Abstract
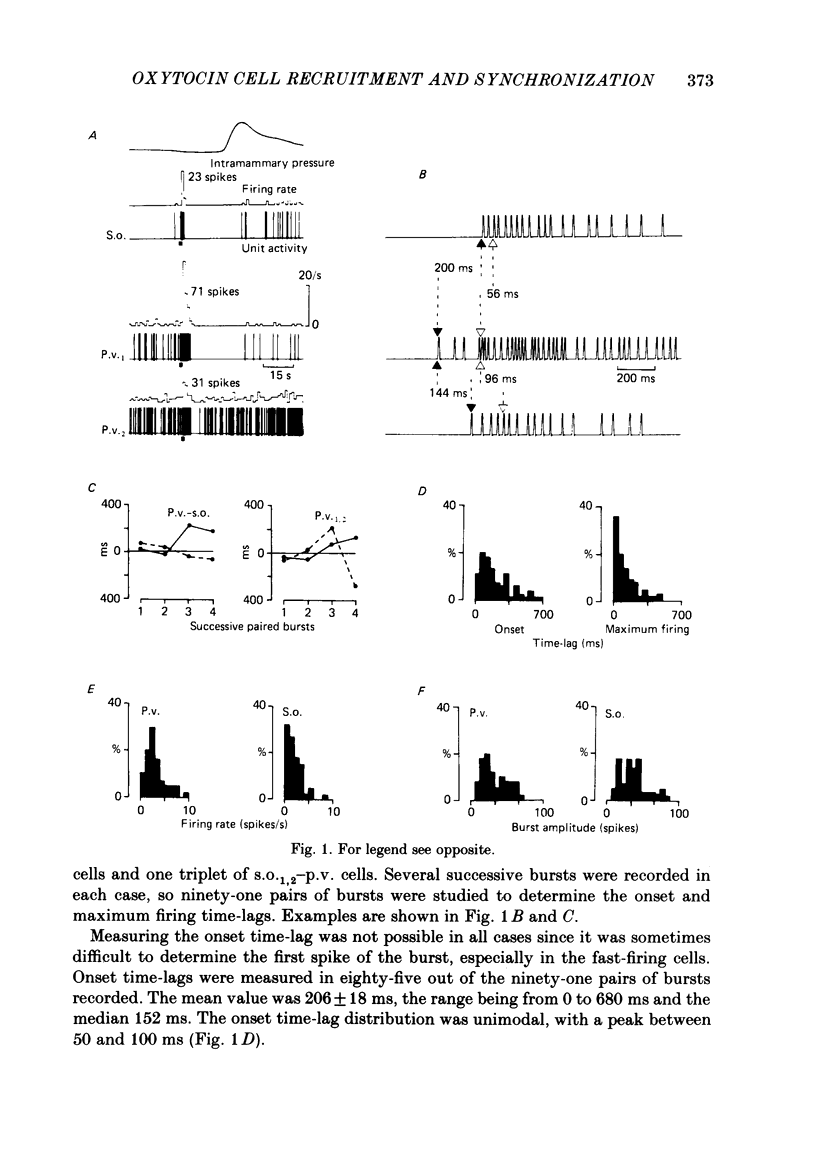
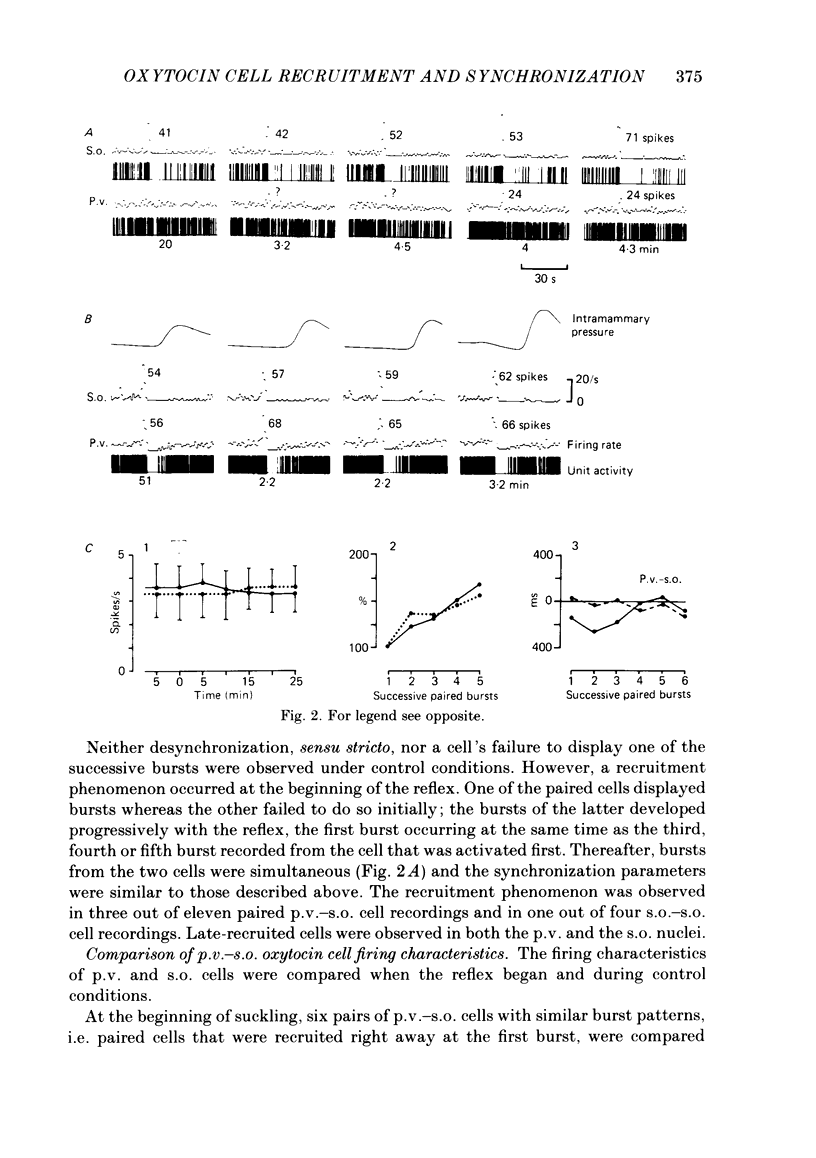
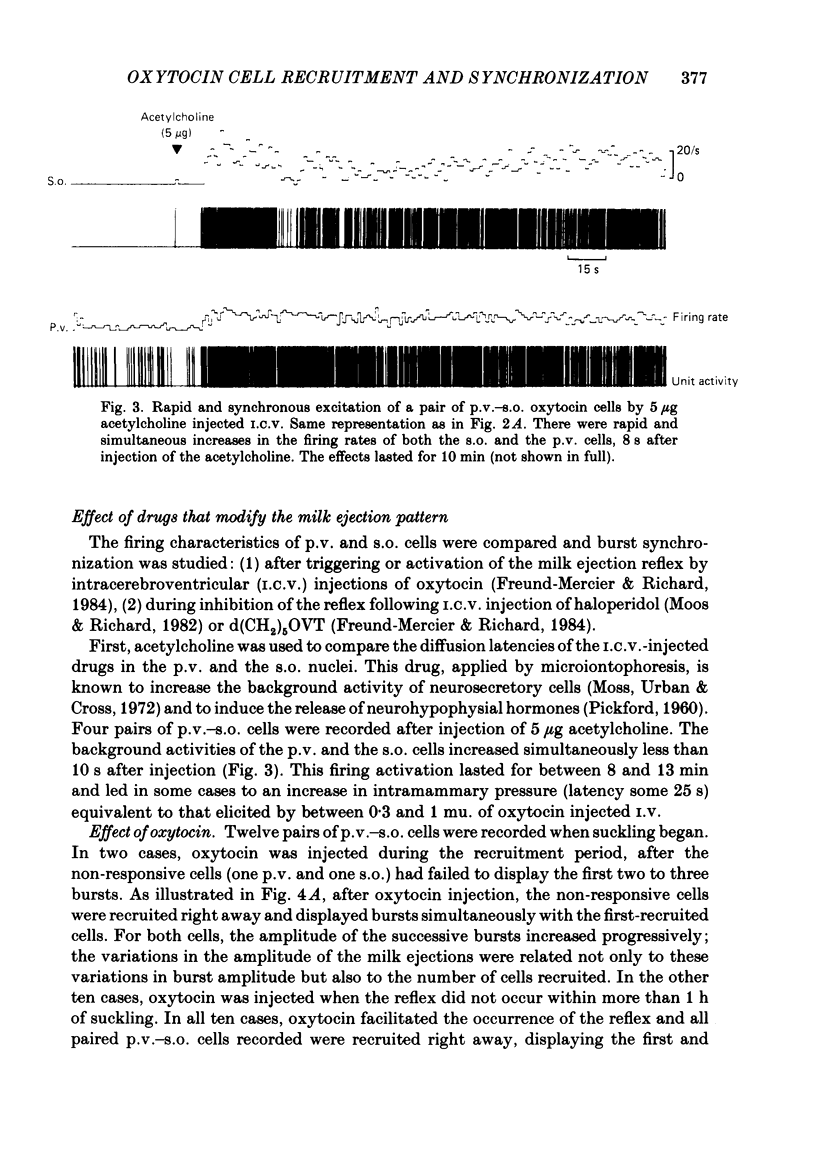
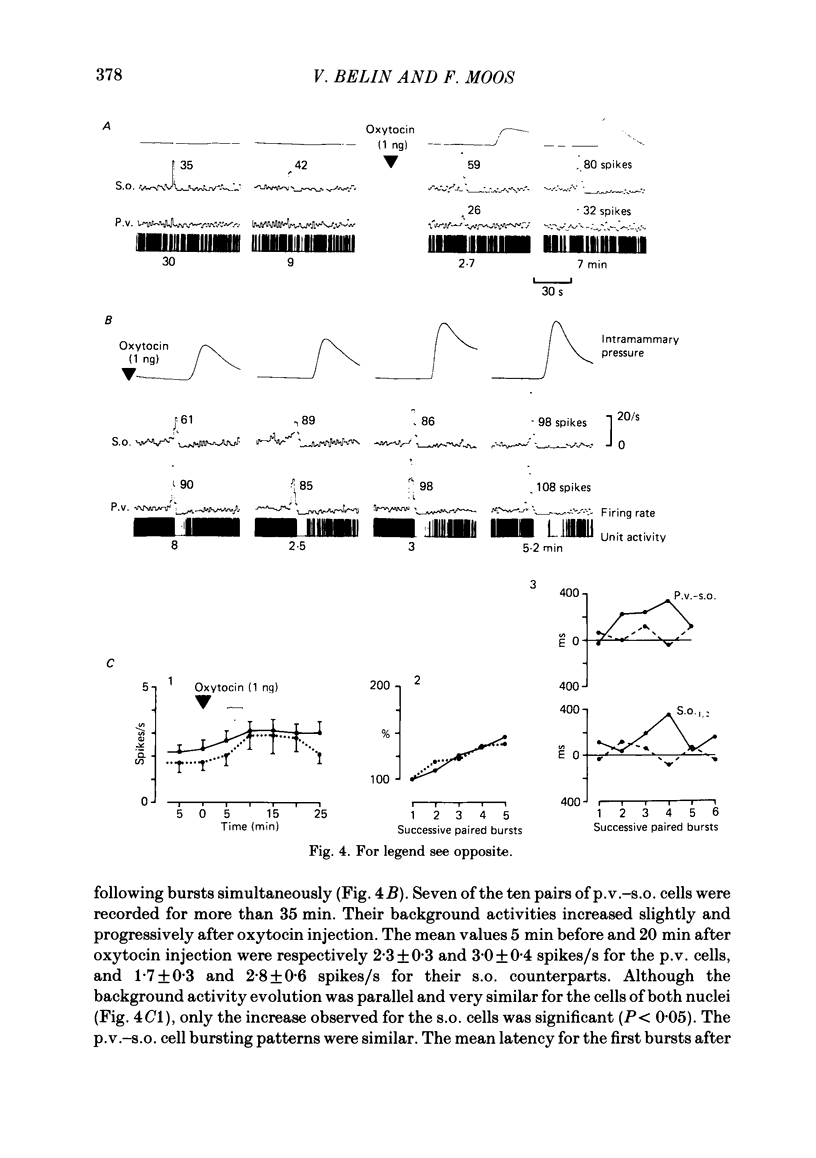
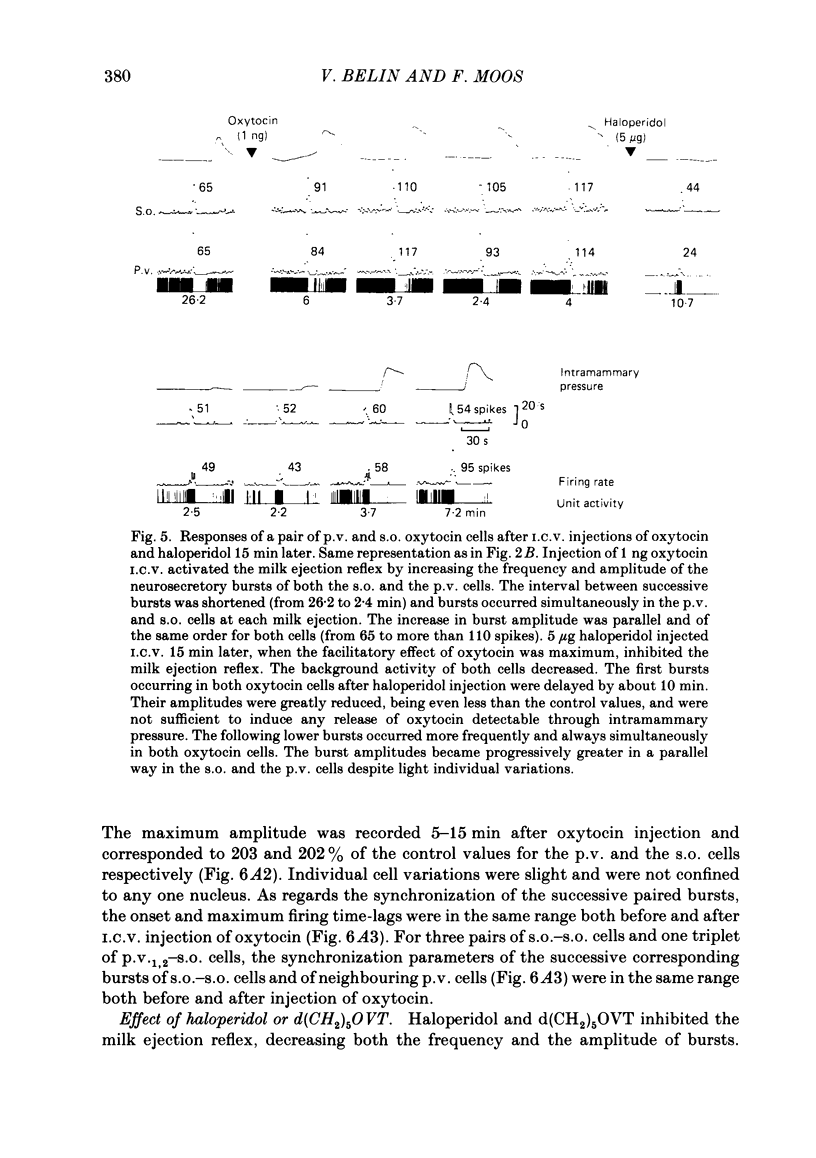
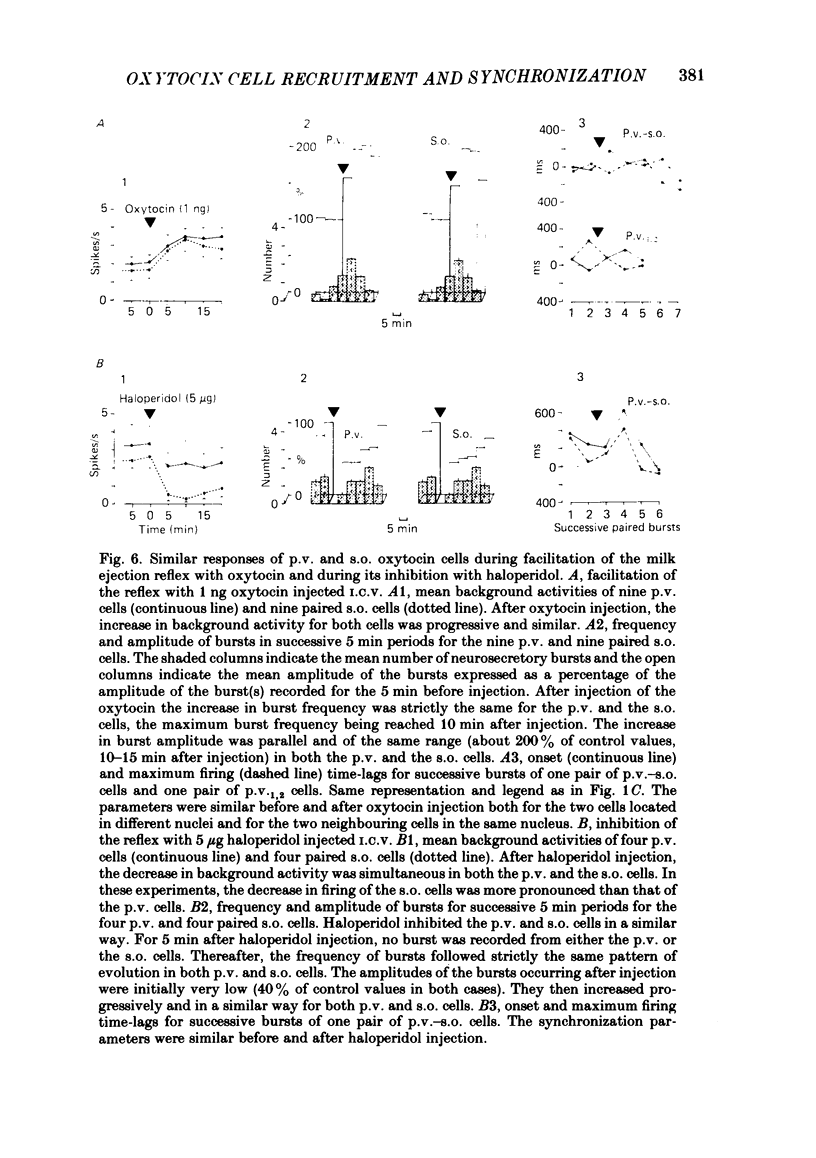
Oxytocin cells in the paraventricular (p.v.) and contralateral supraoptic (s.o.) nuclei were pair-recorded (with two micro-electrodes) in suckled rats after being anaesthetized with urethane (1.2 g/kg), to study the synchronization of their neurosecretory bursts, the importance of cell recruitment and their firing characteristics. The synchronization of paired bursts was determined by measuring the onset time-lag (time in milliseconds between the onset of two corresponding bursts) and the maximum firing time-lag (time in milliseconds between the two shortest interspike intervals for the corresponding bursts). For each cell, the characteristics studied were: the background activity and the frequency and amplitude (total number of spikes) of the neurosecretory bursts. All paired p.v.-s.o. cells recorded were activated simultaneously 12-18 s before each milk ejection. The onset of a burst could vary either way, up to 680 ms, in relation to the other (mean onset time-lag was 206 +/- 18 ms; n = 85) but the maximum activation periods fitted more closely, the mean maximum firing time-lag being 122 +/- 14 ms (n = 64). Both parameters varied randomly, in duration and order from one pair of cells to another, from one pair of bursts to another for successive bursts of a given pair of cells and independently, whether the cells were in the p.v. or the