Abstract
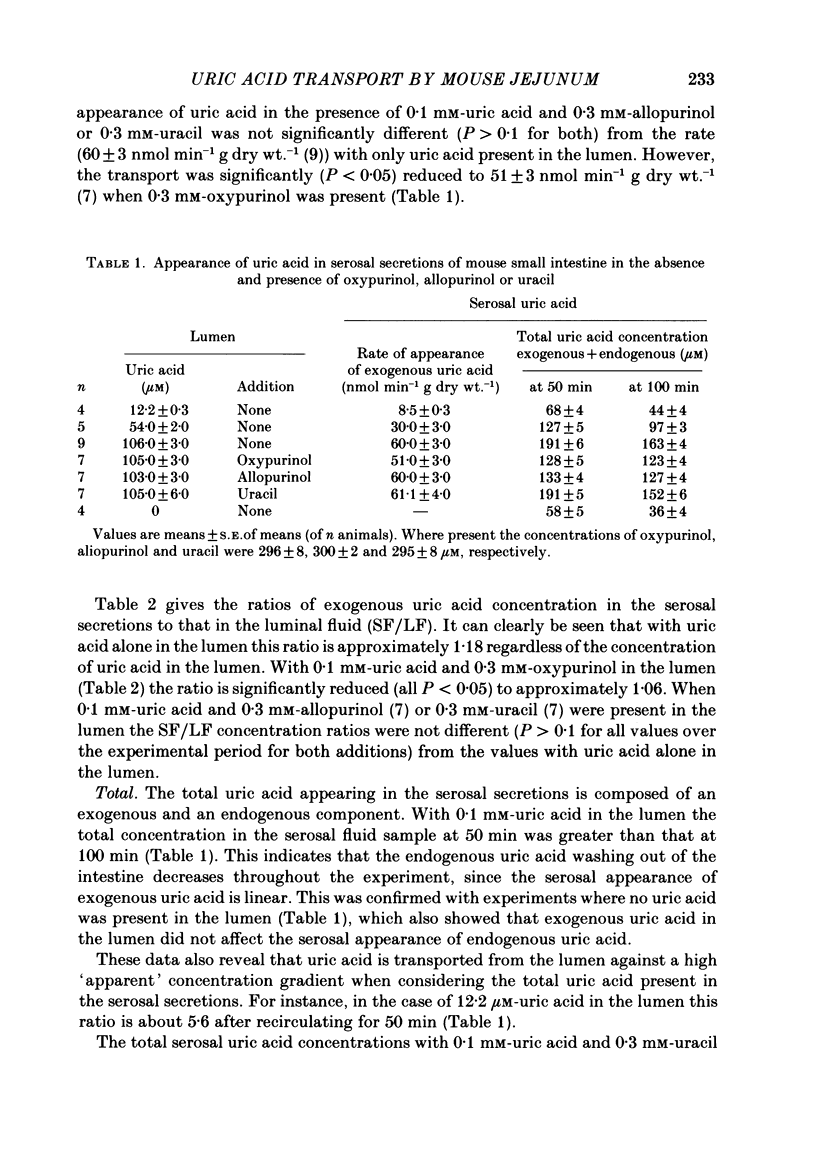
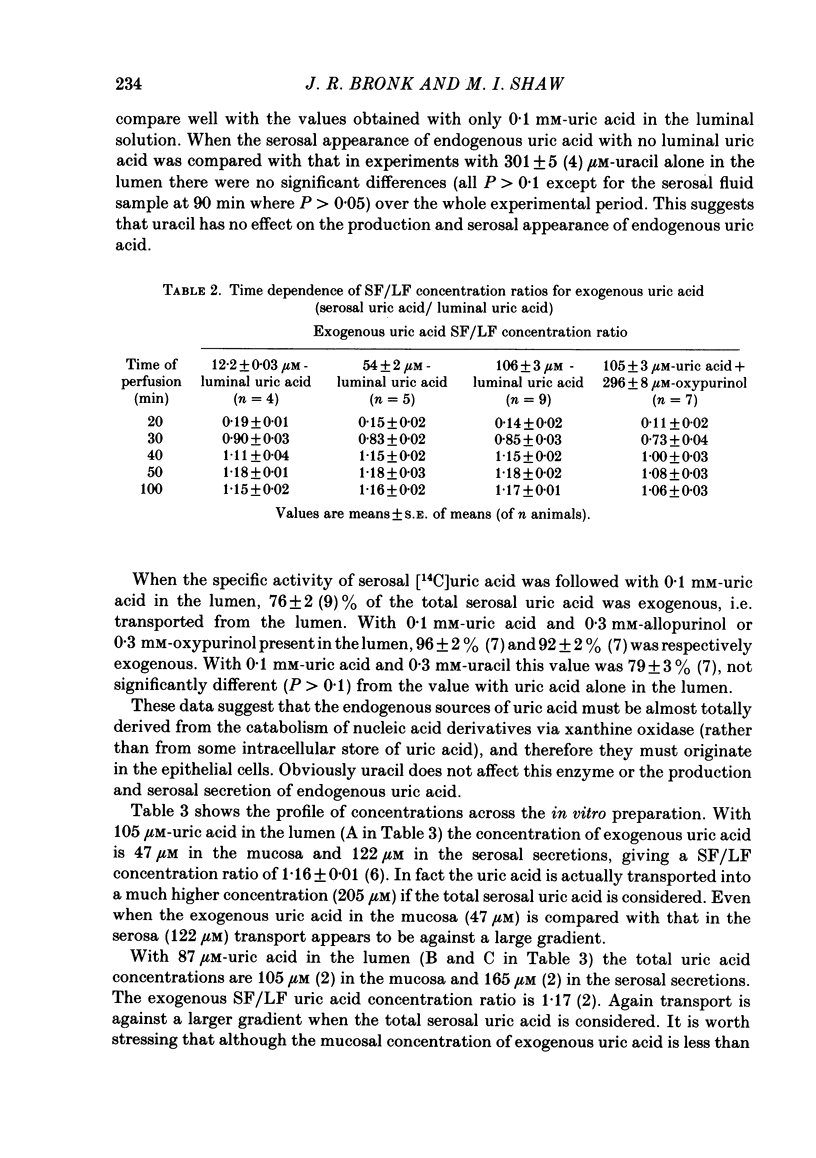
The in vitro recirculation technique was used to study the uptake and transport of uric acid by the jejunum of mouse small intestine. Three components of the serosal secretions appeared to be endogenously derived nucleic acid derivatives; two of these were identified as uric acid and uracil. There was no detectable metabolism of uric acid by the intestine. Uric acid transported from the lumen appeared in the serosal fluid at a concentration higher than that in the lumen. The final serosal/luminal concentration ratio of about 1.18 for exogenous uric acid was found to be constant over the concentration range studied (0.01-0.1 mM). The presence of exogenous uric acid in the lumen did not affect the production of endogenous uric acid by the intestine and its release into the serosal secretions. Mucosal concentration of exogenous uric acid was below, but the total mucosal concentration (exogenous+endogenous) was above, that in the lumen. There was no evidence for the secretion of endogenous uric acid into the lumen. Oxypurinol significantly decreased the rate of serosal appearance of exogenous uric acid. Allopurinol did not affect the transport of exogenous uric acid from the lumen and there was negligible metabolism of allopurinol to oxypurinol by the tissue. Uracil did not affect the transport of exogenous uric acid from the lumen, or the serosal appearance of endogenous uric acid. Likewise uracil transport was unaffected by luminal uric acid.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berlin R. D., Hawkins R. A. Secretion of purines by the small intestine: general characteristics. Am J Physiol. 1968 Oct;215(4):932–941. doi: 10.1152/ajplegacy.1968.215.4.932. [DOI] [PubMed] [Google Scholar]
- CLARKSON T. W., CROSS A. C., TOOLE S. R. Electrical potentials across isolated small intestine of the rat. Am J Physiol. 1961 Jun;200:1233–1235. doi: 10.1152/ajplegacy.1961.200.6.1233. [DOI] [PubMed] [Google Scholar]
