Abstract
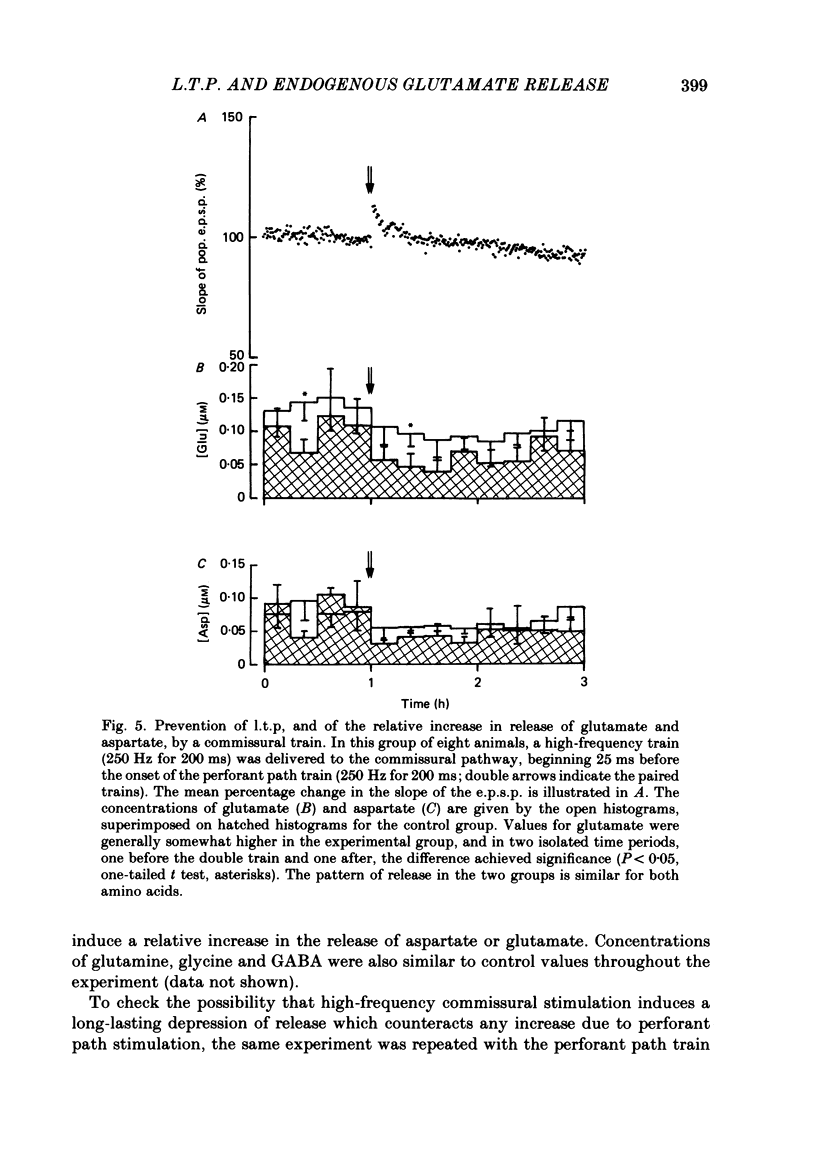
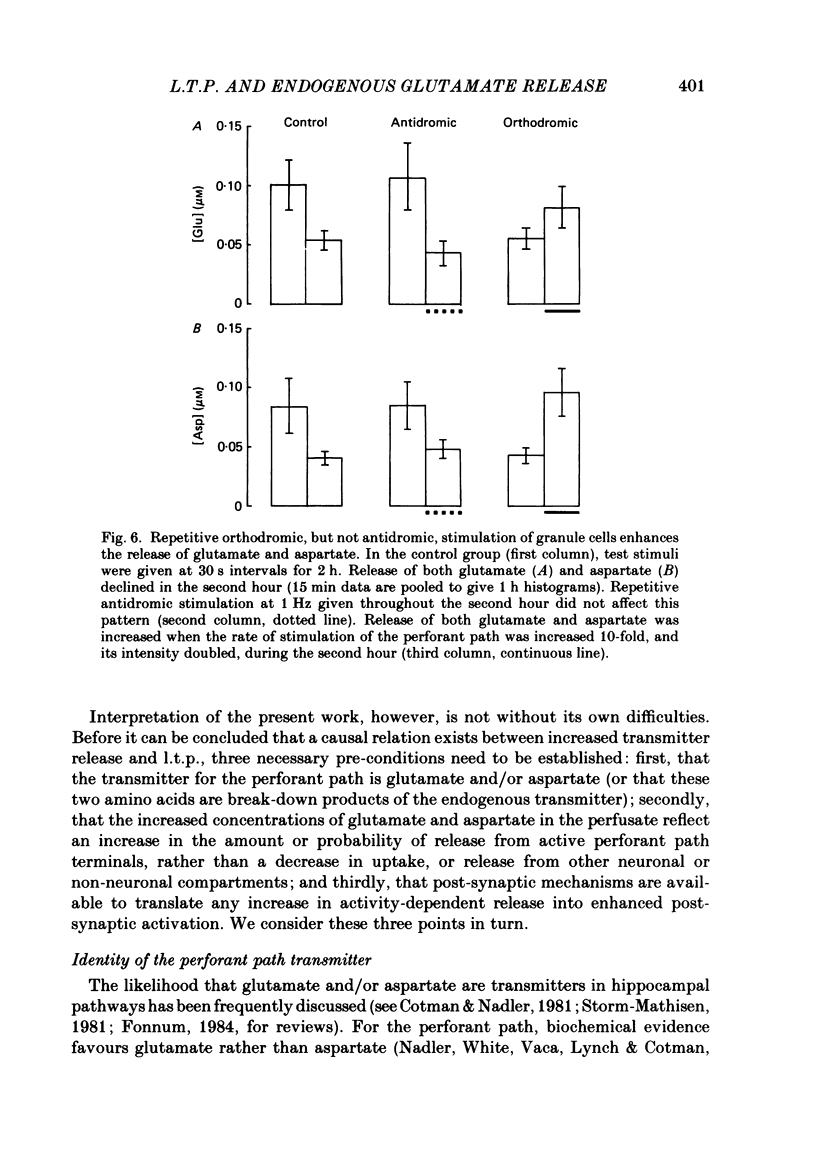
The relationship between long-term potentiation (l.t.p.) and the release of endogenous amino acid transmitters has been investigated in the dentate gyrus of rats anaesthetized with urethane. The molecular layer was perfused with artificial cerebrospinal fluid using a push-pull cannula. The perfusate was collected and analysed for glutamate, aspartate, glycine, glutamine and gamma-aminobutyric acid (GABA) using high-performance liquid chromatography (h.p.l.c.) with fluorometric detection. Recording electrodes were attached to the cannula to enable responses evoked by test stimuli to the perforant path to be monitored in the molecular and cell body layers. Perfusion was continued for 3 h while test stimuli were delivered to the perforant path at 30 s intervals. In the control group (n = 8), no further stimulation was given. In a second group (n = 8), a single high-frequency train (250 Hz for 200 ms) was delivered at the end of the first hour to induce l.t.p. The average potentiation of the slope of the excitatory post-synaptic potential (e.p.s.p.) 2 h later was 15%. In a third group (n = 8), the train to the perforant path was paired with a train to the commissural input to the dentate gyrus, a procedure which blocks the induction of l.t.p. In the potentiated group, there was an increase in the concentrations of glutamate and aspartate following the induction of l.t.p., relative to the decline seen in corresponding periods of the control group. This increase remained statistically significant for 1.5 h in the case of glutamate and for 45 min in the case of aspartate. There were no l.t.p.