we have also shown that targeted therapy with pGensil2-shRNA/FAK alone greatly reduced tumor growth, and FAK-silencing tumor had a significantly greater degree of apoptosis than both HK and NS controls as measured by TUNEL assay.
Survival signaling is a central pathway in cancer progression; as tumor cells proliferate and begin to invade their surrounding substratum, it is necessary to suppress the apoptotic signals associated with the loss of cell–matrix interaction (Frisch and Francis 1994). In breast tumor cells, FAK has two separate functions, one of which is promoting the adhesive interactions between tumor cells and their ECM, and the other is acting as a survival signal that is independent of cellular adhesion (Xu et al. 2000). The molecular mechanism of FAK required for survival signaling is probably associated with the caspase-3 (Xu et al. 2000). Meanwhile, it’s also been suggested that caspase-8 associated with its cofactor FADD (Fas-associated death domain), were involved in the apoptotic process trigged by interruption with FAK (Xu et al. 2000). In addition to apoptosis, other parameters such as cell proliferation has been shown to have a causal relationship with FAK activity (Zhao et al. 1998). This is important, in light of a recent study that demonstrated that a loss of FAK expression in a transgenic model of breast cancer resulted in a diminishment in the formation of hyperplasia formation, which appeared to be due to reduced proliferation in lesions lacking FAK, compared to lesions that retained FAK expression (Lahlou et al. 2007). In this regard, we detected dramatic decreased expression level of proliferation marker Ki-67 in pGensil2-shRNA/FAK treated group, which is in excellent agreement with previous studies showing that up-regulation of FAK activity is a potential requirement for proliferation of cancer cells (Aguirre Ghiso 2002; Hecker et al. 2002). Although FAK silencing has direct anti-tumor activity in breast cancer, there is growing evidence to that the tumor microenvironment may also be affected. Sheta et al. (2000) demonstrated that cell contact-mediated induction of VEGF gene transcription occurs selectively in malignant prostate cells via FAK-dependent MAPK pathway. Accordingly, yet another group illustrated that stable FRNK expression in 4T1 breast carcinoma cells formed small avascular tumors, compared with controls as a result of inhibition of VEGF production (Dow et al. 1999). VEGF, a potent and highly specific angiogenic growth factor, which selectively induces endothelial cell mitogenesis resulting in the sprouting of new capillaries, has been intensively focused on by researchers to design a variety of strategies to block its activity for cancer treatment, as we have previously discussed (Wei et al. 2001). In an effort to examine the effect of pGensil2-shRNA/FAK on antiangiogenesis based on the above mechanism, we performed immunohistochemistry for CD31 and calculated MVD. It turned out that in our experiments, the mean MVD was reduced in tumors treated with pGensil2-shRNA/FAK alone, compared with control groups, suggesting an antivascular effect. On the other hand, FAK is a signaling molecule that provides integrin-mediated signaling, whereas it has been demonstrated that endothelial av integrins, in particular, regulate angiogenesis, are in concert with angiogenic growth factors such as VEGF and bFGF (Friedlander et al. 1995), indicating that FAK might be involved in angiogenesis via endothelial integrin. In favor of this view, later works have confirmed the role for FAK in angiogenesis by coupling to these angiogenic growth factors (Eliceiri et al. 2002; Im et al. 2007; John et al. 2003). Hence, we speculate that pGensil2-shRNA/FAK may strengthen the antivascular effect when applied in humans compared with nude mice.
