Abstract
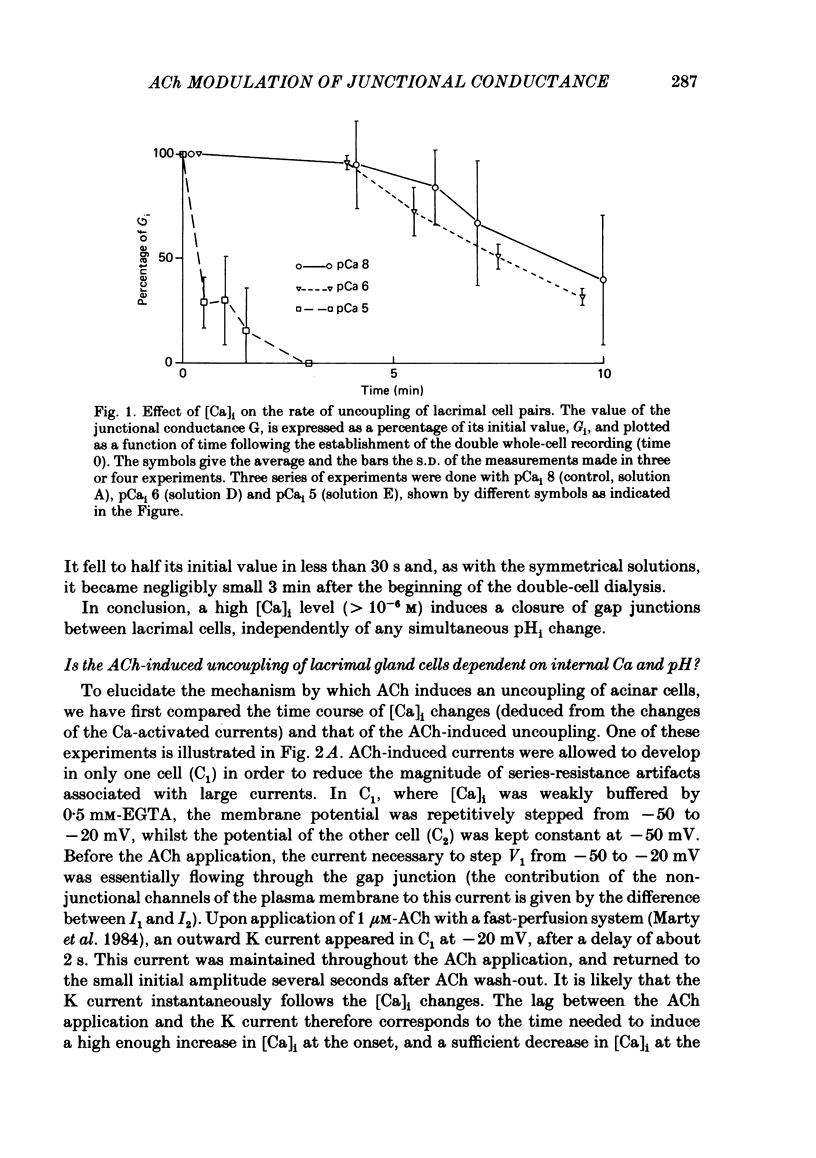
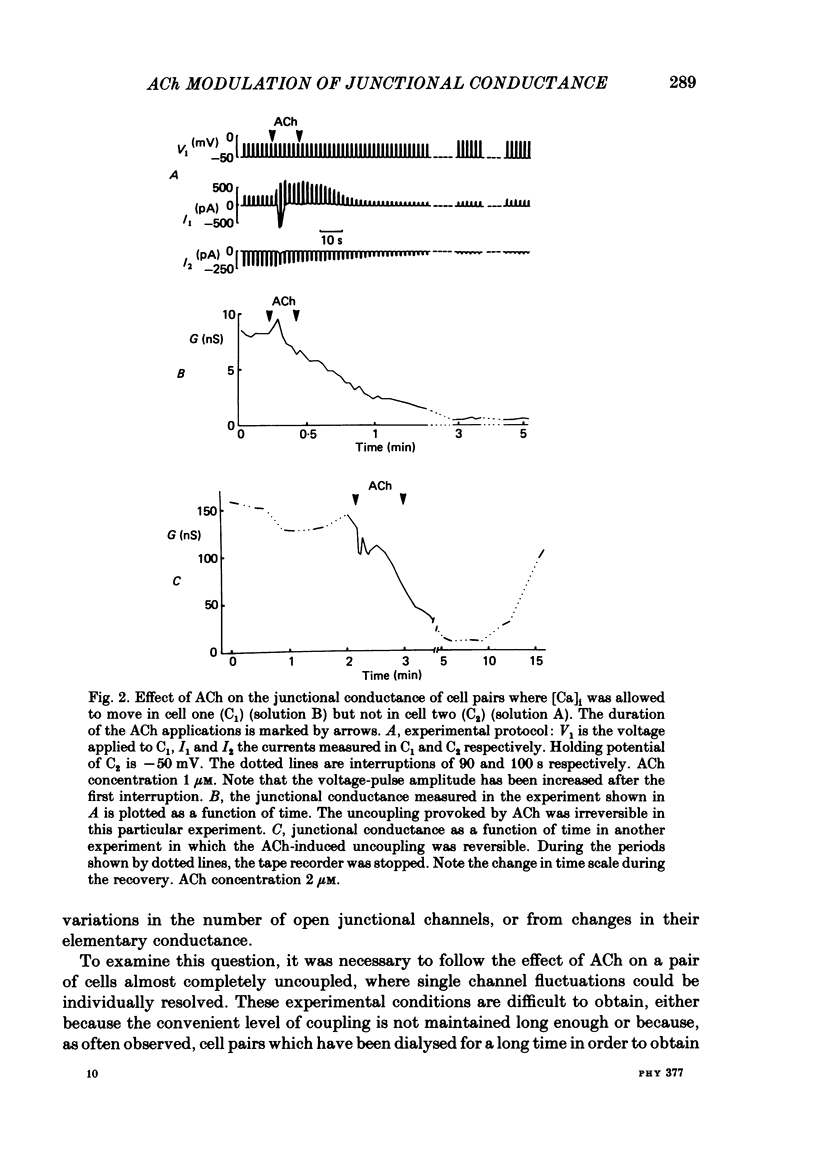
The conductance of intercellular junctions between rat lacrimal cells was studied with the double whole-cell tight-seal recording technique. This conductance decreases spontaneously with time as a result of the double-cell dialysis. The rate of this 'spontaneous' uncoupling is unaffected by changing the internal Ca concentration, [Ca]i, between 10(-8) M and 10(-6) M. This rate of uncoupling is greatly increased when [Ca]i is approximately 10(-5) M, and this effect does not involve changes in the internal proton concentration. When [Ca]i is weakly buffered in one of the two cells, 1-2 microM-acetylcholine (ACh) both activates Ca-dependent channels in that cell (Marty, Tan & Trautmann, 1984) and uncouples the two cells. The uncoupling is not synchronous with the increase in [Ca]i as reflected by the Ca-dependent currents. When [Ca]i is strongly buffered in both cells, ACh fails to activate Ca-dependent currents, but it can still uncouple the cells. This ACh-induced uncoupling is often preceded by a transient enhancing of the coupling. In conclusion, ACh has several distinct effects on lacrimal cells: activation of Ca-dependent channels in the plasma membrane, closure of junctional channels involving a Ca-independent mechanism, and sometimes, an increase in the junctional coupling by a Ca-independent mechanism.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Browne C. L., Wiley H. S., Dumont J. N. Oocyte-follicle cell gap junctions in Xenopus laevis and the effects of gonadotropin on their permeability. Science. 1979 Jan 12;203(4376):182–183. doi: 10.1126/science.569364. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Contractions induced by a calcium-triggered release of calcium from the sarcoplasmic reticulum of single skinned cardiac cells. J Physiol. 1975 Aug;249(3):469–495. doi: 10.1113/jphysiol.1975.sp011026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlay I., Petersen O. H. Acetylcholine-evoked uncoupling restricts the passage of Lucifer Yellow between pancreatic acinar cells. Cell Tissue Res. 1982;225(3):633–638. doi: 10.1007/BF00214809. [DOI] [PubMed] [Google Scholar]
- Giaume C., Spira M. E., Korn H. Uncoupling of invertebrate electrotonic synapses by carbon dioxide. Neurosci Lett. 1980 Apr;17(1-2):197–202. doi: 10.1016/0304-3940(80)90084-1. [DOI] [PubMed] [Google Scholar]