-associated changes in the release of glutamine or glycine; there was an indication that l.t.p. may be associated with a decrease in the release of GABA. Increasing the frequency and intensity of perforant path activation resulted in enhanced concentrations of glutamate and aspartate in the perfusate; no such changes occurred when granule cells were activated antidromically. We discuss the origin of the relative increases in the concentration of glutamate and aspartate which are found in the perfusate following the induction of l.t.p. and conclude that the most likely source is a sustained increase in activity-dependent release of these amino acids from perforant path terminals.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF

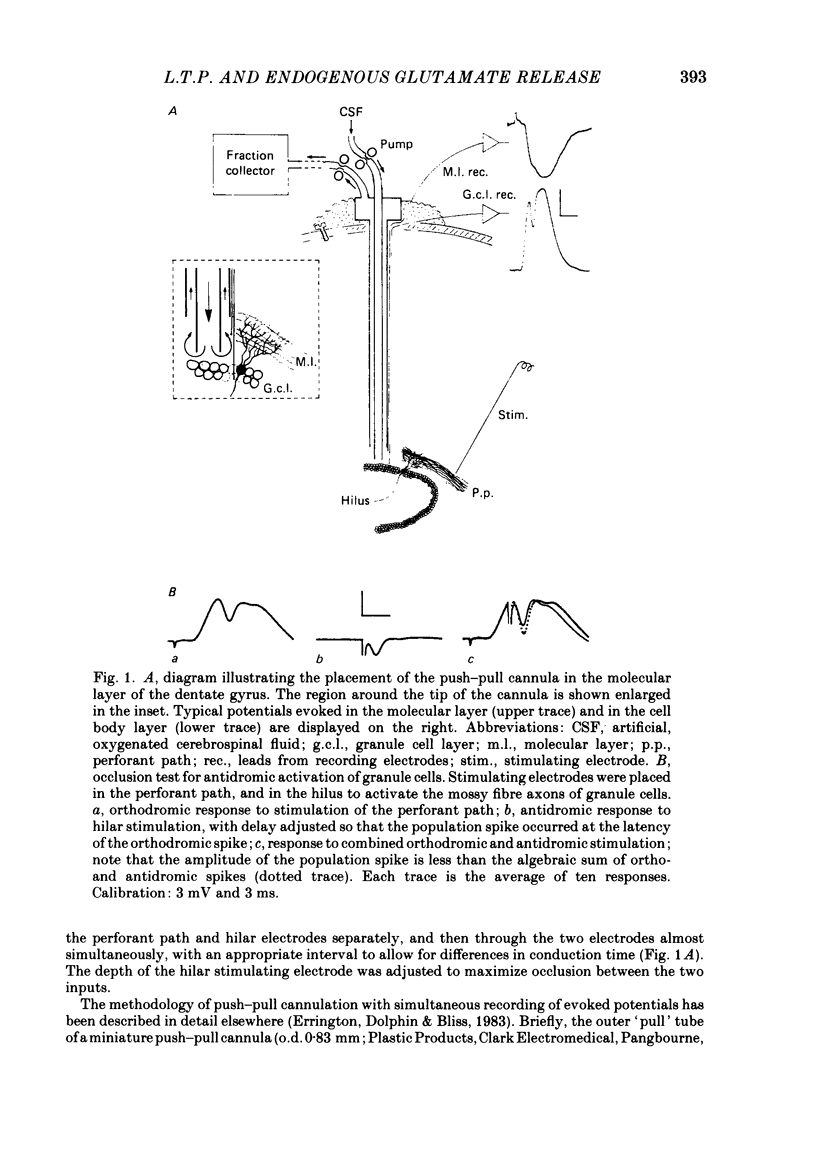


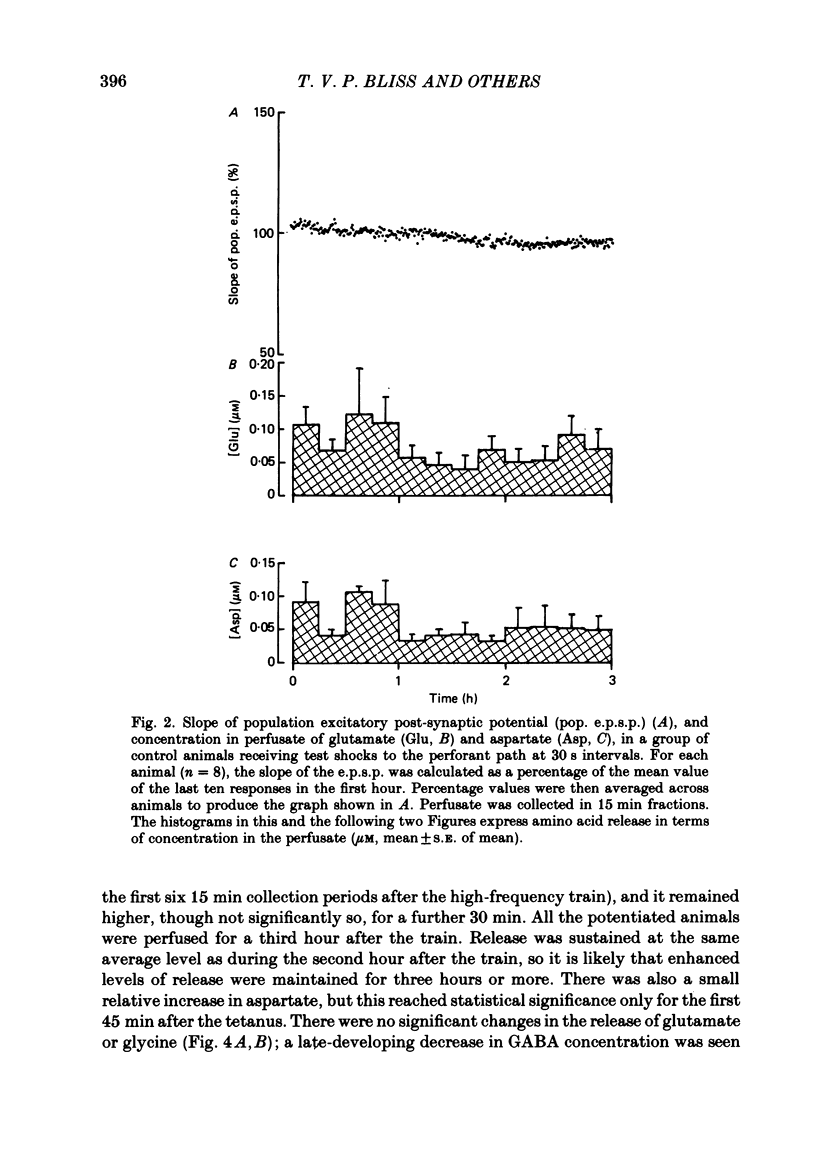
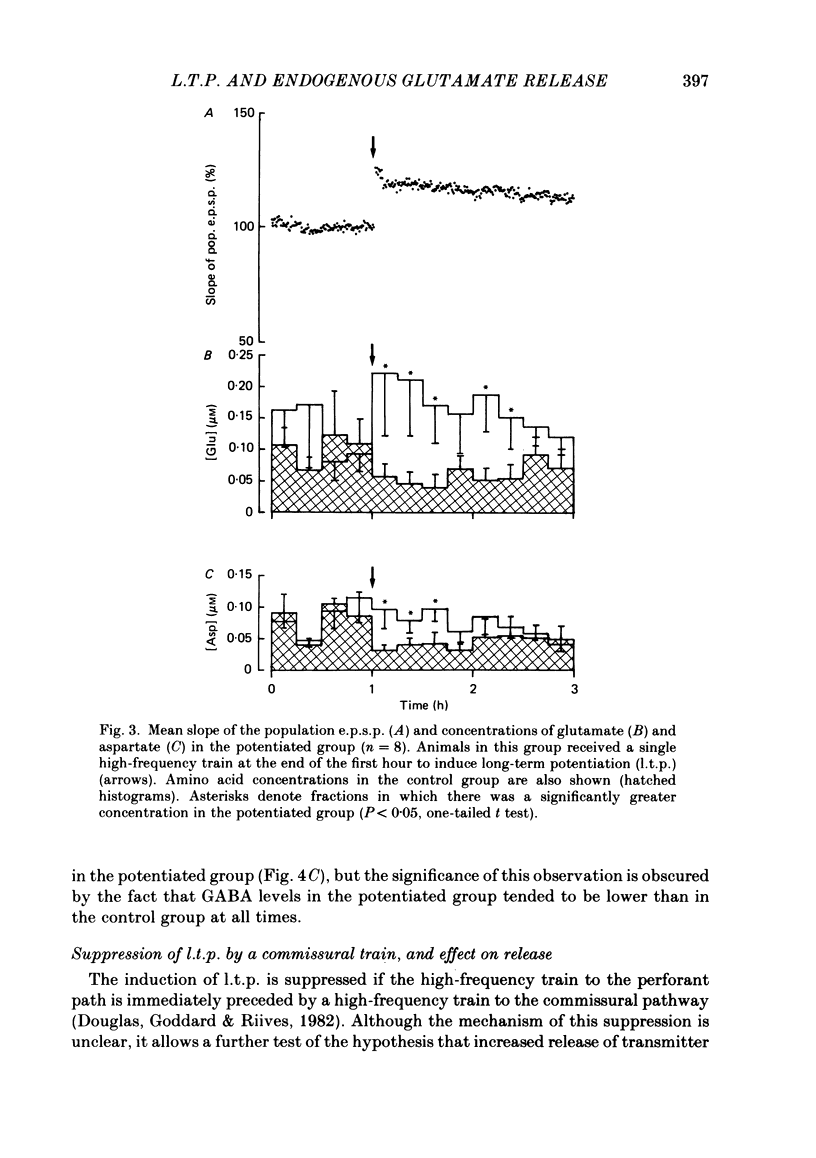
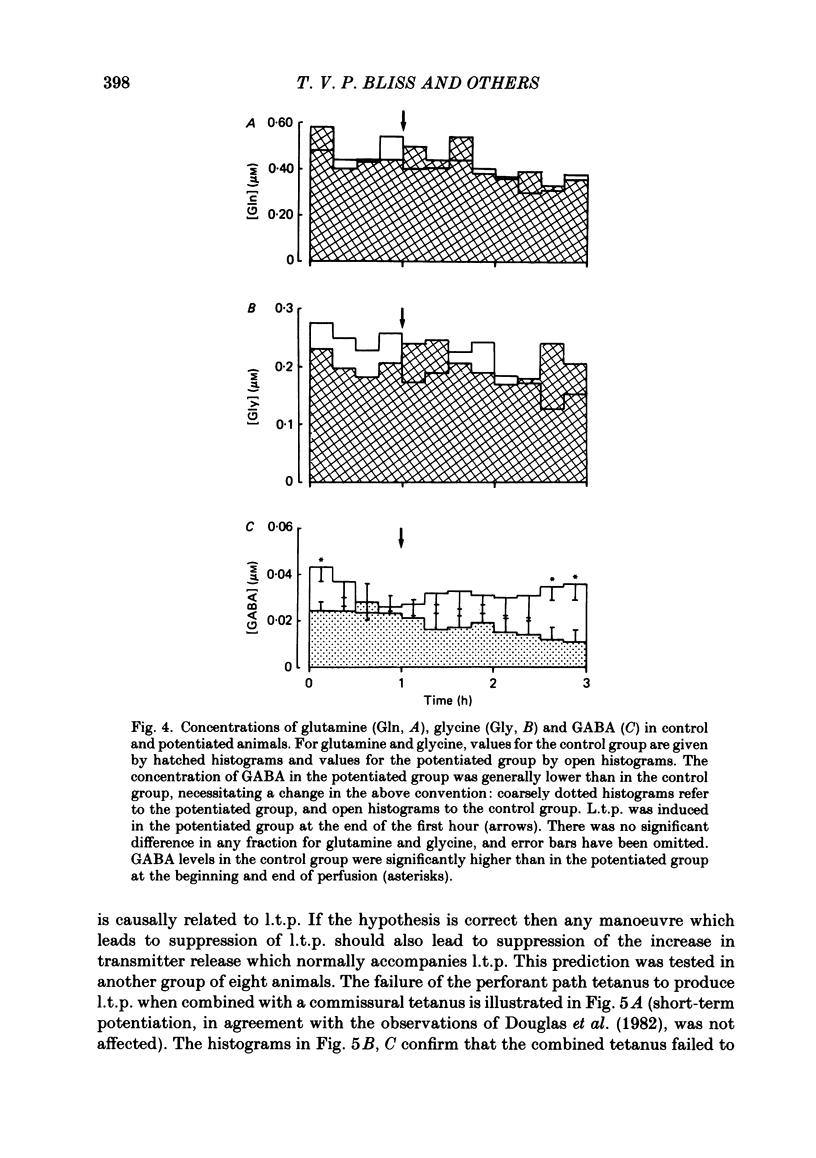








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham W. C., Bliss T. V., Goddard G. V. Heterosynaptic changes accompany long-term but not short-term potentiation of the perforant path in the anaesthetized rat. J Physiol. 1985 Jun;363:335–349. doi: 10.1113/jphysiol.1985.sp015714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba A., Okumura S., Mizuo H., Iwata H. Inhibiton of diazepam and gamma-aminobutyric acid of depolarization-induced release of [14C]cysteine sulfinate and [3H]glutamate in rat hippocampal slices. J Neurochem. 1983 Jan;40(1):280–284. doi: 10.1111/j.1471-4159.1983.tb12683.x. [DOI] [PubMed] [Google Scholar]
