Abstract
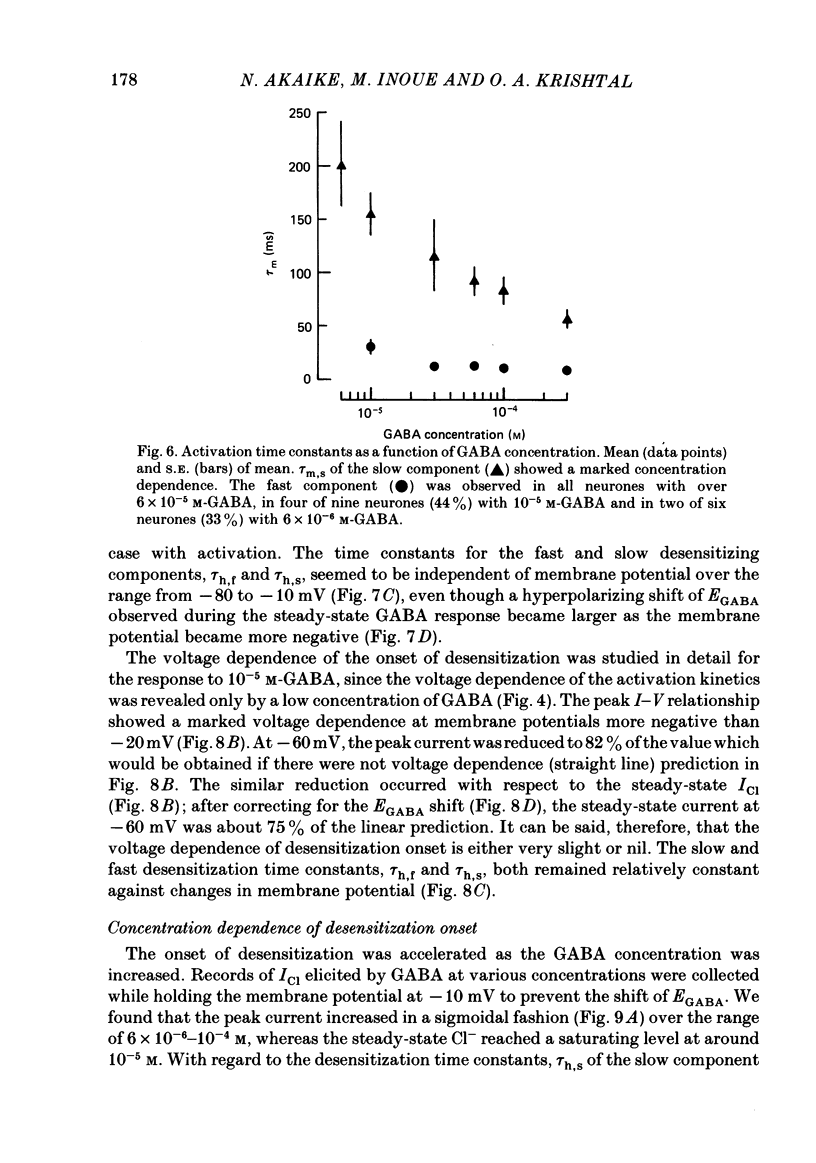
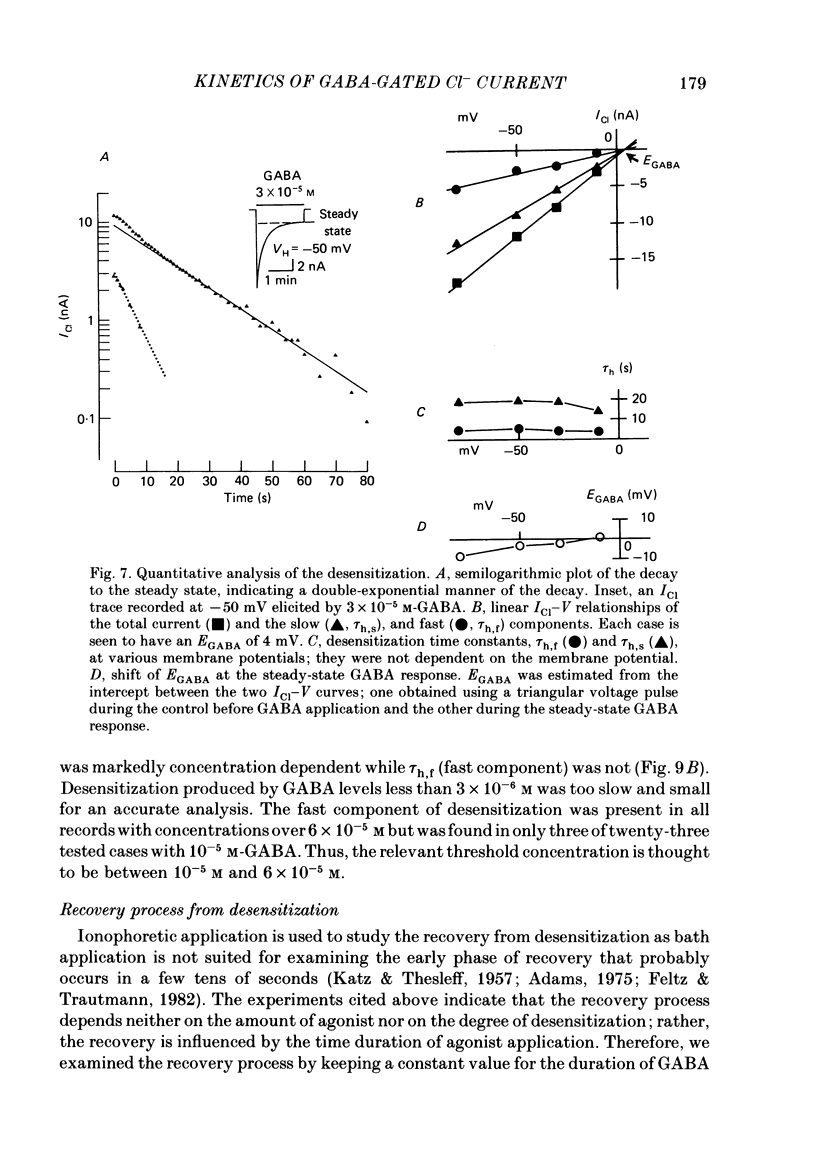
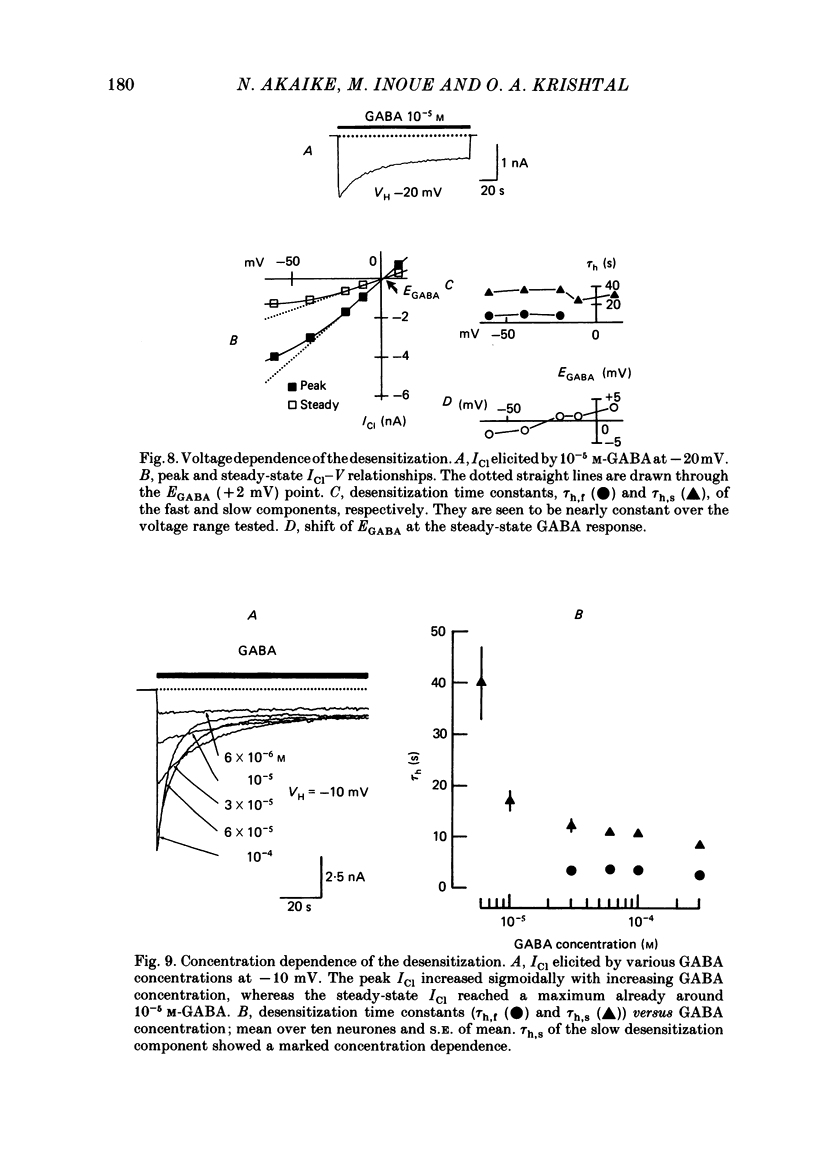
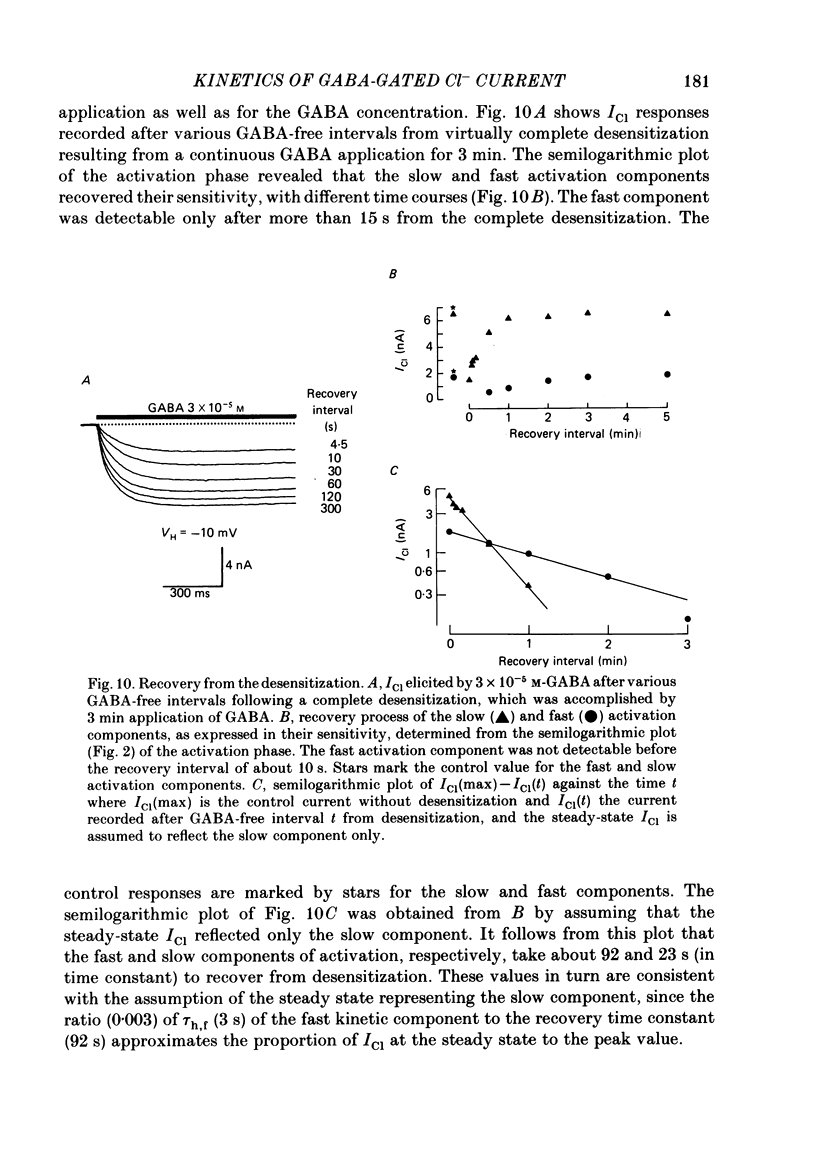
Kinetics of the activation and desensitization phases of gamma-aminobutyric acid (GABA)-induced Cl- current (ICl) were studied in single frog sensory neurones using the 'concentration-clamp' technique which enables perfusion of drugs with the time constant of about 3 ms. Both activation and desensitization phases of GABA response consisted of a single exponential at low concentrations and a double exponential at high concentrations. The time constant of the fast kinetic component in each phase was relatively stable, about 5 ms for activation and 3 s for desensitization over concentrations from 3 X 10(-5) to 3 X 10(-4) M, whereas those of the slow kinetic component decreased with increasing concentrations. The two kinetic components in both phases showed the same reversal potential. The slow and fast activation components recovered sensitivity from desensitization with different time courses: the recovery rate of the fast activation component was slow and that of the slow one, rapid. The peak ICl elicited at GABA concentrations below 10(-5) M increased disproportionally at more negative membrane potentials, thereby suggesting that the activation kinetics is voltage dependent. The steady-state ICl-voltage relationship obtained with less than 10(-5) M-GABA showed a non-linearity, probably due to voltage dependence of activation rather than that of desensitization kinetics. These results suggest the presence of at least two different GABA receptor-Cl- ionophore complexes with a different affinity and kinetics.