- Ginsborg B. L., House C. R. Stimulus-response coupling in gland cells. Annu Rev Biophys Bioeng. 1980;9:55–80. doi: 10.1146/annurev.bb.09.060180.000415. [DOI] [PubMed] [Google Scholar]
- Iwatsuki N., Petersen O. H. Electrical coupling and uncoupling of exocrine acinar cells. J Cell Biol. 1978 Nov;79(2 Pt 1):533–545. doi: 10.1083/jcb.79.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwatsuki N., Petersen O. H. Pancreatic acinar cells: acetylcholine-evoked electrical uncoupling and its ionic dependency. J Physiol. 1978 Jan;274:81–06. doi: 10.1113/jphysiol.1978.sp012135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwatsuki N., Petersen O. H. Pancreatic acinar cells: the effect of carbon dioxide, ammonium chloride and acetylcholine on intercellular communication. J Physiol. 1979 Jun;291:317–326. doi: 10.1113/jphysiol.1979.sp012815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanagasuntheram P., Randle P. J. Calcium metabolism and amylase release in rat parotid acinar cells. Biochem J. 1976 Dec 15;160(3):547–564. doi: 10.1042/bj1600547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler J. A., Spray D. C., Saez J. C., Bennett M. V. Determination of synaptic phenotype: insulin and cAMP independently initiate development of electrotonic coupling between cultured sympathetic neurons. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6235–6239. doi: 10.1073/pnas.81.19.6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasater E. M., Dowling J. E. Dopamine decreases conductance of the electrical junctions between cultured retinal horizontal cells. Proc Natl Acad Sci U S A. 1985 May;82(9):3025–3029. doi: 10.1073/pnas.82.9.3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty A., Tan Y. P., Trautmann A. Three types of calcium-dependent channel in rat lacrimal glands. J Physiol. 1984 Dec;357:293–325. doi: 10.1113/jphysiol.1984.sp015501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meech R. W., Thomas R. C. The effect of calcium injection on the intracellular sodium and pH of snail neurones. J Physiol. 1977 Mar;265(3):867–879. doi: 10.1113/jphysiol.1977.sp011749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyton J., Trautmann A. Single-channel currents of an intercellular junction. 1985 Sep 26-Oct 2Nature. 317(6035):331–335. doi: 10.1038/317331a0. [DOI] [PubMed] [Google Scholar]
- Peracchia C., Girsch S. J. Functional modulation of cell coupling: evidence for a calmodulin-driven channel gate. Am J Physiol. 1985 Jun;248(6 Pt 2):H765–H782. doi: 10.1152/ajpheart.1985.248.6.H765. [DOI] [PubMed] [Google Scholar]