s.o. nucleus. However, in most cases, the neurosecretory burst with the highest amplitude began and reached its peak firing rate before the corresponding burst from the other cell. Cell recruitment was observed when the milk ejection reflex began, for both the p.v. and the s.o. cells. The bursts of the non-responsive cells developed progressively with the reflex, but, as soon as a cell was recruited, all its successive bursts were simultaneous with those of the first-recruited oxytocin cells. During a regular pattern of milk ejections, the mean background activity of sixty p.v. cells (3.1 +/- 0.2 spikes/s) was significantly higher than that of their s.o. counterparts (1.9 +/- 0.2 spikes/s). Nevertheless, the mean amplitude of the neurosecretory bursts of the sixty p.v. cells (49 +/- 3 spikes) did not differ significantly from that of their s.o. counterparts (55 +/- 4 spikes).(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrew R. D., MacVicar B. A., Dudek F. E., Hatton G. I. Dye transfer through gap junctions between neuroendocrine cells of rat hypothalamus. Science. 1981 Mar 13;211(4487):1187–1189. doi: 10.1126/science.7466393. [DOI] [PubMed] [Google Scholar]
- Armstrong W. E., Schöler J., McNeill T. H. Immunocytochemical, Golgi and electron microscopic characterization of putative dendrites in the ventral glial lamina of the rat supraoptic nucleus. Neuroscience. 1982 Mar;7(3):679–694. doi: 10.1016/0306-4522(82)90074-4. [DOI] [PubMed] [Google Scholar]
- Bankowski K., Manning M., Seto J., Haldar J., Sawyer W. H. Design and synthesis of potent in vivo antagonists of oxytocin. Int J Pept Protein Res. 1980 Nov;16(5):382–391. doi: 10.1111/j.1399-3011.1980.tb02962.x. [DOI] [PubMed] [Google Scholar]
