Abstract
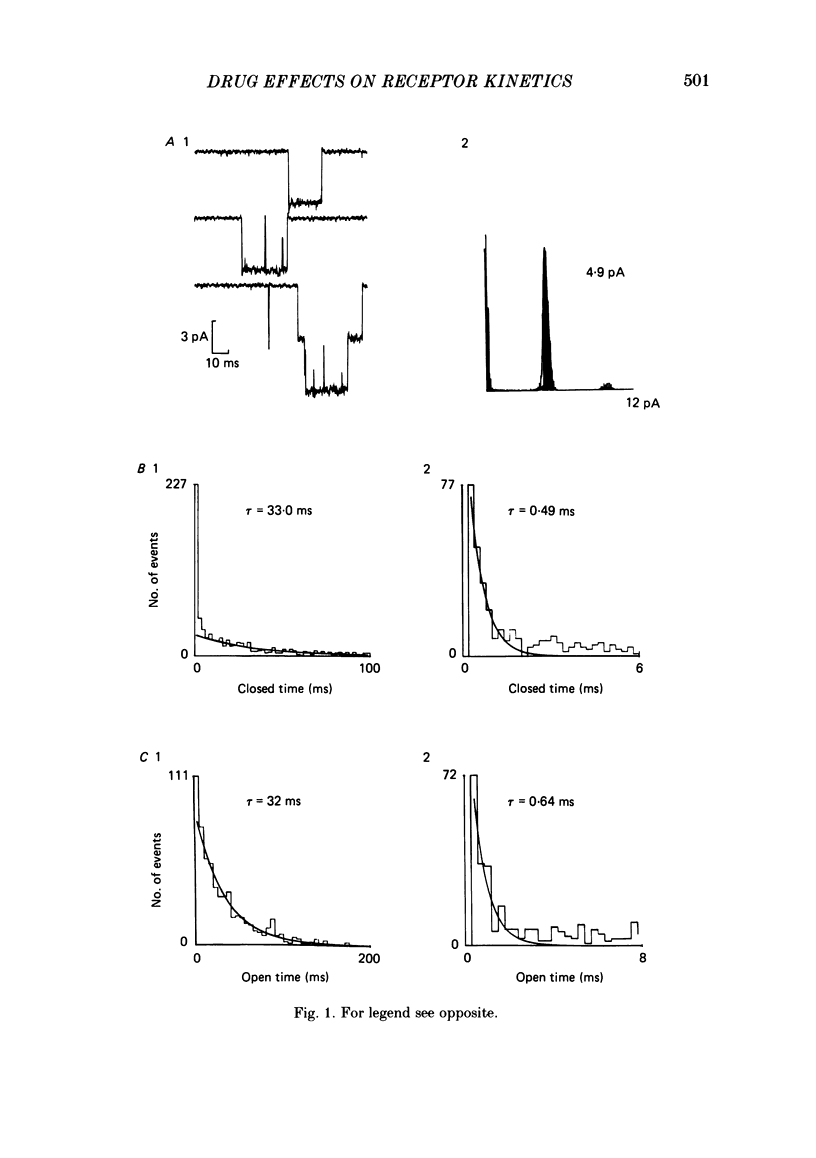
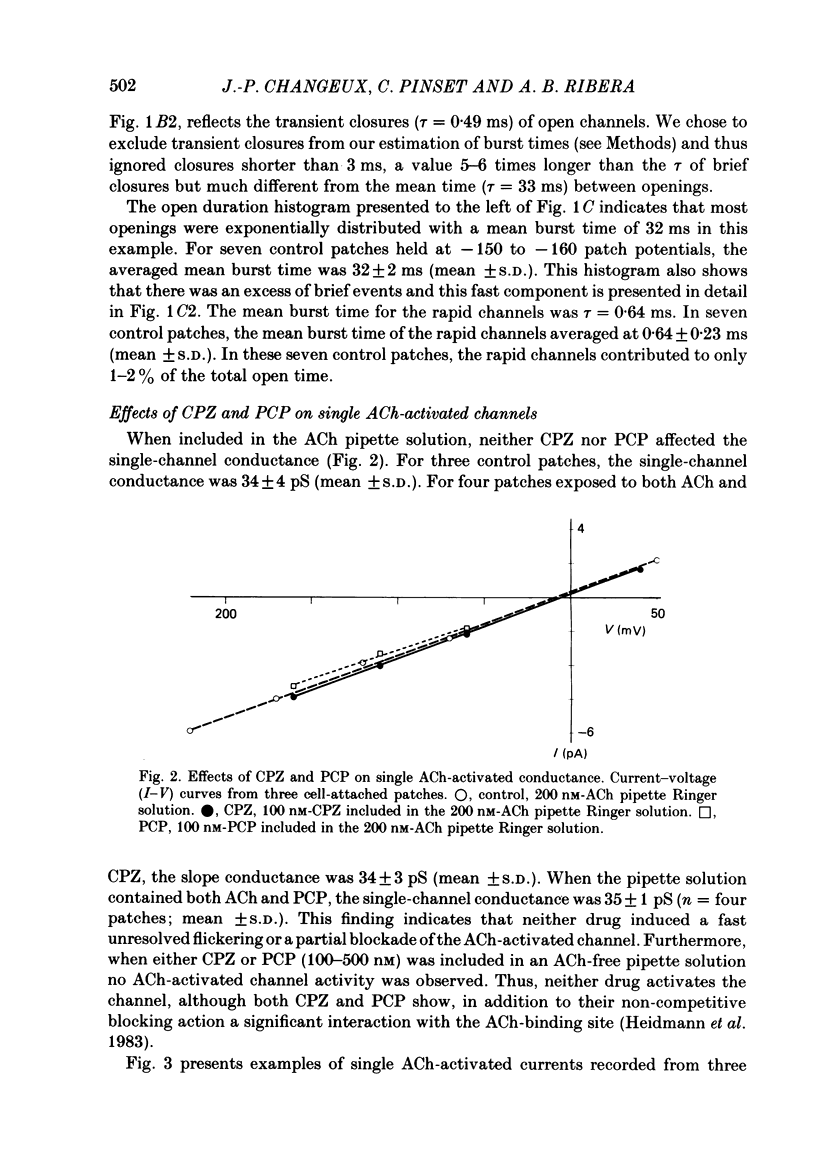
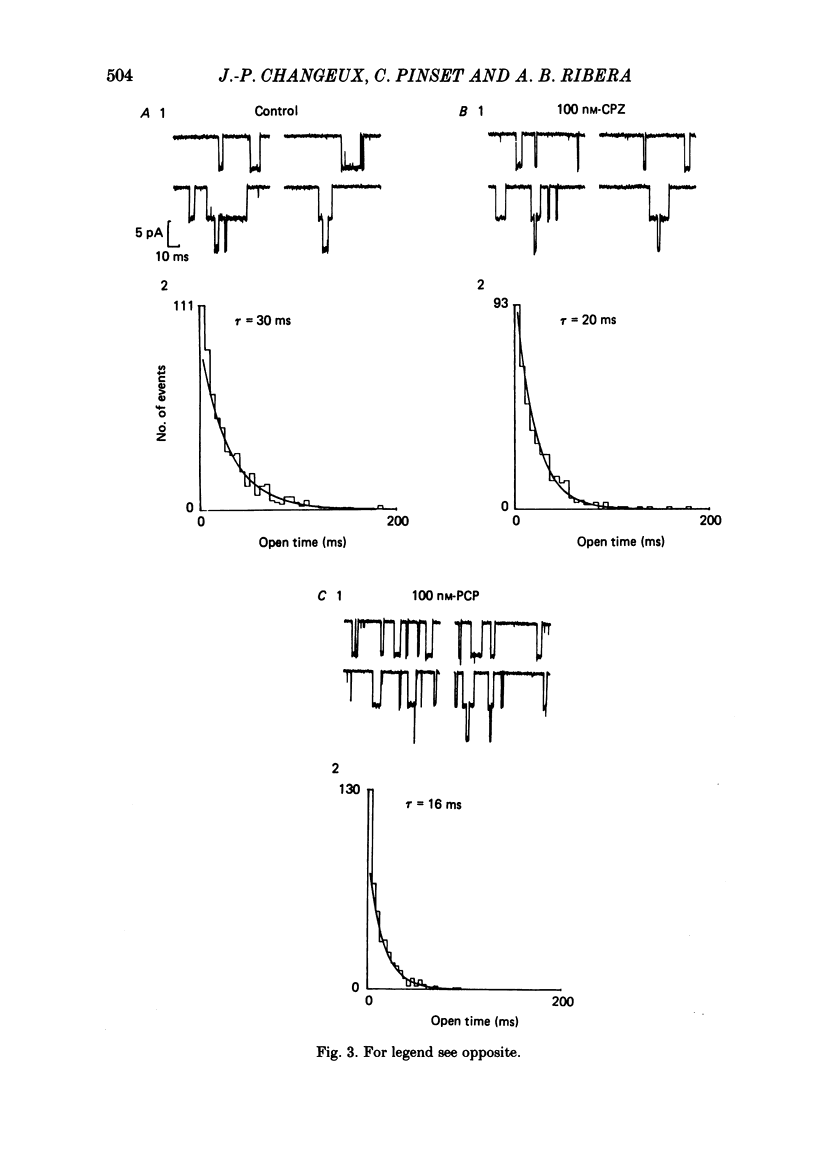
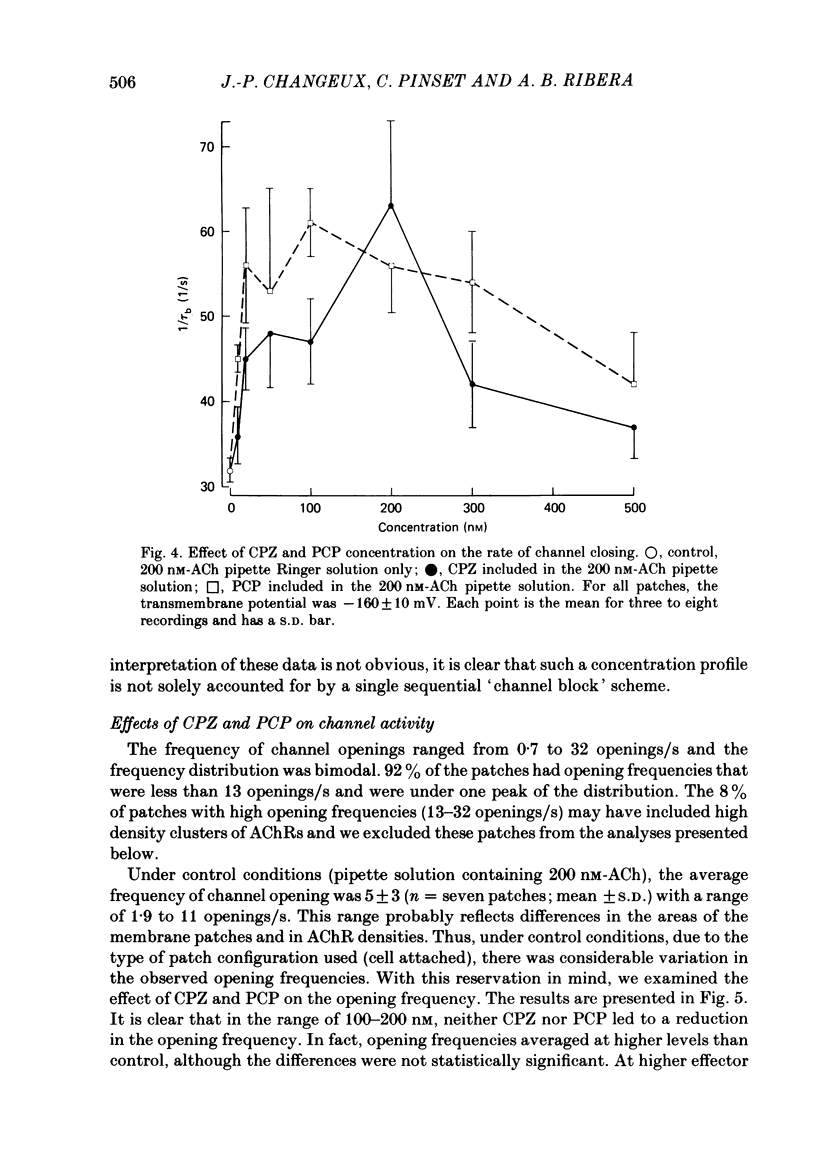
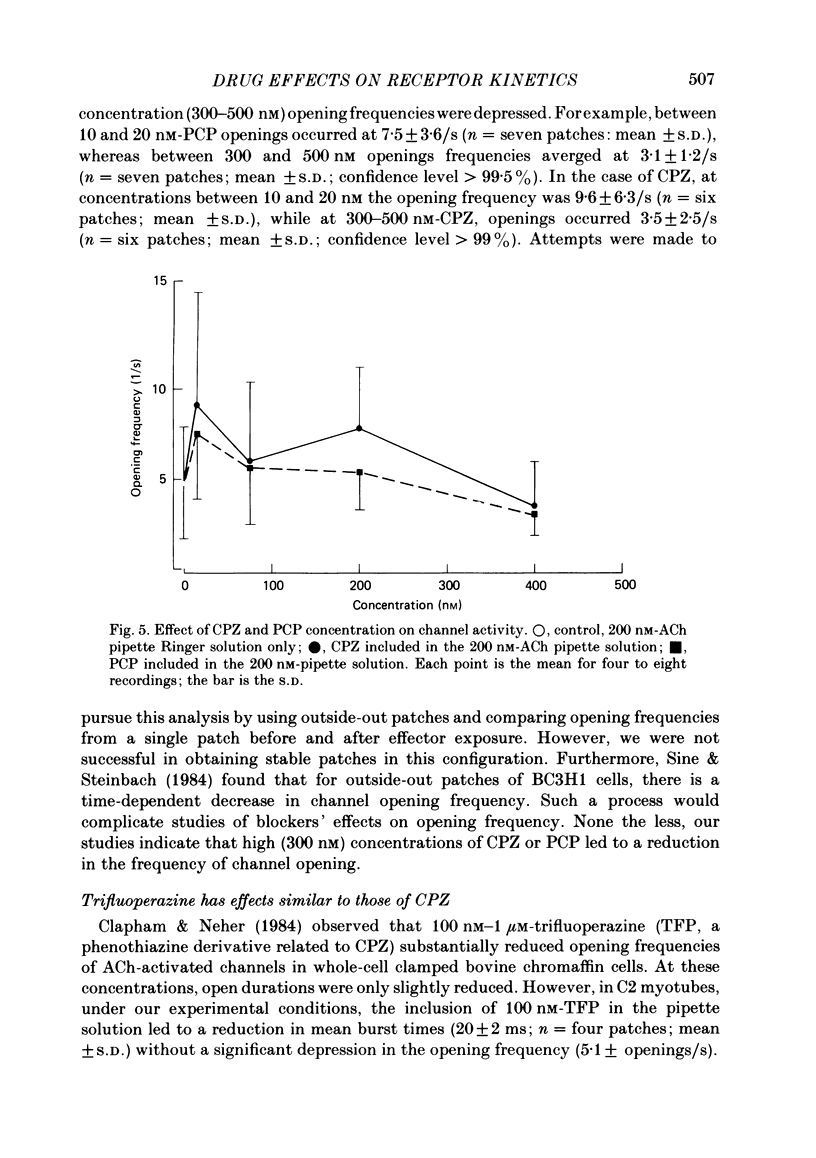
Patch-clamp techniques were used to record acetylcholine- (ACh) activated single-channel currents in cell-attached membrane patches from myotubes of the mouse cell line, C2. The effects of the phenothiazine derivative chlorpromazine (CPZ) and of the hallucinogen phencyclidine (PCP) on ACh-activated single-channel properties were studied under conditions where both compounds are positively charged (pH 7.2). The single-channel conductance was unaffected by either CPZ or PCP at concentrations ranging from 10 to 500 nM. 10-200 nM-CPZ and PCP led to shortened mean burst times. CPZ and PCP effects on mean burst times were voltage independent and did not vary in a simple linear manner with concentration. 10-200 nM-CPZ and PCP did not reduce channel opening frequencies, suggesting that the fraction of non-conducting state (occupied, blocked or desensitized) favoured at equilibrium was not significant at these concentrations. On the other hand, concentrations of CPZ and PCP higher than 300 nM did lead to depressed channel opening frequencies. In addition, we observed that, at these concentrations, the shortened burst duration reverses to the longer values found at lower effector concentrations. The effects of CPZ and PCP on ACh-activated single-channel kinetics are interpreted in terms of current models of ACh-receptor structure and conformational transitions.