Abstract
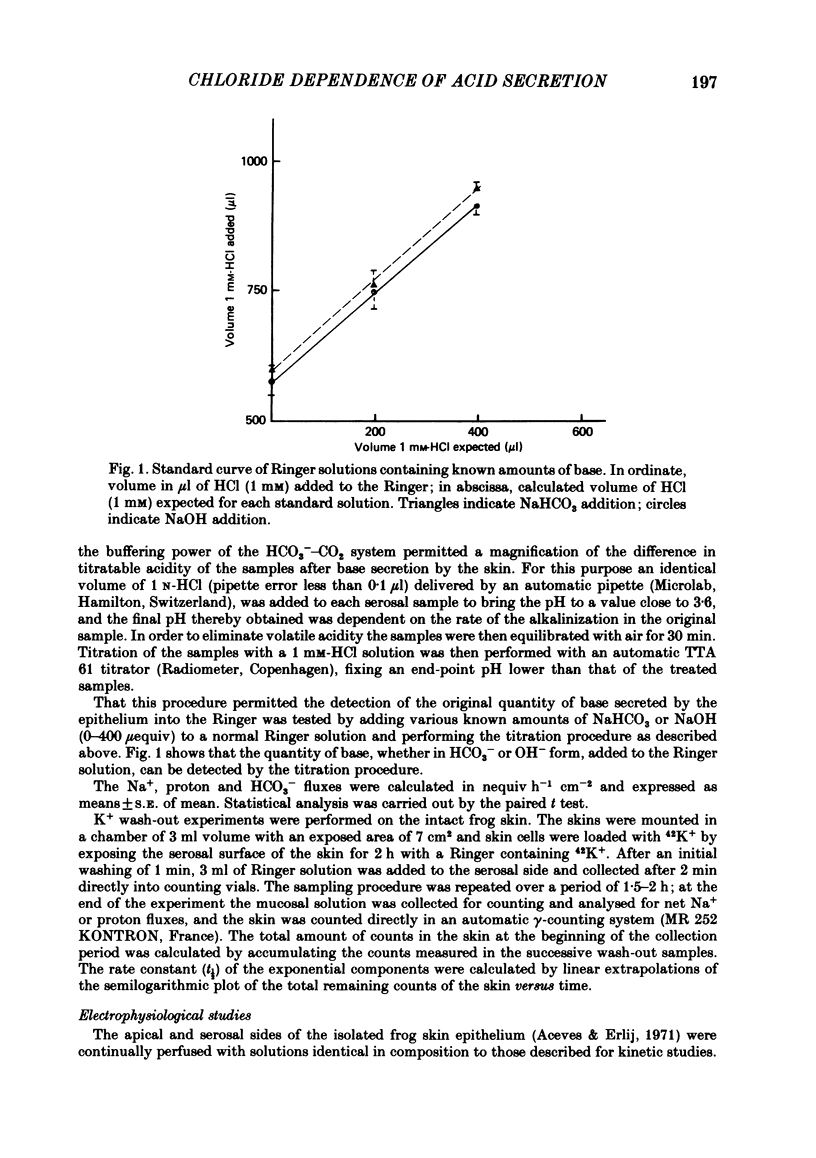
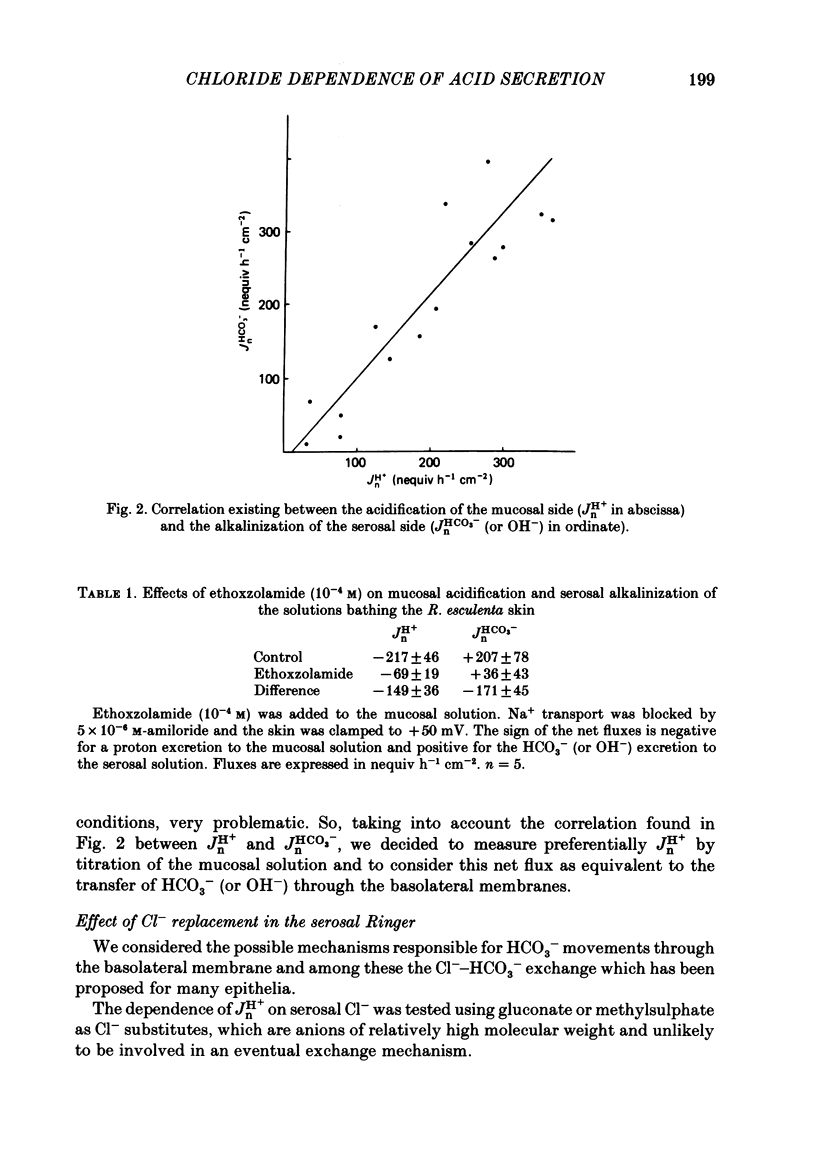
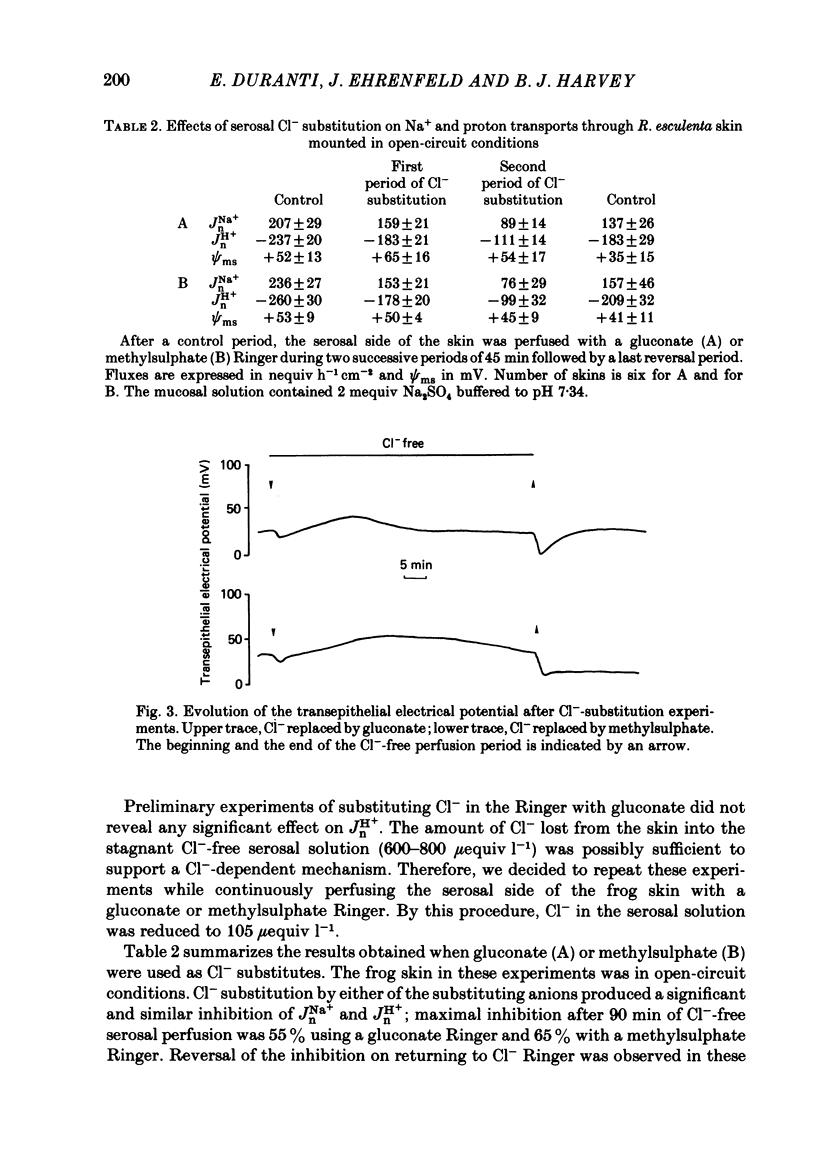
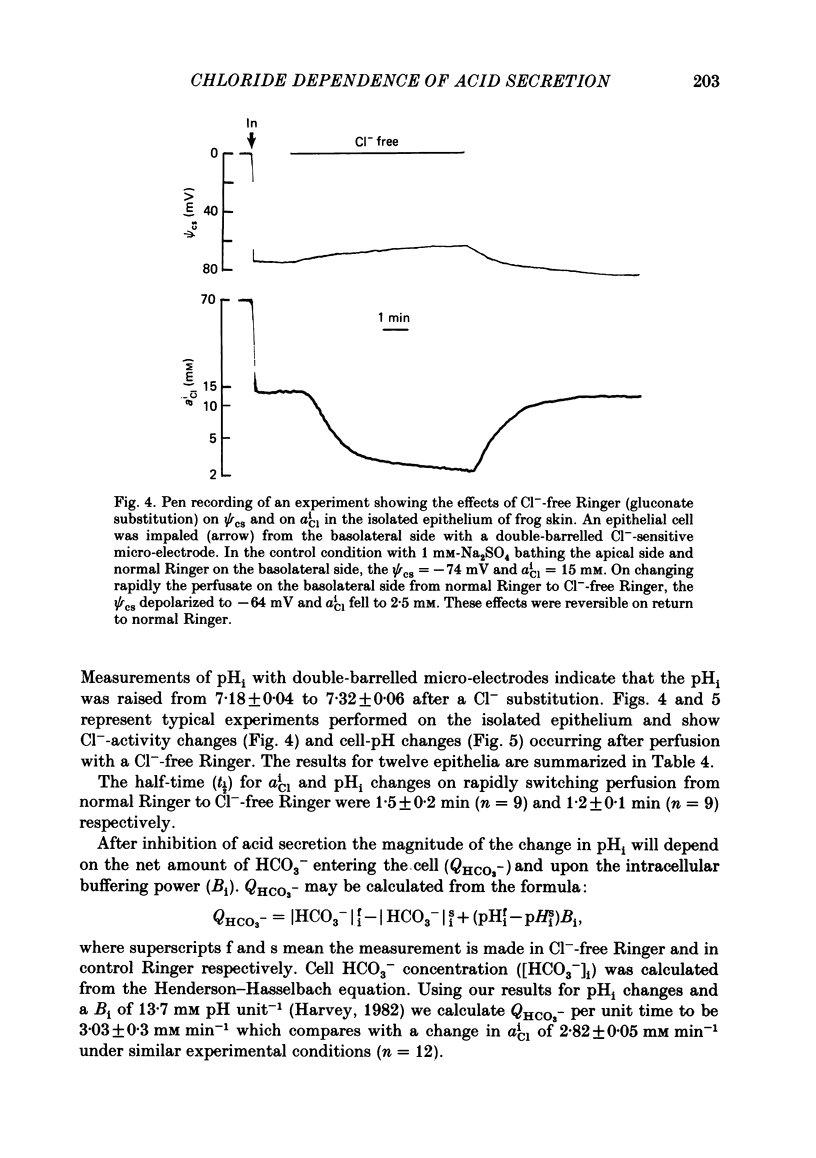
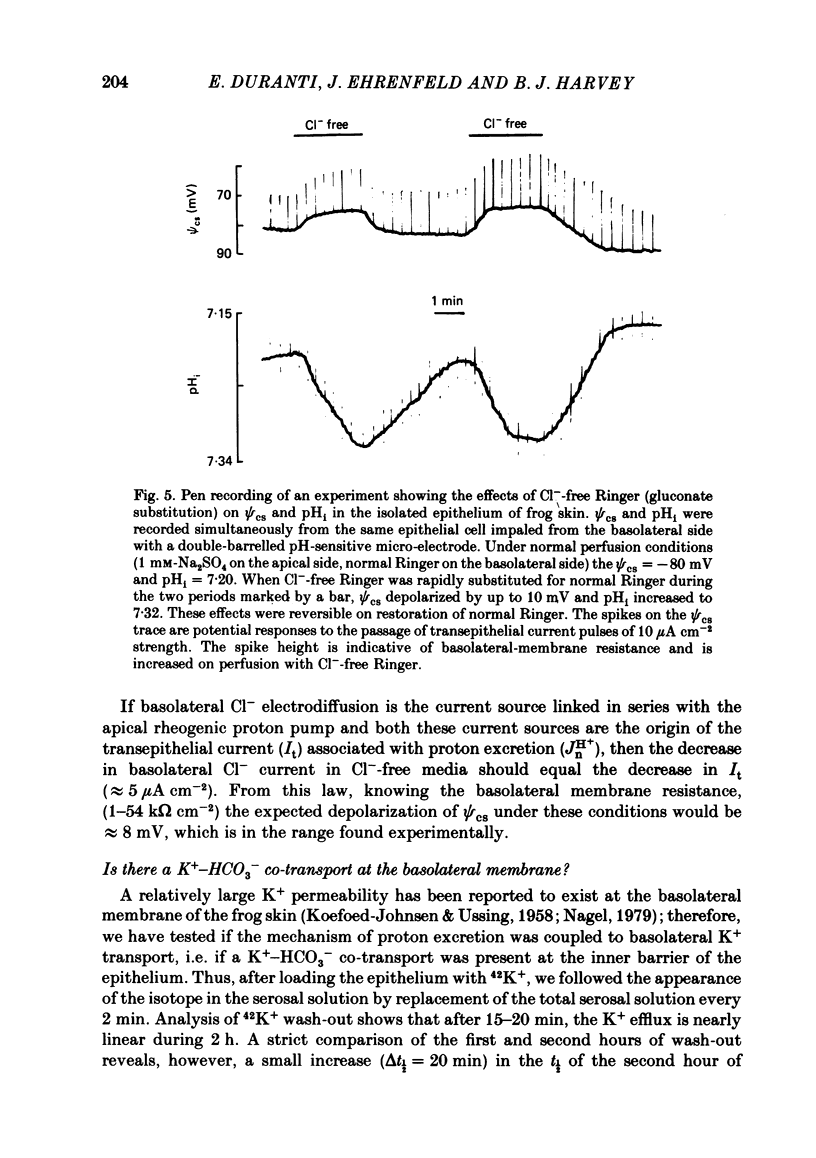
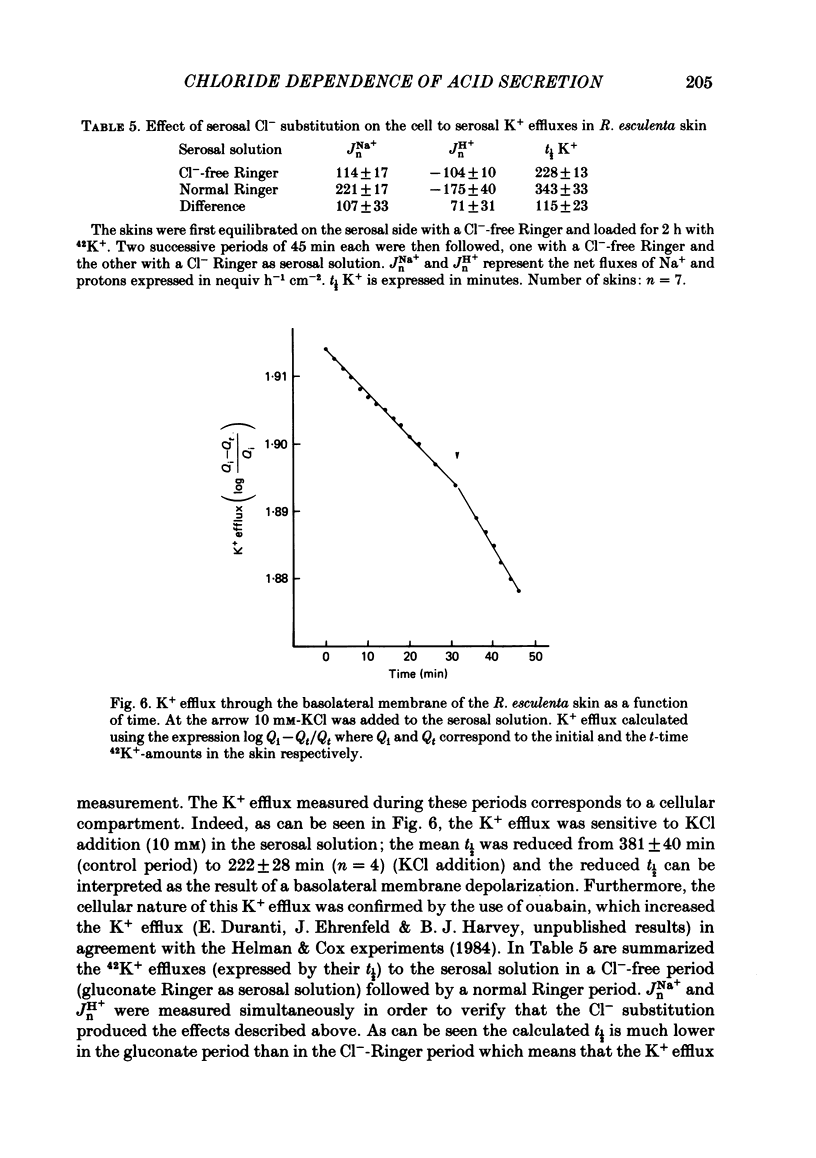
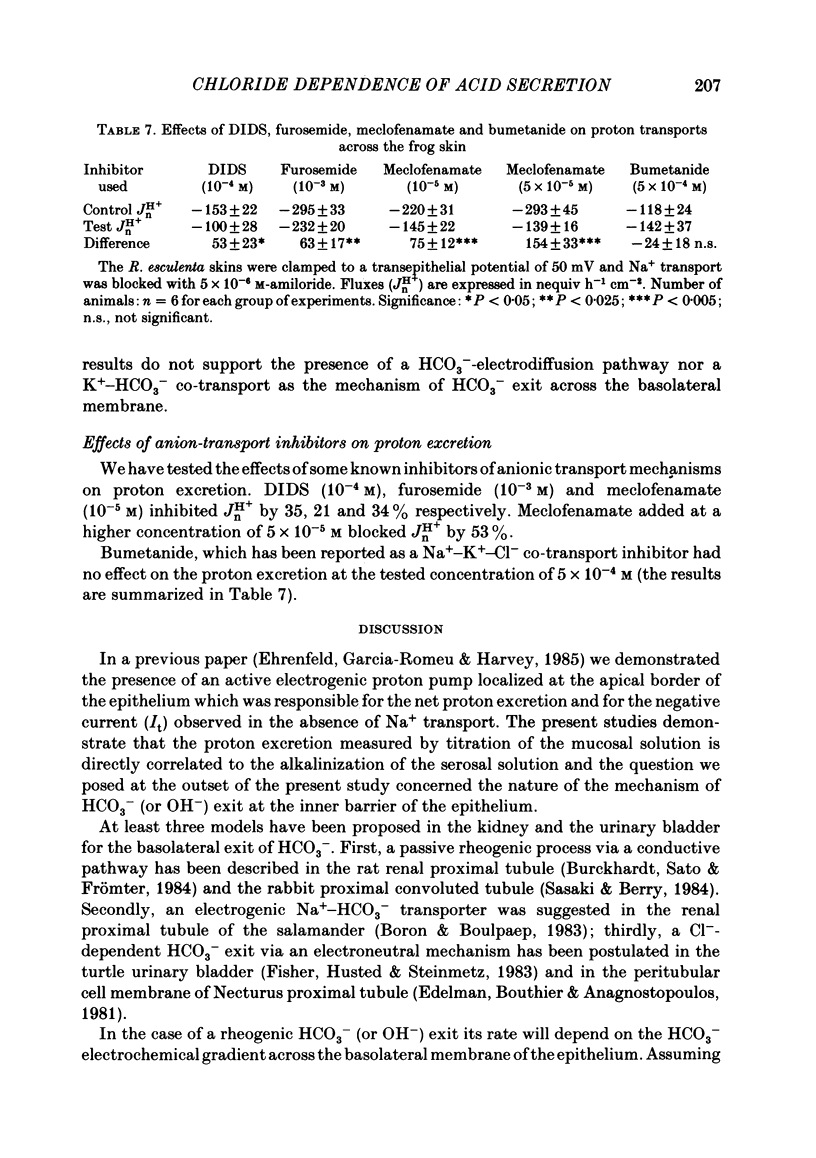
Kinetic and electrophysiological studies were carried out to characterize the efflux of HCO3- (or OH-) across the basolateral membrane of the proton-secreting cells of the frog skin epithelium bathed with dilute saline mucosal solutions. In control conditions, the acidification of the mucosal solution (JnH+) was correlated directly with serosal alkalinization. Cl- substitution in the serosal Ringer (by gluconate or methylsulphate ions) induced an inhibition of proton excretion (70% inhibition). Measurements of the basolateral membrane potential with conventional micro-electrodes and of cell Cl- activity (aCli) and proton activity with double-barrelled ion-sensitive micro-electrodes recorded a basolateral membrane depolarization