Abstract
The uptake of Pb into human red blood cells has been studied using Pb buffers. Passive Pb movements can be studied conveniently when the cells are depleted of adenosine 5'-triphosphate (ATP), to eliminate active transport, and of inorganic phosphate, to prevent precipitation of lead phosphate. Pb can cross the membrane passively in either direction. Influx and efflux show similar properties. Passive Pb transport is strongly stimulated by HCO3-, and is reduced by replacing Cl- with ClO4-. It is inhibited by low concentrations of 4-acetamido-4'-isothiocyanostilbene-2,2'-disulphonic acid (SITS) and 4,4'-diisothiocyanostilbene-2.2'-disulphonic acid (DIDS), characteristic inhibitors of anion transport. Pb uptake is unaffected by varying the external concentrations of Na+, K+ and Ca2+. When Pb enters the cell, it binds mainly to haemoglobin. The ratio of bound Pb:free Pb2+ in the cytosol is estimated to be 6000:1. Pb binding to haemoglobin is unaffected by oxygenation. Binding to albumin is quantitatively similar to binding to haemoglobin. The implications of these results for the transport and binding of Pb in the blood are discussed.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barltrop D., Smith A. Interaction of lead with erythrocytes. Experientia. 1971 Jan 15;27(1):92–93. doi: 10.1007/BF02137760. [DOI] [PubMed] [Google Scholar]
- CLARKSON T. W., KENCH J. E. Uptake of lead by human erythrocytes in vitro. Biochem J. 1958 Jul;69(3):432–439. doi: 10.1042/bj0690432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn I. M., Lew V. L., Lüthi U. Reversal of the potassium entry mechanism in red cells, with and without reversal of the entire pump cycle. J Physiol. 