- Fisher R. B., Gardner M. L. A kinetic approach to the study of absorption of solutes by isolated perfused small intestine. J Physiol. 1974 Aug;241(1):211–234. doi: 10.1113/jphysiol.1974.sp010650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M. J. Epithelial transport of weak electrolytes. Properties of a model of the three compartment system. J Theor Biol. 1977 Feb 21;64(4):771–778. doi: 10.1016/0022-5193(77)90276-4. [DOI] [PubMed] [Google Scholar]
- Jackson M. J., Shiau Y. F., Bane S., Fox M. Intestinal transport of weak electrolytes. Evidence in favor of a three-compartment system. J Gen Physiol. 1974 Feb;63(2):187–213. doi: 10.1085/jgp.63.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M. J., Tai C. Y., Steane J. E. Weak electrolyte permeation in alimentary epithelia. Am J Physiol. 1981 Mar;240(3):G191–G198. doi: 10.1152/ajpgi.1981.240.3.G191. [DOI] [PubMed] [Google Scholar]
- Khan A. H., Wilson S., Crawhall J. C. The influx of uric acid and other purines into everted jejunal sacs of the rat and hamster. Can J Physiol Pharmacol. 1975 Feb;53(1):113–119. doi: 10.1139/y75-015. [DOI] [PubMed] [Google Scholar]
- Kolassa N., Schützenberger W. G., Wiener H., Turnheim K. Active secretion of hypoxanthine and xanthine by guinea pig jejunum in vitro. Am J Physiol. 1980 Feb;238(2):G141–G149. doi: 10.1152/ajpgi.1980.238.2.G141. [DOI] [PubMed] [Google Scholar]
- Leese H. J., Bronk J. R. Automated fluorometric analysis of micromolar quantities of ATP, glucose, and lactic acid. Anal Biochem. 1972 Jan;45(1):211–221. doi: 10.1016/0003-2697(72)90021-8. [DOI] [PubMed] [Google Scholar]
- Lucas M. Determination of acid surface pH in vivo in rat proximal jejunum. Gut. 1983 Aug;24(8):734–739. doi: 10.1136/gut.24.8.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton A. P., Hanson P. J. Monosaccharide transport by the small intestine of lean and genetically obese (ob/ob) mice. Q J Exp Physiol. 1984 Jan;69(1):117–126. doi: 10.1113/expphysiol.1984.sp002772. [DOI] [PubMed] [Google Scholar]
- Morton A. P., Hanson P. J. Transport of leucine by the small intestine of lean and genetically obese (ob/ob) mice. Q J Exp Physiol. 1983 Jan;68(1):29–38. doi: 10.1113/expphysiol.1983.sp002700. [DOI] [PubMed] [Google Scholar]
- Parsons D. S., Shaw M. I. Use of high performance liquid chromatography to study absorption and metabolism of purines by rat jejunum in vitro. Q J Exp Physiol. 1983 Jan;68(1):53–67. doi: 10.1113/expphysiol.1983.sp002702. [DOI] [PubMed] [Google Scholar]