of 5.1 +/- 0.7 mV (n = 12), a decrease in aCli from 14.5 +/- 1.6 mequiv l-1 to 1.8 +/- 0.3 mequiv l-1 (n = 12), and a cell pH increase from 7.18 +/- 0.04 to 7.32 +/- 0.06 (n = 12) after serosal Cl- replacement. 4,4'-diisothiocyanostilbene-2-2'-disulphonic acid (DIDS) (10(-4) M) and meclofenamate (5 X 10(-5) M) inhibit JHn+ by 34% and 53% respectively whereas bumetanide did not block JHn+. Depolarization of the basolateral membrane (2 mM-Ba2+ addition to the serosal solution) did not block proton excretion. We show that cell Cl- activity is maintained at a higher level than that predicted by the equilibrium potential, by a mechanism located at the basolateral membrane of the epithelium since the apical solution was Cl(-)-free. This mechanism is not sensitive to potential changes at the basolateral membrane in the range tested. An electroneutral Cl(-)-HCO3- exchange mechanism is the simplest hypothesis which can account for our results.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aceves J., Erlij D. Sodium transport across the isolated epithelium of the frog skin. J Physiol. 1971 Jan;212(1):195–210. doi: 10.1113/jphysiol.1971.sp009317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aickin C. C., Thomas R. C. An investigation of the ionic mechanism of intracellular pH regulation in mouse soleus muscle fibres. J Physiol. 1977 Dec;273(1):295–316. doi: 10.1113/jphysiol.1977.sp012095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello-Reuss E. Electrical properties of the basolateral membrane of the straight portion of the rabbit proximal renal tubule. J Physiol. 1982 May;326:49–63. doi: 10.1113/jphysiol.1982.sp014176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biagi B., Kubota T., Sohtell M., Giebisch G. Intracellular potentials in rabbit proximal tubules perfused in vitro. Am J Physiol. 1981 Mar;240(3):F200–F210. doi: 10.1152/ajprenal.1981.240.3.F200. [DOI] [PubMed] [Google Scholar]