1970 Apr;207(2):371–391. doi: 10.1113/jphysiol.1970.sp009067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew V. L. On the ATP dependence of the Ca 2+ -induced increase in K + permeability observed in human red cells. Biochim Biophys Acta. 1971 Jun 1;233(3):827–830. doi: 10.1016/0005-2736(71)90185-4. [DOI] [PubMed] [Google Scholar]
- Passow H. Selective enhancement of potassium efflux from red blood cells by lead. A comparison with the effects of calcium. Prog Clin Biol Res. 1981;51:79–104. [PubMed] [Google Scholar]
- Simons T. J. A method for estimating free Ca within human red blood cells, with an application to the study of their Ca-dependent K permeability. J Membr Biol. 1982;66(3):235–247. doi: 10.1007/BF01868498. [DOI] [PubMed] [Google Scholar]
- Simons T. J. Active transport of lead by human red blood cells. FEBS Lett. 1984 Jul 9;172(2):250–254. doi: 10.1016/0014-5793(84)81135-7. [DOI] [PubMed] [Google Scholar]
- Simons T. J. Influence of lead ions on cation permeability in human red cell ghosts. J Membr Biol. 1985;84(1):61–71. doi: 10.1007/BF01871648. [DOI] [PubMed] [Google Scholar]
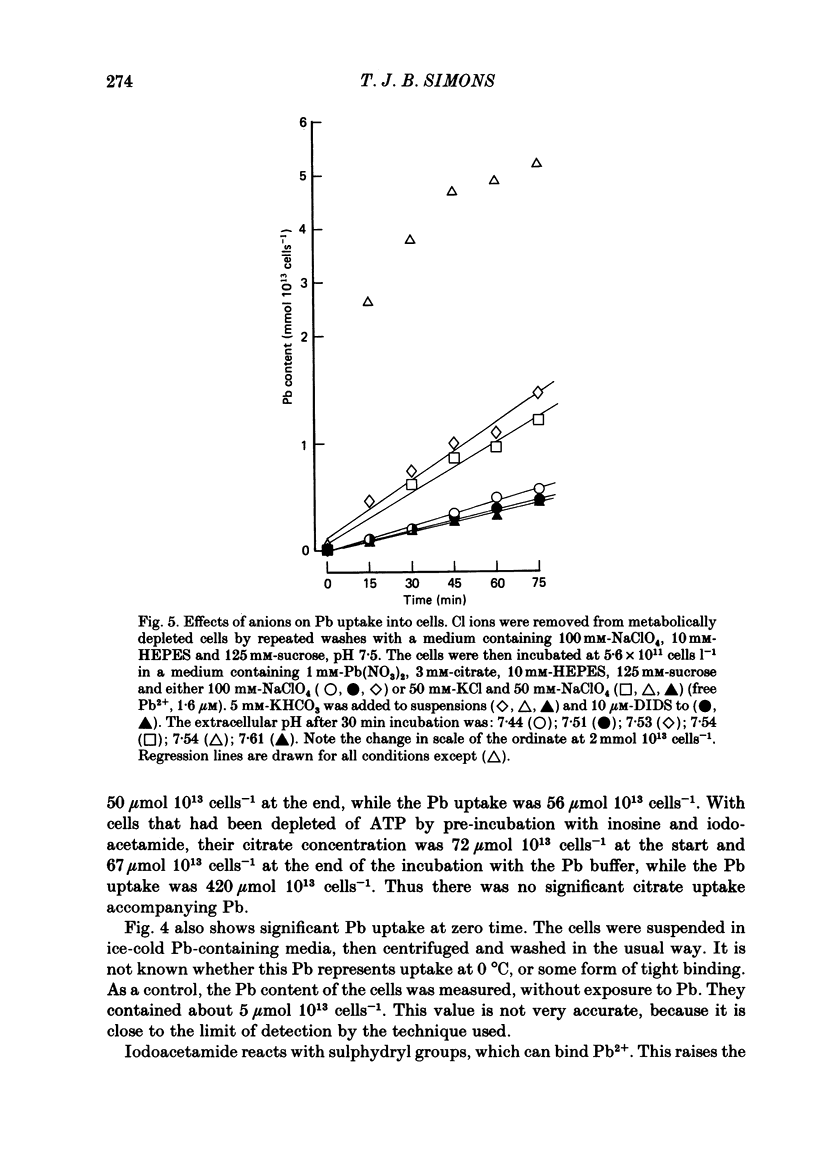
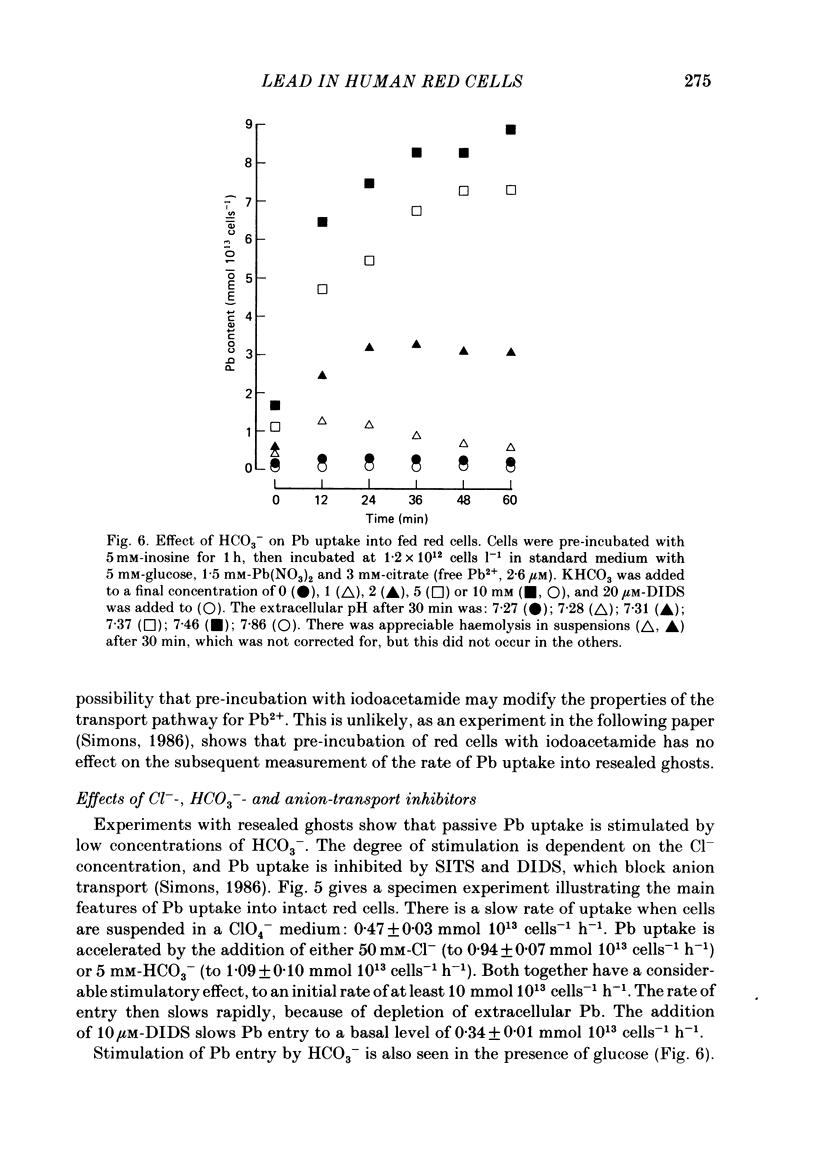
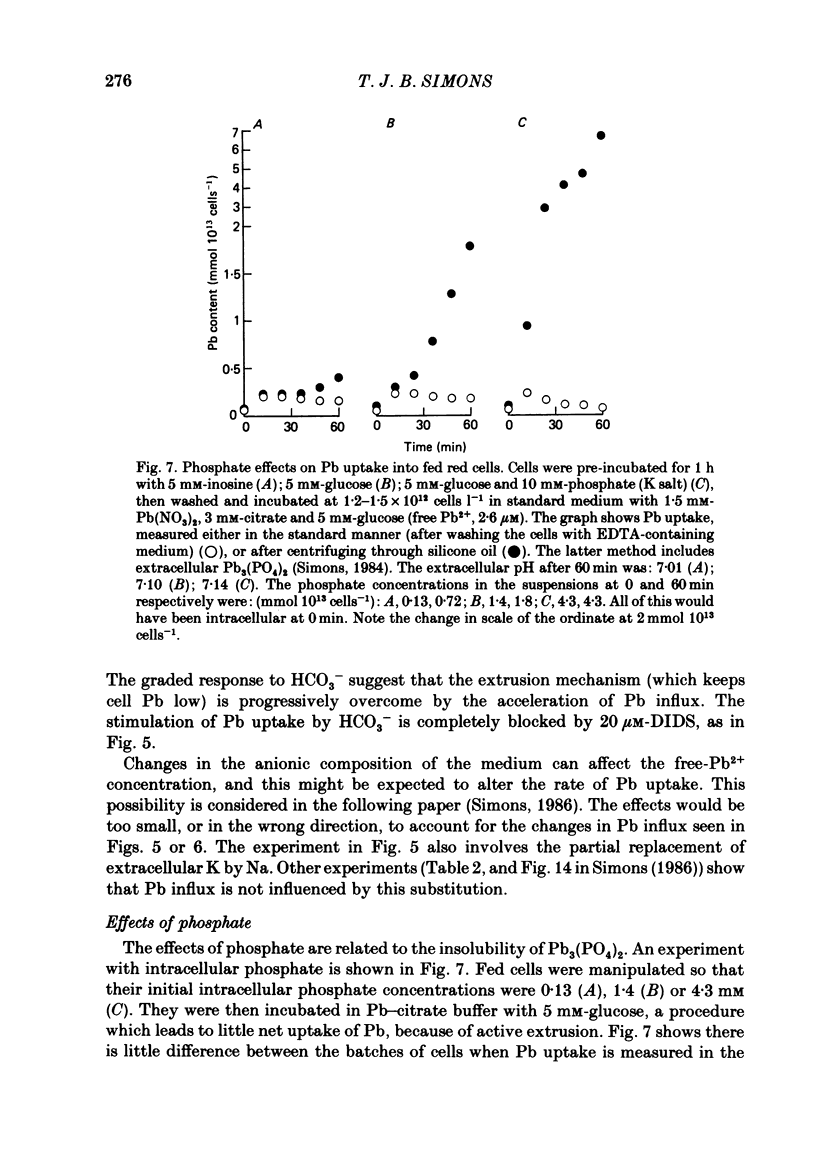
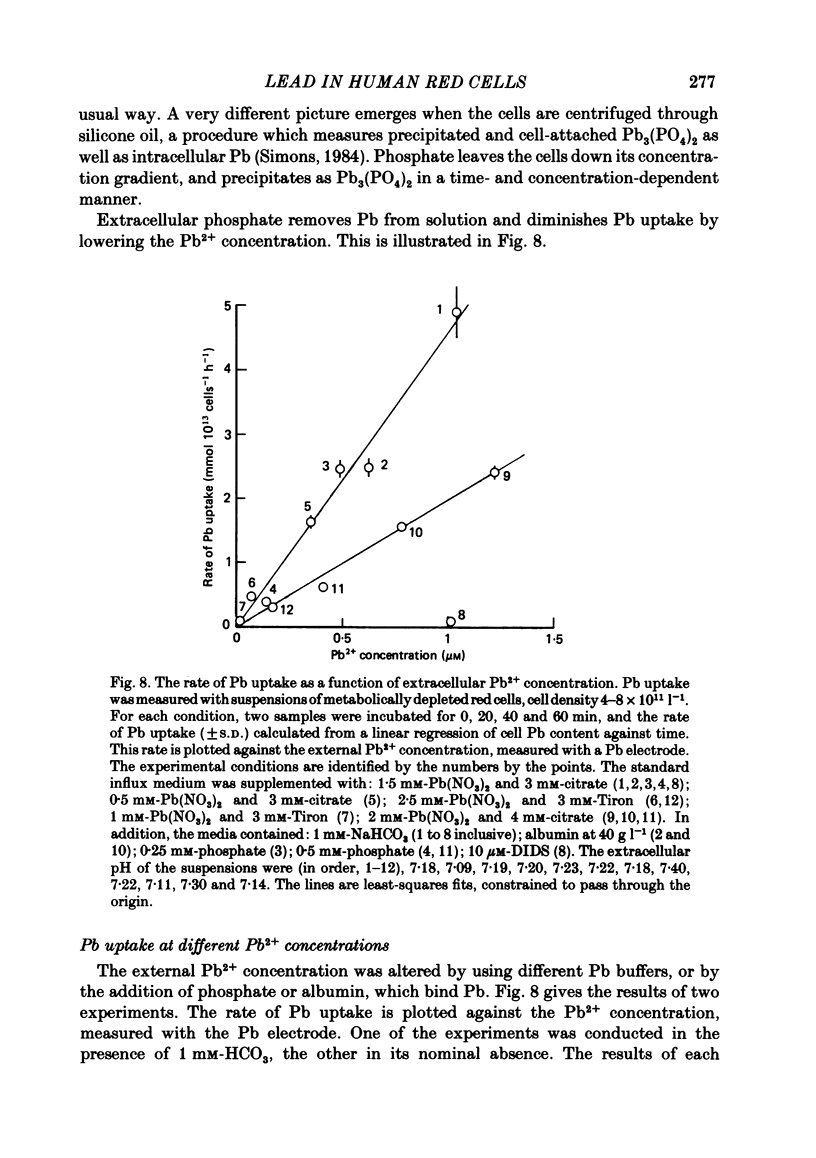
- Simons T. J. The role of anion transport in the passive movement of lead across the human red cell membrane. J Physiol. 1986 Sep;378:287–312. doi: 10.1113/jphysiol.1986.sp016220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIL-MALHERBE H., GREEN R. H. The catalytic effect of molybdate on the hydrolysis of organic phosphate bonds. Biochem J. 1951 Aug;49(3):286–292. [PMC free article] [PubMed] [Google Scholar]
- deSilva P. E. Determination of lead in plasma and studies on its relationship to lead in erythrocytes. Br J Ind Med. 1981 Aug;38(3):209–217. doi: 10.1136/oem.38.3.209. [DOI] [PMC free article] [PubMed] [Google Scholar]