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R. Acetylcholine receptor kinetics. J Membr Biol. 1981 Feb 28;58(3):161–174. doi: 10.1007/BF01870902. [DOI] [PubMed] [Google Scholar]
- Adams P. R. Drug blockade of open end-plate channels. J Physiol. 1976 Sep;260(3):531–552. doi: 10.1113/jphysiol.1976.sp011530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P. R. Voltage jump analysis of procaine action at frog end-plate. J Physiol. 1977 Jun;268(2):291–318. doi: 10.1113/jphysiol.1977.sp011858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albuquerque E. X., Tsai M. C., Aronstam R. S., Eldefrawi A. T., Eldefrawi M. E. Sites of action of phencyclidine. II. Interaction with the ionic channel of the nicotinic receptor. Mol Pharmacol. 1980 Sep;18(2):167–178. [PubMed] [Google Scholar]
- Anwyl R., Narahashi T. Comparison of desensitization and time-dependent block of the acetylcholine receptor responses by chlorpromazine, cytochalasin B, triton X-100 and other agents. Br J Pharmacol. 1980 May;69(1):99–106. doi: 10.1111/j.1476-5381.1980.tb10887.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changeux J. P., Devillers-Thiéry A., Chemouilli P. Acetylcholine receptor: an allosteric protein. Science. 1984 Sep 21;225(4668):1335–1345. doi: 10.1126/science.6382611. [DOI] [PubMed] [Google Scholar]