- Boron W. F., Boulpaep E. L. Intracellular pH regulation in the renal proximal tubule of the salamander. Basolateral HCO3- transport. J Gen Physiol. 1983 Jan;81(1):53–94. doi: 10.1085/jgp.81.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boron W. F. Intracellular pH transients in giant barnacle muscle fibers. Am J Physiol. 1977 Sep;233(3):C61–C73. doi: 10.1152/ajpcell.1977.233.3.C61. [DOI] [PubMed] [Google Scholar]
- Burckhardt B. C., Sato K., Frömter E. Electrophysiological analysis of bicarbonate permeation across the peritubular cell membrane of rat kidney proximal tubule. I. Basic observations. Pflugers Arch. 1984 May;401(1):34–42. doi: 10.1007/BF00581530. [DOI] [PubMed] [Google Scholar]
- Cabantchik Z. I., Rothstein A. The nature of the membrane sites controlling anion permeability of human red blood cells as determined by studies with disulfonic stilbene derivatives. J Membr Biol. 1972 Dec 29;10(3):311–330. doi: 10.1007/BF01867863. [DOI] [PubMed] [Google Scholar]
- Cassola A. C., Mollenhauer M., Frömter E. The intracellular chloride activity of rat kidney proximal tubular cells. Pflugers Arch. 1983 Dec;399(4):259–265. doi: 10.1007/BF00652749. [DOI] [PubMed] [Google Scholar]