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R. A study of desensitization using voltage clamp. Pflugers Arch. 1975 Oct 28;360(2):135–144. doi: 10.1007/BF00580536. [DOI] [PubMed] [Google Scholar]
- Akaike N., Hattori K., Inomata N., Oomura Y. gamma-Aminobutyric-acid- and pentobarbitone-gated chloride currents in internally perfused frog sensory neurones. J Physiol. 1985 Mar;360:367–386. doi: 10.1113/jphysiol.1985.sp015622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson C. R., Stevens C. F. Voltage clamp analysis of acetylcholine produced end-plate current fluctuations at frog neuromuscular junction. J Physiol. 1973 Dec;235(3):655–691. doi: 10.1113/jphysiol.1973.sp010410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwyl R., Narahashi T. Desensitization of the acetylcholine receptor of denervated rat soleus muscle and the effect of calcium. Br J Pharmacol. 1980 May;69(1):91–98. doi: 10.1111/j.1476-5381.1980.tb10886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bregestovksi P. D., Bukharaeva E. A., Iljin V. I. Voltage clamp analysis of acetylcholine receptor desensitization in isolated mollusc neurones. J Physiol. 1979 Dec;297(0):581–595. doi: 10.1113/jphysiol.1979.sp013058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesnut T. J. Two-component desensitization at the neuromuscular junction of the frog. J Physiol. 