- Baudry M., Oliver M., Creager R., Wieraszko A., Lynch G. Increase in glutamate receptors following repetitive electrical stimulation in hippocampal slices. Life Sci. 1980 Jul 28;27(4):325–330. doi: 10.1016/0024-3205(80)90200-3. [DOI] [PubMed] [Google Scholar]
- Baxter D. A., Bittner G. D., Brown T. H. Quantal mechanism of long-term synaptic potentiation. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5978–5982. doi: 10.1073/pnas.82.17.5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs C. A., McAfee D. A., McCaman R. E. Long-term potentiation of synaptic acetylcholine release in the superior cervical ganglion of the rat. J Physiol. 1985 Jun;363:181–190. doi: 10.1113/jphysiol.1985.sp015703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang F. L., Greenough W. T. Transient and enduring morphological correlates of synaptic activity and efficacy change in the rat hippocampal slice. Brain Res. 1984 Aug 20;309(1):35–46. doi: 10.1016/0006-8993(84)91008-4. [DOI] [PubMed] [Google Scholar]
- Collingridge G. L., Kehl S. J., McLennan H. Excitatory amino acids in synaptic transmission in the Schaffer collateral-commissural pathway of the rat hippocampus. J Physiol. 1983 Jan;334:33–46. doi: 10.1113/jphysiol.1983.sp014478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crunelli V., Forda S., Collingridge G. L., Kelly J. S. Intracellular recorded synaptic antagonism in the rat dentate gyrus. Nature. 1982 Dec 2;300(5891):450–452. doi: 10.1038/300450a0. [DOI] [PubMed] [Google Scholar]