- Piccolino M., Neyton J., Gerschenfeld H. M. Decrease of gap junction permeability induced by dopamine and cyclic adenosine 3':5'-monophosphate in horizontal cells of turtle retina. J Neurosci. 1984 Oct;4(10):2477–2488. doi: 10.1523/JNEUROSCI.04-10-02477.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose B., Loewenstein W. R. Permeability of a cell junction and the local cytoplasmic free ionized calcium concentration: a study with aequorin. J Membr Biol. 1976 Aug 27;28(1):87–119. doi: 10.1007/BF01869692. [DOI] [PubMed] [Google Scholar]
- Rose B., Rick R. Intracellular pH, intracellular free Ca, and junctional cell-cell coupling. J Membr Biol. 1978 Dec 29;44(3-4):377–415. doi: 10.1007/BF01944230. [DOI] [PubMed] [Google Scholar]
- Sims S. M., Daniel E. E., Garfield R. E. Improved electrical coupling in uterine smooth muscle is associated with increased numbers of gap junctions at parturition. J Gen Physiol. 1982 Sep;80(3):353–375. doi: 10.1085/jgp.80.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spray D. C., Harris A. L., Bennett M. V. Gap junctional conductance is a simple and sensitive function of intracellular pH. Science. 1981 Feb 13;211(4483):712–715. doi: 10.1126/science.6779379. [DOI] [PubMed] [Google Scholar]
- Spray D. C., Stern J. H., Harris A. L., Bennett M. V. Gap junctional conductance: comparison of sensitivities to H and Ca ions. Proc Natl Acad Sci U S A. 1982 Jan;79(2):441–445. doi: 10.1073/pnas.79.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teranishi T., Negishi K., Kato S. Dopamine modulates S-potential amplitude and dye-coupling between external horizontal cells in carp retina. Nature. 1983 Jan 20;301(5897):243–246. doi: 10.1038/301243a0. [DOI] [PubMed] [Google Scholar]
- Trautmann A., Marty A. Activation of Ca-dependent K channels by carbamoylcholine in rat lacrimal glands. Proc Natl Acad Sci U S A. 1984 Jan;81(2):611–615. doi: 10.1073/pnas.81.2.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turin L., Warner A. E. Intracellular pH in early Xenopus embryos: its effect on current flow between blastomeres. J Physiol. 1980 Mar;300:489–504. doi: 10.1113/jphysiol.1980.sp013174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turin L., Warner A. Carbon dioxide reversibly abolishes ionic communication between cells of early amphibian embryo. Nature. 1977 Nov 3;270(5632):56–57. doi: 10.1038/270056a0. [DOI] [PubMed] [Google Scholar]