1983 Mar;336:229–241. doi: 10.1113/jphysiol.1983.sp014578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy S. G., Miledi R. Junctional and extrajunctional membrane channels activated by GABA in locust muscle fibres. Proc R Soc Lond B Biol Sci. 1981 Mar 27;211(1185):527–535. doi: 10.1098/rspb.1981.0021. [DOI] [PubMed] [Google Scholar]
- Dudel J., Finger W., Stettmeier H. Inhibitory synaptic channels activated by gamma-aminobutyric acid (GABA) in crayfish muscle. Pflugers Arch. 1980 Sep;387(2):143–151. doi: 10.1007/BF00584265. [DOI] [PubMed] [Google Scholar]
- Feltz A., Trautmann A. Desensitization at the frog neuromuscular junction: a biphasic process. J Physiol. 1982 Jan;322:257–272. doi: 10.1113/jphysiol.1982.sp014036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori K., Akaike N., Oomura Y., Kuraoka S. Internal perfusion studies demonstrating GABA-induced chloride responses in frog primary afferent neurons. Am J Physiol. 1984 Mar;246(3 Pt 1):C259–C265. doi: 10.1152/ajpcell.1984.246.3.C259. [DOI] [PubMed] [Google Scholar]
- Ishizuka S., Hattori K., Akaike N. Separation of ionic currents in the somatic membrane of frog sensory neurons. J Membr Biol. 1984;78(1):19–28. doi: 10.1007/BF01872528. [DOI] [PubMed] [Google Scholar]
- KATZ B., THESLEFF S. A study of the desensitization produced by acetylcholine at the motor end-plate. J Physiol. 1957 Aug 29;138(1):63–80. doi: 10.1113/jphysiol.1957.sp005838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The statistical nature of the acetycholine potential and its molecular components. J Physiol. 