- Crunelli V., Forda S., Kelly J. S. The reversal potential of excitatory amino acid action on granule cells of the rat dentate gyrus. J Physiol. 1984 Jun;351:327–342. doi: 10.1113/jphysiol.1984.sp015248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lauro A., Schmid R. W., Meek J. L. Is aspartic acid the neurotransmitter of the perforant pathway? Brain Res. 1981 Mar 2;207(2):476–480. doi: 10.1016/0006-8993(81)90382-6. [DOI] [PubMed] [Google Scholar]
- Dolphin A. C., Errington M. L., Bliss T. V. Long-term potentiation of the perforant path in vivo is associated with increased glutamate release. Nature. 1982 Jun 10;297(5866):496–498. doi: 10.1038/297496a0. [DOI] [PubMed] [Google Scholar]
- Douglas R. M., Goddard G. V., Riives M. Inhibitory modulation of long-term potentiation: evidence for a postsynaptic locus of control. Brain Res. 1982 May 27;240(2):259–272. doi: 10.1016/0006-8993(82)90221-9. [DOI] [PubMed] [Google Scholar]
- Douglas R. M., McNaughton B. L., Goddard G. V. Commissural inhibition and facilitation of granule cell discharge in fascia dentata. J Comp Neurol. 1983 Sep 20;219(3):285–294. doi: 10.1002/cne.902190304. [DOI] [PubMed] [Google Scholar]
- Dunwiddie T. V., Lynch G. The relationship between extracellular calcium concentrations and the induction of hippocampal long-term potentiation. Brain Res. 1979 Jun 15;169(1):103–110. doi: 10.1016/0006-8993(79)90377-9. [DOI] [PubMed] [Google Scholar]
- Errington M. L., Dolphin A. C., Bliss T. V. A method for combining field potential recording with local perfusion in the hippocampus of the anaesthetized rat. J Neurosci Methods. 1983 Apr;7(4):353–357. doi: 10.1016/0165-0270(83)90027-4. [DOI] [PubMed] [Google Scholar]