- Changeux J. P. The acetylcholine receptor: an "allosteric" membrane protein. Harvey Lect. 1979 1980;75:85–254. [PubMed] [Google Scholar]
- Clapham D. E., Neher E. Trifluoperazine reduces inward ionic currents and secretion by separate mechanisms in bovine chromaffin cells. J Physiol. 1984 Aug;353:541–564. doi: 10.1113/jphysiol.1984.sp015350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Sakmann B. Fluctuations in the microsecond time range of the current through single acetylcholine receptor ion channels. Nature. 1981 Dec 3;294(5840):464–466. doi: 10.1038/294464a0. [DOI] [PubMed] [Google Scholar]
- Eldefrawi M. E., Eldefrawi A. T., Aronstam R. S., Maleque M. A., Warnick J. E., Albuquerque E. X. [3H]Phencyclidine: a probe for the ionic channel of the nicotinic receptor. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7458–7462. doi: 10.1073/pnas.77.12.7458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finer-Moore J., Stroud R. M. Amphipathic analysis and possible formation of the ion channel in an acetylcholine receptor. Proc Natl Acad Sci U S A. 1984 Jan;81(1):155–159. doi: 10.1073/pnas.81.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraudat J., Dennis M., Heidmann T., Chang J. Y., Changeux J. P. Structure of the high-affinity binding site for noncompetitive blockers of the acetylcholine receptor: serine-262 of the delta subunit is labeled by [3H]chlorpromazine. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2719–2723. doi: 10.1073/pnas.83.8.2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grünhagen H. H., Changeux J. P. Studies on the electrogenic action of acetylcholine with Torpedo marmorata electric organ. IV. Quinacrine: a fluorescent probe for the conformational transitions of the cholinergic receptor protein in its membrane-bound state. J Mol Biol. 1976 Sep 25;106(3):497–516. doi: 10.1016/0022-2836(76)90249-7. [DOI] [PubMed] [Google Scholar]
- Grünhagen H. H., Iwatsubo M., Changeux J. P. Fast kinetic studies on the interaction of cholinergic agonists with the membrane-bound acetylcholine receptor from Torpedo marmorata as revealed by quinacrine fluorescence. Eur J Biochem. 1977 Oct 17;80(1):225–242. doi: 10.1111/j.1432-1033.1977.tb11875.x. [DOI] [PubMed] [Google Scholar]
- Guy H. R. A structural model of the acetylcholine receptor channel based on partition energy and helix packing calculations. Biophys J. 1984 Jan;45(1):249–261. doi: 10.1016/S0006-3495(84)84152-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidmann T., Changeux J. P. Time-resolved photolabeling by the noncompetitive blocker chlorpromazine of the acetylcholine receptor in its transiently open and closed ion channel conformations. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1897–1901. doi: 10.1073/pnas.81.6.