FAK is over-expressed in a broad variety of tumors, including cervical, ovarian, and colorectal (McCormack et al. 1997; Judson et al. 1999; Amy et al. 2003). Specifically in breast cancer, FAK is overexpressed in approximately 88% of tumors and is associated with the invasive potential of a tumor (Owens et al. 1995), although the mechanisms behind FAK overexpression have yet to be fully elucidated. Differences in gene expression between normal cells and cancer cells often provide interesting targets for antineoplastic therapy. In the past decades, chemical inhibitors have been given priority to exploit such differences and to cause growth disadvantage to cancer cells. Chemical inhibitors, however, have the intrinsic disadvantage that they often evoke numerous, significant, and nonspecific interactions that may contribute to the toxic side effects and narrow therapeutic windows. On the contrary, RNA interference (RNAi) provides an attractive alternative to down-regulate the expression of specific target genes in tumor cells without the associated off-target effects of chemical agents, especially in long-term studies (Hannon and Rossi 2004). Since the first demonstration of RNAi activity in vivo (McCaffrey et al. 2002), there have been numerous studies aimed towards using RNAi to treat diseases, including cancer (Hannon and Rossi 2004). In the case of FAK, there is some evidence that RNAi might be a valuable therapeutic tool. Systemic administration of FAK siRNA to mice suppressed metastasis in a model of human pancreatic cancer (Duxbury et al. 2004). In addition, FAK siRNA potentiates gemcitabine induced cytotoxicity in mice with pancreatic adenocarcinoma (Duxbury et al. 2003), and in a like manner augments the effect of docetaxel-mediated ovarian carcinoma therapy (Halder et al. 2006), raising the possibility that FAK silencing could be potentially useful in combination with conventional cytotoxic agents. All these former FAK-targeting studies have done a good job by using small interfering RNA (siRNA), as the method of gene silencing. Vitro-synthesized siRNA, the 21- and 22-nucleotide double-stranded RNA that is long enough to induce gene-specific suppression, but short enough to evade the host interferon response (Elbashir et al. 2001), has been harnessed as a powerful tool for analysis of gene function and gene-specific therapeutics. However, high production costs and transient nature limit this technology’s utility for many laboratories and experimental situations (Tuschl 2002). In an attempt to address these limitations, several DNA-based plasmid vectors have been developed that direct transcription of siRNA templates, which are intracellularly processed into functional siRNAs (Paul et al. 2002; Miyagishi and Taira 2002). What’s more, the endogenous expression of siRNA from introduced DNA templates, is thought to not only overcome some limitations of exogenous siRNA delivery, in particular the transient loss of phenotype, but also to maintain the potency of siRNA, that is siRNAs can function as primers for an RNA-dependent RNA polymerase that synthesizes additional dsRNA, which in turn is processed into siRNAs, amplifying the effects of the original siRNAs (Sijen et al. 2001; Lipardi et al. 2001). Owing a great deal to the former investigators who have confirmed the ready-to-use duplex RNAs of defined sequence and length targeting FAK (Duxbury et al. 2004), in this study, we carried out the experiment, and tested the therapeutic efficacy of i.v. RNAi-based gene therapy directed at the human FAK in a model of human breast cancer, with U6 snRNA promoter-driven plasmid encodes for a short hairpin RNA (shRNA). When employed with cationic liposomes, both our in vitro transfection and in vivo systemic delivery studies, have exhibited that CLDC resulted in efficient FAK down-regulation and therapeutic efficacy. The importance of this work is that the CLDC is rapidly transferable to a clinical setting for cancer therapeutics (Nagel et al. 1993). Although this technique is not tissue specific, further modifications of the liposome may allow for tumor-selective delivery (Zhang et al. 2004).
We investigated FAK silencing using a cationic liposome delivery method for several reasons. Liposomes are already being clinically used for chemotherapy. Cationic liposomes are particularly attractive due to their favorable characteristics such as biodegradability, minimal immunogenicity compared with viral vectors, relative ease of large-scale production, and simplicity of use. Several studies have shown that intravenous administration of the CLDC leads to systemic gene expression (Templeton et al. 1997; Liu et al. 1997). Factors that control the efficiency of cationic liposome-mediated transfection in vivo via intravenous administration, include proper cationic lipids, multilamellar structure, inclusion of cholesterol as a helper lipid, et cetera (Templeton et al. 1997; Liu et al. 1997). More importantly, when administered i.v., the leaky microvasculature in tumors could facilitate extravasation, and thus, increase the permeability of tumor vessels to liposomes (Yuan et al. 1994). According to these brilliant researches, the cationic liposome we prepared for i.v. administration is composed of DOTAP and cholesterol (1:1 M ratio); when mixed with plasmid, the size of CLDC vary from 150 to 170 nm, with PDI <0.1 as measured by Malvern Mastersizer. Biodistribution studies showed that CLDC was high concentration accumulated in liver, spleen and tumor tissues (data not shown). However, there have been recent publications that raise potential problems due to the non-specific effects of the liposome carriers on the interferon response, regardless of the siRNA or shRNA target (Ma et al. 2005). This is supported by our finding that therapy with a HK control results in some reduction in tumor growth compared with NS control. Nonetheless, in the treatment of cancer, proinflammatory cytokines induction may be of additional benefit, as long as toxicities are limited (Dow et al. 1999).