- Chan Y. L., Biagi B., Giebisch G. Control mechanisms of bicarbonate transport across the rat proximal convoluted tubule. Am J Physiol. 1982 May;242(5):F532–F543. doi: 10.1152/ajprenal.1982.242.5.F532. [DOI] [PubMed] [Google Scholar]
- Cohen L. H., Mueller A., Steinmetz P. R. Inhibition of the bicarbonate exit step in urinary acidification by a disulfonic stilbene. J Clin Invest. 1978 Apr;61(4):981–986. doi: 10.1172/JCI109023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousin J. L., Motais R. Inhibition of anion permeability by amphiphilic compounds in human red cell: evidence for an interaction of niflumic acid with the band 3 protein. J Membr Biol. 1979 Apr 20;46(2):125–153. doi: 10.1007/BF01961377. [DOI] [PubMed] [Google Scholar]
- Ehrenfeld J., Garcia-Romeu F. Active hydrogen excretion and sodium absorption through isolated frog skin. Am J Physiol. 1977 Jul;233(1):F46–F54. doi: 10.1152/ajprenal.1977.233.1.F46. [DOI] [PubMed] [Google Scholar]
- Ehrenfeld J., Garcia-Romeu F. Coupling between chloride absorption and base excretion in isolated skin of Rana esculenta. Am J Physiol. 1978 Jul;235(1):F33–F39. doi: 10.1152/ajprenal.1978.235.1.F33. [DOI] [PubMed] [Google Scholar]
- Ehrenfeld J., Garcia-Romeu F., Harvey B. J. Electrogenic active proton pump in Rana esculenta skin and its role in sodium ion transport. J Physiol. 1985 Feb;359:331–355. doi: 10.1113/jphysiol.1985.sp015588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira K. T., Ferreira H. G. The regulation of volume and ion composition in frog skin. Biochim Biophys Acta. 1981 Aug 20;646(2):193–202. doi: 10.1016/0005-2736(81)90325-4. [DOI] [PubMed] [Google Scholar]
- Fischer J. L., Husted R. F., Steinmetz P. R. Chloride dependence of the HCO3 exit step in urinary acidification by the turtle bladder. Am J Physiol. 1983 Nov;245(5 Pt 1):F564–F568. doi: 10.1152/ajprenal.1983.245.5.F564. [DOI] [PubMed] [Google Scholar]