- Feasey K. J., Lynch M. A., Bliss T. V. Long-term potentiation is associated with an increase in calcium-dependent, potassium-stimulated release of [14C]glutamate from hippocampal slices: an ex vivo study in the rat. Brain Res. 1986 Jan 29;364(1):39–44. doi: 10.1016/0006-8993(86)90985-6. [DOI] [PubMed] [Google Scholar]
- Ferkany J. W., Coyle J. T. Evoked release of aspartate and glutamate: disparities between prelabeling and direct measurement. Brain Res. 1983 Nov 14;278(1-2):279–282. doi: 10.1016/0006-8993(83)90254-8. [DOI] [PubMed] [Google Scholar]
- Ffrench-Mullen J. M., Koller K., Zaczek R., Coyle J. T., Hori N., Carpenter D. O. N-Acetylaspartylglutamate: possible role as the neurotransmitter of the lateral olfactory tract. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3897–3900. doi: 10.1073/pnas.82.11.3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fifková E., Van Harreveld A. Long-lasting morphological changes in dendritic spines of dentate granular cells following stimulation of the entorhinal area. J Neurocytol. 1977 Apr;6(2):211–230. doi: 10.1007/BF01261506. [DOI] [PubMed] [Google Scholar]
- Fonnum F. Glutamate: a neurotransmitter in mammalian brain. J Neurochem. 1984 Jan;42(1):1–11. doi: 10.1111/j.1471-4159.1984.tb09689.x. [DOI] [PubMed] [Google Scholar]
- Haas H. L., Rose G. The role of inhibitory mechanisms in hippocampal long-term potentiation. Neurosci Lett. 1984 Jun 29;47(3):301–306. doi: 10.1016/0304-3940(84)90530-5. [DOI] [PubMed] [Google Scholar]
- Haycock J. W., Levy W. B., Denner L. A., Cotman C. W. Effects of elevated [K+]O on the release of neurotransmitters from cortical synaptosomes: efflux or secretion? J Neurochem. 1978 May;30(5):1113–1125. doi: 10.1111/j.1471-4159.1978.tb12406.x. [DOI] [PubMed] [Google Scholar]