- Belin V., Moos F., Richard P. Synchronization of oxytocin cells in the hypothalamic paraventricular and supraoptic nuclei in suckled rats: direct proof with paired extracellular recordings. Exp Brain Res. 1984;57(1):201–203. doi: 10.1007/BF00231147. [DOI] [PubMed] [Google Scholar]
- Bruni J. E., Perumal P. M. Cytoarchitecture of the rat's supraoptic nucleus. Anat Embryol (Berl) 1984;170(2):129–138. doi: 10.1007/BF00318997. [DOI] [PubMed] [Google Scholar]
- Freund-Mercier M. J., Richard P. Electrophysiological evidence for facilitatory control of oxytocin neurones by oxytocin during suckling in the rat. J Physiol. 1984 Jul;352:447–466. doi: 10.1113/jphysiol.1984.sp015302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund-Mercier M. J., Richard P. Excitatory effects of intraventricular injections of oxytocin on the milk ejection reflex in the rat. Neurosci Lett. 1981 May 6;23(2):193–198. doi: 10.1016/0304-3940(81)90039-2. [DOI] [PubMed] [Google Scholar]
- Gutnick M. J., Connors B. W., Prince D. A. Mechanisms of neocortical epileptogenesis in vitro. J Neurophysiol. 1982 Dec;48(6):1321–1335. doi: 10.1152/jn.1982.48.6.1321. [DOI] [PubMed] [Google Scholar]
- Hatton G. I., Tweedle C. D. Magnocellular neuropeptidergic neurons in hypothalamus: increases in membrane apposition and number of specialized synapses from pregnancy to lactation. Brain Res Bull. 1982 Feb;8(2):197–204. doi: 10.1016/0361-9230(82)90046-6. [DOI] [PubMed] [Google Scholar]