- Parsons D. S., Volman-Mitchell H. The transamination of glutamate and aspartate during absorption in vitro by small intestine of chicken, guinea-pig and rat. J Physiol. 1974 Jun;239(3):677–694. doi: 10.1113/jphysiol.1974.sp010589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rundles R. W., Wyngaarden J. B. Drugs and uric acid. Annu Rev Pharmacol. 1969;9:345–362. doi: 10.1146/annurev.pa.09.040169.002021. [DOI] [PubMed] [Google Scholar]
- SCHANKER L. S., JEFFREY J. J., TOCCO D. J. INTERACTION OF PURINES WITH THE PYRIMIDINE TRANSPORT PROCESS OF THE SMALL INTESTINE. Biochem Pharmacol. 1963 Sep;12:1047–1053. doi: 10.1016/0006-2952(63)90028-5. [DOI] [PubMed] [Google Scholar]
- SCHANKER L. S., TOCCO D. J., BRODIE B. B., HOGBEN C. A. Absorption of drugs from the rat small intestine. J Pharmacol Exp Ther. 1958 May;123(1):81–88. [PubMed] [Google Scholar]
- Shaw M. I., Parsons D. S. Absorption and metabolism of allopurinol and oxypurinol by rat jejunum in vitro: effects on uric acid transport. Clin Sci (Lond) 1984 Mar;66(3):257–267. doi: 10.1042/cs0660257. [DOI] [PubMed] [Google Scholar]
- Shaw M. I., Parsons D. S. Uptake of uric acid, xanthine and hypoxanthine by brush-border membrane vesicles from mouse small intestine. Biochim Biophys Acta. 1984 Dec 19;778(3):530–538. doi: 10.1016/0005-2736(84)90404-8. [DOI] [PubMed] [Google Scholar]
- Simmonds H. A., Rising T. J., Cadenhead A., Hatfield P. J., Jones A. S., Cameron J. S. Radioisotope studies of purine metabolism during administration of guanine and allopurinol in the pig. Biochem Pharmacol. 1973 Oct 15;22(20):2553–2563. [PubMed] [Google Scholar]
- Tai C. Y., Jackson M. J. Weak-acid transport in the small intestine: discrimination in the lamina propria. J Membr Biol. 1981 Mar 15;59(1):35–43. doi: 10.1007/BF01870819. [DOI] [PubMed] [Google Scholar]
- WILSON T. H., KAZYAK L. Acid-base changes across the wall of hamster and rat intestine. Biochim Biophys Acta. 1957 Apr;24(1):124–132. doi: 10.1016/0006-3002(57)90154-3. [DOI] [PubMed] [Google Scholar]
- Wyngaarden J. B. Gout. Adv Metab Disord. 1965;2:1–78. doi: 10.1016/b978-1-4831-6750-3.50006-8. [DOI] [PubMed] [Google Scholar]