- Hirst G. D., Redman S. J., Wong K. Post-tetanic potentiation and facilitation of synaptic potentials evoked in cat spinal motoneurones. J Physiol. 1981 Dec;321:97–109. doi: 10.1113/jphysiol.1981.sp013973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack J. J., Redman S. J., Wong K. The components of synaptic potentials evoked in cat spinal motoneurones by impulses in single group Ia afferents. J Physiol. 1981 Dec;321:65–96. doi: 10.1113/jphysiol.1981.sp013972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferys J. G. Initiation and spread of action potentials in granule cells maintained in vitro in slices of guinea-pig hippocampus. J Physiol. 1979 Apr;289:375–388. doi: 10.1113/jphysiol.1979.sp012742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph M. H., Davies P. Electrochemical activity of o-phthalaldehyde-mercaptoethanol derivatives of amino acids. Application to high-performance liquid chromatographic determination of amino acids in plasma and other biological materials. J Chromatogr. 1983 Oct 14;277:125–136. [PubMed] [Google Scholar]
- Kawagoe R., Onodera K., Takeuchi A. The uptake and release of glutamate at the crayfish neuromuscular junction. J Physiol. 1984 Sep;354:69–78. doi: 10.1113/jphysiol.1984.sp015362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyano K., Kuba K., Minota S. Long-term potentiation of transmitter release induced by repetitive presynaptic activities in bull-frog sympathetic ganglia. J Physiol. 