1972 Aug;224(3):665–699. doi: 10.1113/jphysiol.1972.sp009918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishtal O. A., Marchenko S. M., Pidoplichko V. I. Receptor for ATP in the membrane of mammalian sensory neurones. Neurosci Lett. 1983 Jan 31;35(1):41–45. doi: 10.1016/0304-3940(83)90524-4. [DOI] [PubMed] [Google Scholar]
- Kuba K., Nishi S. Characteristics of fast excitatory postsynaptic current in bullfrog sympathetic ganglion cells. Effects of membrane potential, temperature and Ca ions. Pflugers Arch. 1979 Jan 31;378(3):205–212. doi: 10.1007/BF00592737. [DOI] [PubMed] [Google Scholar]
- Lester H. A., Chang H. W. Response of acetylcholine receptors to rapid photochemically produced increases in agonist concentration. Nature. 1977 Mar 24;266(5600):373–374. doi: 10.1038/266373a0. [DOI] [PubMed] [Google Scholar]
- Magazanik L. G., Vyskocil F. Dependence of acetylcholine desensitization on the membrane potential of frog muscle fibre and on the ionic changes in the medium. J Physiol. 1970 Oct;210(3):507–518. doi: 10.1113/jphysiol.1970.sp009223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Stevens C. F. The effect of voltage on the time course of end-plate currents. J Physiol. 1972 May;223(1):151–171. doi: 10.1113/jphysiol.1972.sp009839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onodera K., Takeuchi A. Inhibitory postsynaptic current in voltage-clamped crayfish muscle. Nature. 1976 Sep 9;263(5573):153–154. doi: 10.1038/263153a0. [DOI] [PubMed] [Google Scholar]