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidmann T., Oswald R. E., Changeux J. P. Multiple sites of action for noncompetitive blockers on acetylcholine receptor rich membrane fragments from torpedo marmorata. Biochemistry. 1983 Jun 21;22(13):3112–3127. doi: 10.1021/bi00282a014. [DOI] [PubMed] [Google Scholar]
- Koblin D. D., Lester H. A. Voltage-dependent and voltage-independent blockade of acetylcholine receptors by local anesthetics in Electrophorus electroplaques. Mol Pharmacol. 1979 May;15(3):559–580. [PubMed] [Google Scholar]
- Krodel E. K., Beckman R. A., Cohen J. B. Identification of a local anesthetic binding site in nicotinic post-synaptic membranes isolated from Torpedo marmorata electric tissue. Mol Pharmacol. 1979 Mar;15(2):294–312. [PubMed] [Google Scholar]
- Magleby K. L., Pallotta B. S. A study of desensitization of acetylcholine receptors using nerve-released transmitter in the frog. J Physiol. 1981 Jul;316:225–250. doi: 10.1113/jphysiol.1981.sp013784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishina M., Kurosaki T., Tobimatsu T., Morimoto Y., Noda M., Yamamoto T., Terao M., Lindstrom J., Takahashi T., Kuno M. Expression of functional acetylcholine receptor from cloned cDNAs. Nature. 1984 Feb 16;307(5952):604–608. doi: 10.1038/307604a0. [DOI] [PubMed] [Google Scholar]