1985 Feb;359:219–233. doi: 10.1113/jphysiol.1985.sp015582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. S., Schottler F., Oliver M., Lynch G. Brief bursts of high-frequency stimulation produce two types of structural change in rat hippocampus. J Neurophysiol. 1980 Aug;44(2):247–258. doi: 10.1152/jn.1980.44.2.247. [DOI] [PubMed] [Google Scholar]
- Lynch G., Halpain S., Baudry M. Effects of high-frequency synaptic stimulation on glumate receptor binding studied with a modified in vitro hippocampal slice preparation. Brain Res. 1982 Jul 22;244(1):101–111. doi: 10.1016/0006-8993(82)90908-8. [DOI] [PubMed] [Google Scholar]
- Lynch G., Larson J., Kelso S., Barrionuevo G., Schottler F. Intracellular injections of EGTA block induction of hippocampal long-term potentiation. Nature. 1983 Oct 20;305(5936):719–721. doi: 10.1038/305719a0. [DOI] [PubMed] [Google Scholar]
- McNaughton B. L., Douglas R. M., Goddard G. V. Synaptic enhancement in fascia dentata: cooperativity among coactive afferents. Brain Res. 1978 Nov 24;157(2):277–293. doi: 10.1016/0006-8993(78)90030-6. [DOI] [PubMed] [Google Scholar]
- Morris R. G., Anderson E., Lynch G. S., Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. 1986 Feb 27-Mar 5Nature. 319(6056):774–776. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- Nadler J. V., Vaca K. W., White W. F., Lynch G. S., Cotman C. W. Aspartate and glutamate as possible transmitters of excitatory hippocampal afferents. Nature. 1976 Apr 8;260(5551):538–540. doi: 10.1038/260538a0. [DOI] [PubMed] [Google Scholar]
- Nowak L., Bregestovski P., Ascher P., Herbet A., Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984 Feb 2;307(5950):462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Sellström A., Hamberger A. Potassium-stimulated gamma-aminobutyric acid release from neurons and glia. Brain Res. 1977 Jan 1;119(1):189–198. doi: 10.1016/0006-8993(77)90099-3. [DOI] [PubMed] [Google Scholar]