In summary, the present studies demonstrate that a U6 snRNA promoter-driven plasmid encodes for a short hairpin RNA (shRNA), directed against the human FAK, is highly effective for down-regulating FAK expression. Furthermore, treatment with pGensil2-shRNA/FAK was highly effective in inhibiting breast cancer growth by directly killing breast tumors, inhibiting tumor cell proliferation, as well as indirect antivascular mechanisms, implicating pGensil2-shRNA/FAK as an attractive therapeutic approach in breast cancer.
Acknowledgments
This investigation was supported by the National Basic Research Program of China (2004CB518800), the projects of National Natural Science Foundation of China, and the National 863 Program.
Abbreviations
- FAK
Focal adhesion kinase
- FRNK
FAK-related non-kinase
- CLDC
Cationic lipid–DNA complexes
- shRNA
Short hairpin RNA
- MAPK
Mitogen-activated protein kinase
- MVD
Microvessel density
- H&E
Hematoxylin and eosin
- TUNEL
TdT-mediated dUTP-biotin nick end labeling
- PI
Propidium iodide
- VEGF
Vascular endothelial growth factor
- bFGF
Basic fibroblast growth factor
- PDI
Particle distribution index
Footnotes
A. Huang, Y. Wan, H. Deng and Y. Wei contributed equally to this work.
Contributor Information
Hong-xin Deng, Email: huangannleon@163.com.
Yu-quan Wei, Phone: +86-28-851640630, Email: yuquawei@vip.sina.com.
References
- Aguirre Ghiso JA (2002) Inhibition of FAK signaling activated by urokinase receptor induces dormancy in human carcinoma cells in vivo. Oncogene 21:2513–2524 [DOI] [PubMed] [Google Scholar]
- Amy L, Chad A, Calvo B et al (2003) Overexpression of focal adhesion kinase in primary colorectal carcinomas and colorectal liver metastasis: immunohistochemistry and real time PCR analysis. Clin Cancer Res 9:215–222 [PubMed] [Google Scholar]
- Bertrand JR, Pottier M, Vekris A, Opolon P, Maksimenko A, Malvy C (2002) Comparison of antisense oligonucleotides and siRNAs in cell culture and in vivo. Biochem Biophys Res Commun 296:1000–1004 [DOI] [PubMed] [Google Scholar]
- Beviglia L, Golubovskaya V, Cance WG (2003) Focal adhesion kinase N-terminus in breast carcinoma cells induces rounding, detachment and apoptosis. Biochem J 373:201–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cance WG, Harris JE, Iacocca MV, Roche E et al (2000) Immunohistochemical analyses of focal adhesion kinase expression in benign and malignant human breast and colon tissues: correlation with preinvasive and invasive phenotypes. Clin Cancer Res 6:2417–2423 [PubMed] [Google Scholar]
- Damsky D, Ilic C, Yamamoto T (1997) Focal adhesion kinase: at the crossroads of signal transduction. J Cell Sci 110:401–407 [DOI] [PubMed] [Google Scholar]
- Dazin P, Aizawa S, Damsky CH et al (1998) Extracellular matrix survival signals transduced by focal adhesion kinase suppress p53-mediated apoptosis. J Cell Biol 143:547–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dow SW, Fradkin LG, Liggitt DH, Potter TA et al (1999) Lipid–DNA complexes induce potent activation of innate immune responses and antitumor activity when administered intravenously. J Immunol 163:1552–1561 [PubMed] [Google Scholar]
- Duxbury MS, Ito H, Benoit E, Zinner MJ, Whang EE (2003) RNA interference targeting focal adhesion kinase enhances pancreatic adenocarcinoma gemcitabine chemosensitivity. Biochem Biophys Res Commun 311:786–792 [DOI] [PubMed] [Google Scholar]
- Duxbury MS, Ito H, Whang EE et al (2004) Focal adhesion kinase gene silencing promotes anoikis and suppresses metastasis of human pancreatic adenocarcinoma cells. Surgery 135:555–562 [DOI] [PubMed] [Google Scholar]
- Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Tuschl T (2001) Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 411:494–498 [DOI] [PubMed] [Google Scholar]
- Eliceiri BP, Puente XS, Sheppard D, Cheresh DA et al (2002) Src-mediated coupling of focal adhesion kinase to integrin alpha(v)beta5 in vascular endothelial growth factor signaling. J Cell Biol 157:149–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedlander M, Brooks PC, Shaffer RW et al (1995) Definition of two angiogenic pathways by distinct alpha v integrins. Science 270:1500–1502 [DOI] [PubMed] [Google Scholar]
- Frisch SM, Francis H (1994) Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol 124:619–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisch SM, Vuori K, Ruoslahti E, Chan-Hui PY et al (1996) Control of adhesion-dependent cell survival by focal adhesion kinase. J Cell Biol 134:793–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golubovskaya V, Beviglia L, Xu LH, Cance W (2002) Dual inhibition of focal adhesion kinase and epidermal growth factor receptor pathways cooperatively induces death receptor-mediated apoptosis in human breast cancer cells. J Biol Chem 277:38978–38987 [DOI] [PubMed] [Google Scholar]
- Golubovskaya VM, Gross S, Kaur AS, Cance WG et al (2003) Simultaneous. Inhibition of focal adhesion kinase and src enhances detachment and apoptosis in colon cancer cell lines. Mol Cancer Res 1:755–764 [PubMed] [Google Scholar]
- Halder J, Kamat AA, Sood AK et al (2006) Focal adhesion kinase targeting using in vivo short interfering RNA delivery in neutral liposomes for ovarian carcinoma therapy. Clin Cancer Res 12:4916–4924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon GJ, Rossi JJ (2004) Unlocking the potential of the human genome with RNA interference. Nature 431:371–378 [DOI] [PubMed] [Google Scholar]
- Hauck CR, Sieg DJ, Hsia DA, Schlaepfer DD et al (2001) Inhibition of focal adhesion kinase expression or activity disrupts epidermal growth factor-stimulated signaling promoting the migration of invasive human carcinoma cells. Cancer Res 61:7079–7090 [PubMed] [Google Scholar]
- Hecker TP, Robert Grammer J Jr, Gladson CL et al (2002) Focal adhesion kinase enhances signaling through the Shc/Extracellular signal regulated kinase pathway in anaplastic astrocytoma tumor biopsy samples. Cancer Res 62:2699–2707 [PubMed] [Google Scholar]
- Hood JD, Frausto R, David A et al (2003) Differential alphav integrin-mediated Ras-ERK signaling during two pathways of angiogenesis. J Cell Biol 162:933–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hungerford JE, Compton MT, Otey CA et al (1996) Inhibition of pp125FAK in cultured fibroblasts results in apoptosis. J Cell Biol 135:1383–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilic D, Furuta Y, Kanazawa S, Aizawa S et al (1995) Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature 377:539–544 [DOI] [PubMed] [Google Scholar]
- Im H-J, Muddasani P, Richard F et al (2007) Basic fibroblast growth factor stimulates matrix metalloproteinase-13 via the molecular cross-talk between the mitogen-activated protein kinases and protein kinase C{delta} pathways in human adult articular chondrocytes. J Biol Chem 282:11110–11121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G, Machado J Jr, Merlo A et al (2001) Loss of focal adhesion kinase (FAK) inhibits epidermal growth factor receptor-dependent migration and induces aggregation of NH2-terminal FAK in the nuclei of apoptotic glioblastoma cells. Cancer Res 61:4978–4981 [PubMed] [Google Scholar]