- Juss T. S., Wakerley J. B. Mesencephalic areas controlling pulsatile oxytocin release in the suckled rat. J Endocrinol. 1981 Nov;91(2):233–244. doi: 10.1677/joe.0.0910233. [DOI] [PubMed] [Google Scholar]
- Kiss J. Z., Palkovits M., Záborszky L., Tribollet E., Szabó D., Makara G. B. Quantitative histological studies on the hypothalamic paraventricular nucleus in rats: I. Number of cells and synaptic boutons. Brain Res. 1983 Mar 7;262(2):217–224. doi: 10.1016/0006-8993(83)91011-9. [DOI] [PubMed] [Google Scholar]
- Leng G., Dyball R. E. Intercommunication in the rat supraoptic nucleus. Q J Exp Physiol. 1983 Jul;68(3):493–504. doi: 10.1113/expphysiol.1983.sp002742. [DOI] [PubMed] [Google Scholar]
- Leng G. The effects of neural stalk stimulation upon firing patterns in rat supraoptic neurones. Exp Brain Res. 1981;41(2):135–145. doi: 10.1007/BF00236603. [DOI] [PubMed] [Google Scholar]
- Lincoln D. W., Hill A., Wakerley J. B. The milk-ejection reflex of the rat: an intermittent function not abolished by surgical levels of anaesthesia. J Endocrinol. 1973 Jun;57(3):459–476. doi: 10.1677/joe.0.0570459. [DOI] [PubMed] [Google Scholar]
- Lincoln D. W., Wakerley J. B. Electrophysiological evidence for the activation of supraoptic neurones during the release of oxytocin. J Physiol. 1974 Oct;242(2):533–554. doi: 10.1113/jphysiol.1974.sp010722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln D. W., Wakerley J. B. Factors governing the periodic activation of supraoptic and paraventricular neurosecretory cells during suckling in the rat. J Physiol. 