- Skrede K. K., Malthe-Sørenssen D. Increased resting and evoked release of transmitter following repetitive electrical tetanization in hippocampus: a biochemical correlate to long-lasting synaptic potentiation. Brain Res. 1981 Mar 16;208(2):436–441. doi: 10.1016/0006-8993(81)90573-4. [DOI] [PubMed] [Google Scholar]
- Turner R. W., Baimbridge K. G., Miller J. J. Calcium-induced long-term potentiation in the hippocampus. Neuroscience. 1982 Jun;7(6):1411–1416. doi: 10.1016/0306-4522(82)90254-8. [DOI] [PubMed] [Google Scholar]
- Vizi E. S., Vyskocil F. Changes in total and quantal release of acetylcholine in the mouse diaphragm during activation and inhibition of membrane ATPase. J Physiol. 1979 Jan;286:1–14. doi: 10.1113/jphysiol.1979.sp012603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins J. C., Evans R. H. Excitatory amino acid transmitters. Annu Rev Pharmacol Toxicol. 1981;21:165–204. doi: 10.1146/annurev.pa.21.040181.001121. [DOI] [PubMed] [Google Scholar]
- White W. F., Nadler J. V., Hamberger A., Cotman C. W., Cummins J. T. Glutamate as transmitter of hippocampal perforant path. Nature. 1977 Nov 24;270(5635):356–357. doi: 10.1038/270356a0. [DOI] [PubMed] [Google Scholar]
- Wigström H., Swann J. W., Andersen P. Calcium dependency of synaptic long-lasting potentiation in the hippocampal slice. Acta Physiol Scand. 1979 Jan;105(1):126–128. doi: 10.1111/j.1748-1716.1979.tb06323.x. [DOI] [PubMed] [Google Scholar]