- Harvey B. J., Kernan R. P. Intracellular ion activities in frog skin in relation to external sodium and effects of amiloride and/or ouabain. J Physiol. 1984 Apr;349:501–517. doi: 10.1113/jphysiol.1984.sp015170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey B. J., Kernan R. P. Sodium-selective micro-electrode study of apical permeability in frog skin: effects of sodium, amiloride and ouabain. J Physiol. 1984 Nov;356:359–374. doi: 10.1113/jphysiol.1984.sp015470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOEFOED-JOHNSEN V., USSING H. H. The nature of the frog skin potential. Acta Physiol Scand. 1958 Jun 2;42(3-4):298–308. doi: 10.1111/j.1748-1716.1958.tb01563.x. [DOI] [PubMed] [Google Scholar]
- Kleinman J. G., Ware R. A., Schwartz J. H. Anion transport regulates intracellular pH in renal cortical tissue. Biochim Biophys Acta. 1981 Oct 20;648(1):87–92. doi: 10.1016/0005-2736(81)90127-9. [DOI] [PubMed] [Google Scholar]
- Kristensen P. Chloride transport across isolated frog skin. Acta Physiol Scand. 1972 Mar;84(3):338–346. doi: 10.1111/j.1748-1716.1972.tb05185.x. [DOI] [PubMed] [Google Scholar]