1975 Sep;250(2):443–461. doi: 10.1113/jphysiol.1975.sp011064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Léránth C., Záborszky L., Marton J., Palkovits M. Quantitative studies on the supraoptic nucleus in the rat. I. Synaptic organization. Exp Brain Res. 1975 May 22;22(5):509–523. doi: 10.1007/BF00237351. [DOI] [PubMed] [Google Scholar]
- Mason W. T. Electrical properties of neurons recorded from the rat supraoptic nucleus in vitro. Proc R Soc Lond B Biol Sci. 1983 Jan 22;217(1207):141–161. doi: 10.1098/rspb.1983.0003. [DOI] [PubMed] [Google Scholar]
- Mason W. T., Leng G. Complex action potential waveform recorded from supraoptic and paraventricular neurones of the rat: evidence for sodium and calcium spike components at different membrane sites. Exp Brain Res. 1984;56(1):135–143. doi: 10.1007/BF00237449. [DOI] [PubMed] [Google Scholar]
- Miles R., Wong R. K. Single neurones can initiate synchronized population discharge in the hippocampus. Nature. 1983 Nov 24;306(5941):371–373. doi: 10.1038/306371a0. [DOI] [PubMed] [Google Scholar]
- Miles R., Wong R. K., Traub R. D. Synchronized afterdischarges in the hippocampus: contribution of local synaptic interactions. Neuroscience. 1984 Aug;12(4):1179–1189. doi: 10.1016/0306-4522(84)90012-5. [DOI] [PubMed] [Google Scholar]
- Minors D. S., Waterhouse J. M. Circadian rhythms of urinary excretion: the relationship between the amount excreted and the circadian changes. J Physiol. 1982 Jun;327:39–51. doi: 10.1113/jphysiol.1982.sp014218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moos F., Freund-Mercier M. J., Guerné Y., Guerné J. M., Stoeckel M. E., Richard P. Release of oxytocin and vasopressin by magnocellular nuclei in vitro: specific facilitatory effect of oxytocin on its own release. J Endocrinol. 1984 Jul;102(1):63–72. doi: 10.1677/joe.0.1020063. [DOI] [PubMed] [Google Scholar]