- Judson PL, He X, Cance WG, Van Le L et al (1999) Overexpression of focal adhesion kinase, a protein tyrosine kinase, in ovarian carcinoma. Cancer 86:1551–1556 [DOI] [PubMed] [Google Scholar]
- Kanner SB, Reynolds AB, Vines RR, Parsons JT et al (1990) Monoclonal antibodies to individual tyrosine-phosphorylated protein substrates of oncogene-encoded tyrosine kinases. Proc Natl Acad Sci USA 87:3328–3332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahlou H, Sanguin-Gendreau V, Zuo D, Cardiff RD, Muller WJ et al (2007) Mammary epithelial-specific disruption of the focal adhesion kinase blocks mammary tumor progression. Proc Natl Acad Sci USA 104:20302–20307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipardi C, Wei Q, Paterson BM (2001) RNAi as random degradative PCR: siRNA primers convert mRNA into dsRNAs that are degraded to generate new siRNAs. Cell 107:297–307 [DOI] [PubMed] [Google Scholar]
- Liu Y, Mounkes LC, Liggitt HD, Debs RJ et al (1997) Factors influencing the efficiency of cationic liposome-mediated intravenous gene delivery. Nat Biotechnol 15:167–173 [DOI] [PubMed] [Google Scholar]
- Livak K, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆Ct method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- Ma Z, Li J, He F, Wilson A, Pitt B, Li S (2005) Cationic lipids enhance siRNA-mediated interferon response in mice. Biochem Biophys Res Commun 330:755–759 [DOI] [PubMed] [Google Scholar]
- McCaffrey AP, Meuse L, Pham TT, Kay MA et al (2002) RNA interference in adult mice. Nature 418:38–39 [DOI] [PubMed] [Google Scholar]
- McCormack SJ, Brazinski SE, Goldstein DJ et al (1997) Activation of the focal adhesion kinase signal transduction pathway in cervical carcinoma cell lines and human genital epithelial cells immortalized with human papillomavirus type 18. Oncogene 15:265–274 [DOI] [PubMed] [Google Scholar]
- Mello CC, Conte D Jr (2002) Revealing the world of RNA interference. Nature 431:338–342 [DOI] [PubMed] [Google Scholar]
- Mitra SK, Hanson DA, Schlaepfer DD (2005) Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol 6:56–68 [DOI] [PubMed] [Google Scholar]
- Mitra SK, Mikolon D, Molina JE, Schlaepfer DD et al (2006) Intrinsic FAK activity and Y925 phosphorylation facilitate an angiogenic switch in tumors. Oncogene 25:5969–5984 [DOI] [PubMed] [Google Scholar]
- Miyagishi M, Taira K (2002) U6-promoter-driven siRNAs with four uridine 3’ overhangs efficiently suppress targeted gene expression in mammalian cells. Nature Biotechnol 20:497–500 [DOI] [PubMed] [Google Scholar]
- Nagel GJ, Nabel EG, Yang ZY et al (1993) Direct gene transfer with DNA–liposome complexes in melanoma: expression, biologic activity and lack of toxicity in humans. Proc Natl Acad Sci USA 90:11307–11311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens LV, Xu L, Craven RJ, Dent GA (1995) Overexpression of the focal adhesion kinase (p125FAK) in invasive human tumors. Cancer Res 55:2752–2755 [PubMed] [Google Scholar]
- Paul CP, Good PD, Winer I, Engelke DR (2002) Effective expression of small interfering RNA in human cells. Nat Biotechnol 5:505–508 [DOI] [PubMed] [Google Scholar]
- Riccardi C, Nicoletti I (2006) Analysis of apoptosis by propidium iodide staining and flow cytometry. Nat Protoc 1:1458–1461 [DOI] [PubMed] [Google Scholar]
- Richardson A, Parsons TA (1996) Mechanism for regulation of the adhesion-associated proteintyrosine kinase pp125FAK. Nature 380:538–540 [DOI] [PubMed] [Google Scholar]
