Abstract
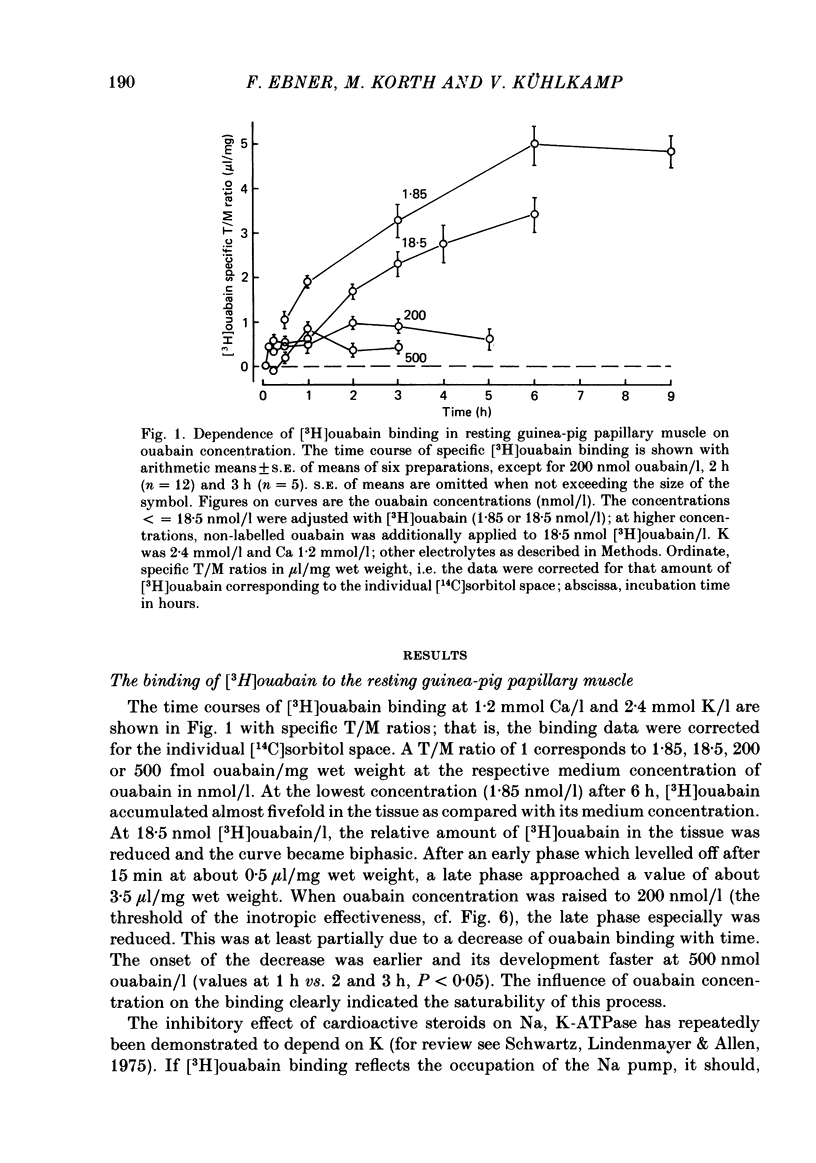