- Moos F., Richard P. Excitatory effect of dopamine on oxytocin and vasopressin reflex releases in the rat. Brain Res. 1982 Jun 10;241(2):249–260. doi: 10.1016/0006-8993(82)91061-7. [DOI] [PubMed] [Google Scholar]
- Moos F., Richard P. Serotonergic control of oxytocin release during suckling in the rat: opposite effects in conscious and anesthetized rats. Neuroendocrinology. 1983;36(4):300–306. doi: 10.1159/000123471. [DOI] [PubMed] [Google Scholar]
- Moss R. L., Urban I., Cross B. A. Microelectrophoresis of cholinergic and aminergic drugs on paraventricular neurons. Am J Physiol. 1972 Aug;223(2):310–318. doi: 10.1152/ajplegacy.1972.223.2.310. [DOI] [PubMed] [Google Scholar]
- PICKFORD M. Factors affecting milk release in the dog and the quantity of oxytocin liberated by suckling. J Physiol. 1960 Jul;152:515–526. doi: 10.1113/jphysiol.1960.sp006506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulain D. A., Wakerley J. B., Dyball R. E. Electrophysiological differentiation of oxytocin- and vasopressin-secreting neurones. Proc R Soc Lond B Biol Sci. 1977 Apr;196(1125):367–384. doi: 10.1098/rspb.1977.0046. [DOI] [PubMed] [Google Scholar]
- Richardson T. L., Turner R. W., Miller J. J. Extracellular fields influence transmembrane potentials and synchronization of hippocampal neuronal activity. Brain Res. 1984 Mar 5;294(2):255–262. doi: 10.1016/0006-8993(84)91037-0. [DOI] [PubMed] [Google Scholar]