- Nagel W. Inhibition of potassium conductance by barium in frog skin epithelium. Biochim Biophys Acta. 1979 Apr 4;552(2):346–357. doi: 10.1016/0005-2736(79)90289-x. [DOI] [PubMed] [Google Scholar]
- Russell J. M., Boron W. F. Role of choloride transport in regulation of intracellular pH. Nature. 1976 Nov 4;264(5581):73–74. doi: 10.1038/264073a0. [DOI] [PubMed] [Google Scholar]
- Sasaki S., Berry C. A. Mechanism of bicarbonate exit across basolateral membrane of the rabbit proximal convoluted tubule. Am J Physiol. 1984 Jun;246(6 Pt 2):F889–F896. doi: 10.1152/ajprenal.1984.246.6.F889. [DOI] [PubMed] [Google Scholar]
- Thomas R. C. Ionic mechanism of the H+ pump in a snail neurone. Nature. 1976 Jul 1;262(5563):54–55. doi: 10.1038/262054a0. [DOI] [PubMed] [Google Scholar]
- Ullrich K. J., Capasso G., Rumrich G., Papavassiliou F., Klöss S. Coupling between proximal tubular transport processes. Studies with ouabain, SITS and HCO3-free solutions. Pflugers Arch. 1977 Apr 25;368(3):245–252. doi: 10.1007/BF00585203. [DOI] [PubMed] [Google Scholar]