- Neher E., Steinbach J. H. Local anaesthetics transiently block currents through single acetylcholine-receptor channels. J Physiol. 1978 Apr;277:153–176. doi: 10.1113/jphysiol.1978.sp012267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswald R. E., Heidmann T., Changeux J. P. Multiple affinity states for noncompetitive blockers revealed by [3H]phencyclidine binding to acetylcholine receptor rich membrane fragments from Torpedo marmorata. Biochemistry. 1983 Jun 21;22(13):3128–3136. doi: 10.1021/bi00282a015. [DOI] [PubMed] [Google Scholar]
- Oswald R., Changeux J. P. Ultraviolet light-induced labeling by noncompetitive blockers of the acetylcholine receptor from Torpedo marmorata. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3925–3929. doi: 10.1073/pnas.78.6.3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinset C., Whalen R. G. Induction of myogenic differentiation in serum-free medium does not require DNA synthesis. Dev Biol. 1985 Apr;108(2):284–289. doi: 10.1016/0012-1606(85)90032-6. [DOI] [PubMed] [Google Scholar]
- Sine S. M., Steinbach J. H. Activation of a nicotinic acetylcholine receptor. Biophys J. 1984 Jan;45(1):175–185. doi: 10.1016/S0006-3495(84)84146-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K., Trautmann A. A patch-clamp study of the partial agonist actions of tubocurarine on rat myotubes. J Physiol. 1984 Apr;349:353–374. doi: 10.1113/jphysiol.1984.sp015160. [DOI] [PMC free article] [PubMed] [Google Scholar]