- Saphier D., Feldman S. Electrophysiologic evidence for neural connections between the paraventricular nucleus and neurons of the supraoptic nucleus in the rat. Exp Neurol. 1985 Jul;89(1):289–294. doi: 10.1016/0014-4886(85)90287-0. [DOI] [PubMed] [Google Scholar]
- Sofroniew M. V., Glasmann W. Golgi-like immunoperoxidase staining of hypothalamic magnocellular neurons that contain vasopressin, oxytocin or neurophysin in the rat. Neuroscience. 1981;6(4):619–643. doi: 10.1016/0306-4522(81)90147-0. [DOI] [PubMed] [Google Scholar]
- Taylor C. P., Dudek F. E. Excitation of hippocampal pyramidal cells by an electrical field effect. J Neurophysiol. 1984 Jul;52(1):126–142. doi: 10.1152/jn.1984.52.1.126. [DOI] [PubMed] [Google Scholar]
- Theodosis D. T. Oxytocin-immunoreactive terminals synapse on oxytocin neurones in the supraoptic nucleus. Nature. 1985 Feb 21;313(6004):682–684. doi: 10.1038/313682a0. [DOI] [PubMed] [Google Scholar]
- Theodosis D. T., Poulain D. A. Evidence for structural plasticity in the supraoptic nucleus of the rat hypothalamus in relation to gestation and lactation. Neuroscience. 1984 Jan;11(1):183–193. doi: 10.1016/0306-4522(84)90222-7. [DOI] [PubMed] [Google Scholar]
- Theodosis D. T., Poulain D. A. Evidence that oxytocin-secreting neurones are involved in the ultrastructural reorganisation of the rat supraoptic nucleus apparent at lactation. Cell Tissue Res. 1984;235(1):217–219. doi: 10.1007/BF00213745. [DOI] [PubMed] [Google Scholar]
- Theodosis D. T., Poulain D. A., Vincent J. D. Possible morphological bases for synchronisation of neuronal firing in the rat supraoptic nucleus during lactation. Neuroscience. 1981;6(5):919–929. doi: 10.1016/0306-4522(81)90173-1. [DOI] [PubMed] [Google Scholar]