- Schlaepfer DD, Hanks SK, Hunter T, vander-Geer P et al (1994) Integrin-mediated signal transduction linked to Ras pathway by GRB2 binding to focal adhesion kinase. Nature 372:786–791 [DOI] [PubMed] [Google Scholar]
- Sheta EA, Harding MA, Conaway MR (2000) Focal adhesion kinase, Rap1, and transcriptional induction of vascular endothelial growth factor. J Natl Cancer Inst 92:1065–1073 [DOI] [PubMed] [Google Scholar]
- Sieg DJ, Hauck CR, Ilic D et al (2000) FAK integrates growth-factor and integrin signals to promote cell migration. Nat Cell Biol 2:249–256 [DOI] [PubMed] [Google Scholar]
- Sijen T, Fleenor J, Simmer F, Thijssen KL, Fire A et al (2001) On the role of RNA amplification in dsRNA-triggered gene silencing. Cell 107:465–476 [DOI] [PubMed] [Google Scholar]
- Sonoda Y, Matsumoto Y, Funakoshi M, Yamamoto D, Kasahara T et al (2000) Anti-apoptotic role of focal adhesion kinase (FAK): induction of inhibitor-of-apoptosis proteins and apoptosis suppression by the overexpression of FAK in a human leukemic cell line, HL-60. J Biol Chem 275:16309–16315 [DOI] [PubMed] [Google Scholar]
- Steeg PS (2006) Tumor metastasis: mechanistic insights and clinical challenges. Nat Med 12:895–904 [DOI] [PubMed] [Google Scholar]
- Templeton NS, Lasic DD, Pavlakis GN et al (1997) Improved DNA: liposome complexes for increased systemic delivery and gene expression. Nat Biotechnol 157:647–652 [DOI] [PubMed] [Google Scholar]
- Thomas Parsons J (1996) Integrin-mediated signalling: regulation by protein tyrosine kinases and small GTP-binding proteins. Curr Opin Cell Biol 2:146–152 [DOI] [PubMed] [Google Scholar]
- Tuschl T (2002) Expanding small RNA interference. Nat Biotechnol 5:446–448 [DOI] [PubMed] [Google Scholar]
- Wei YQ, Zhao X, Kariya Y, Fukata H, Teshigawara K, Uchida A (1994) Induction of apoptosis by quercetin: Involvement of heat shock protein. Cancer Res 54:4952–4957 [PubMed] [Google Scholar]
- Wei YQ, Huang MJ, Yang L et al (2001) Immunogene therapy of tumors with vaccine based on xenopus homologous vascular endothelial growth factor as a model antigen. Proc Natl Acad Sci USA 98:11545–11550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu LH, Owens LV, Sturge GC, Cance WG et al (1996) Attenuation of the expression of the focal adhesion kinase induces apoptosis in tumor cells. Cell Growth Differ 4:413–418 [PubMed] [Google Scholar]
- Xu LH, Yang X, Craven RJ, Cance WG et al (1998) The COOH-terminal domain of the focal adhesion kinase induces loss of adhesion and cell death in human tumor cells. Cell Growth Differ 12:999–1005 [PubMed] [Google Scholar]
- Xu L-H, Yang X-H, Bradham A, Cance G et al (2000) The focal adhesion kinase suppresses transformation-associated, anchorage-independent apoptosis in human breast cancer cells. J Biol Chem 275:30597–30604 [DOI] [PubMed] [Google Scholar]
- Yuan F, Leunig M, Huang SK, Jain RK et al (1994) Microvascular permeability and interstitial penetration of sterically stabilized (stealth) liposomes in a human tumor xenograft. Cancer Res 54:3352–3356 [PubMed] [Google Scholar]
- Zhang Y, Zhang YF, Bryant J, Pardridge WM et al (2004) Intravenous RNA interference gene therapy targeting the human epidermal growth factor receptor prolongs survival in intracranial brain cancer. Clin Cancer Res 10:3667–3677 [DOI] [PubMed] [Google Scholar]
- Zhao JH, Reiske H, Guan JL et al (1998) Regulation of the cell cycle by focal adhesion kinase. J Cell Biol 143:1997–2008 [DOI] [PMC free article] [PubMed] [Google Scholar]