- Rang H. P. The characteristics of synaptic currents and responses to acetylcholine of rat submandibular ganglion cells. J Physiol. 1981 Feb;311:23–55. doi: 10.1113/jphysiol.1981.sp013571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scubon-Mulieri B., Parsons R. L. Desensitization and recovery at the frog neuromuscular junction. J Gen Physiol. 1977 Apr;69(4):431–447. doi: 10.1085/jgp.69.4.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scubon-Mulieri B., Parsons R. L. Desensitization onset and recovery at the potassium-depolarized frog neuromuscular junction are voltage sensitive. J Gen Physiol. 1978 Mar;71(3):285–299. doi: 10.1085/jgp.71.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal M., Barker J. L. Rat hippocampal neurons in culture: properties of GABA-activated Cl- ion conductance. J Neurophysiol. 1984 Mar;51(3):500–515. doi: 10.1152/jn.1984.51.3.500. [DOI] [PubMed] [Google Scholar]
- Segal M., Barker J. L. Rat hippocampal neurons in culture: voltage-clamp analysis of inhibitory synaptic connections. J Neurophysiol. 1984 Sep;52(3):469–487. doi: 10.1152/jn.1984.52.3.469. [DOI] [PubMed] [Google Scholar]
- Weinstock M. M. Activation and desensitization of acetylcholine receptors in fish muscle with a photoisomerizable agonist. J Physiol. 1983 May;338:423–433. doi: 10.1113/jphysiol.1983.sp014681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasui S., Ishizuka S., Akaike N. GABA activates different types of chloride-conducting receptor-ionophore complexes in a dose-dependent manner. Brain Res. 1985 Sep 30;344(1):176–180. doi: 10.1016/0006-8993(85)91206-5. [DOI] [PubMed] [Google Scholar]