- Thomson A. M. Correlations between the firing of supraoptic neurones in slices of rat brain. Exp Brain Res. 1984;54(2):217–224. doi: 10.1007/BF00236221. [DOI] [PubMed] [Google Scholar]
- Traub R. D., Wong R. K. Cellular mechanism of neuronal synchronization in epilepsy. Science. 1982 May 14;216(4547):745–747. doi: 10.1126/science.7079735. [DOI] [PubMed] [Google Scholar]
- Tribollet E., Dreifuss J. J. Localization of neurones projecting to the hypothalamic paraventricular nucleus area of the rat: a horseradish peroxidase study. Neuroscience. 1981;6(7):1315–1328. doi: 10.1016/0306-4522(81)90190-1. [DOI] [PubMed] [Google Scholar]
- Voloschin L. M., Tramezzani J. H. The neural input of the milk ejection reflex in the hypothalamus. Endocrinology. 1973 Apr;92(4):973–983. doi: 10.1210/endo-92-4-973. [DOI] [PubMed] [Google Scholar]
- Wakerley J. B., Lincoln D. W. The milk-ejection reflex of the rat: a 20- to 40-fold acceleration in the firing of paraventricular neurones during oxytocin release. J Endocrinol. 1973 Jun;57(3):477–493. doi: 10.1677/joe.0.0570477. [DOI] [PubMed] [Google Scholar]
- Yaari Y., Konnerth A., Heinemann U. Spontaneous epileptiform activity of CA1 hippocampal neurons in low extracellular calcium solutions. Exp Brain Res. 1983;51(1):153–156. doi: 10.1007/BF00236813. [DOI] [PubMed] [Google Scholar]
- Yarom Y., Spira M. E. Extracellular potassium ions mediate specific neuronal interaction. Science. 1982 Apr 2;216(4541):80–82. doi: 10.1126/science.6278595. [DOI] [PubMed] [Google Scholar]