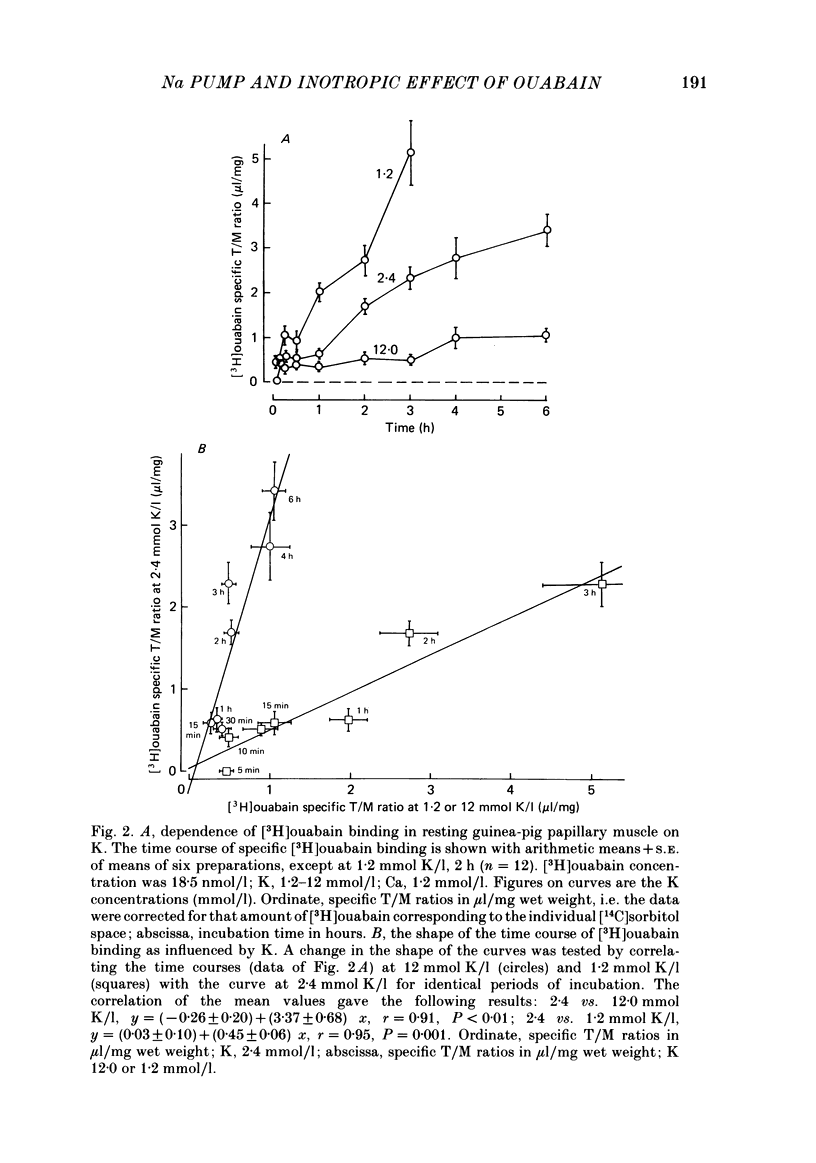
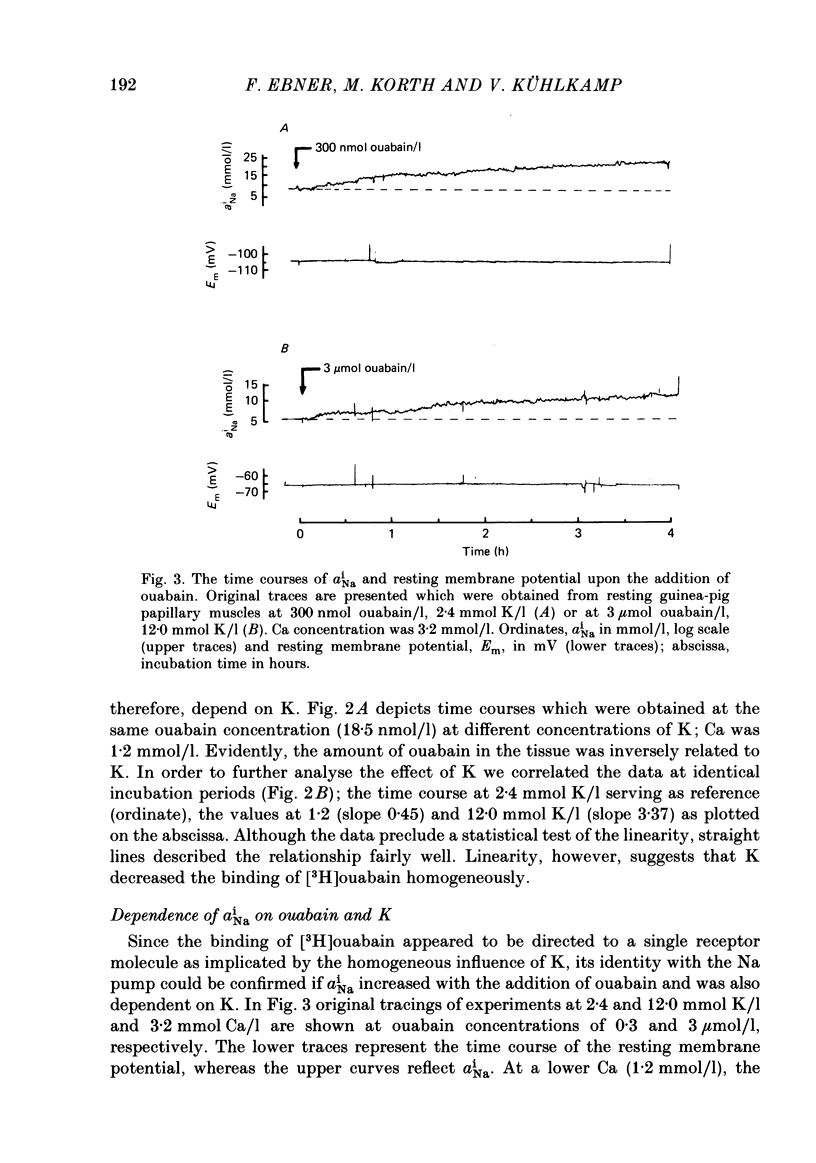
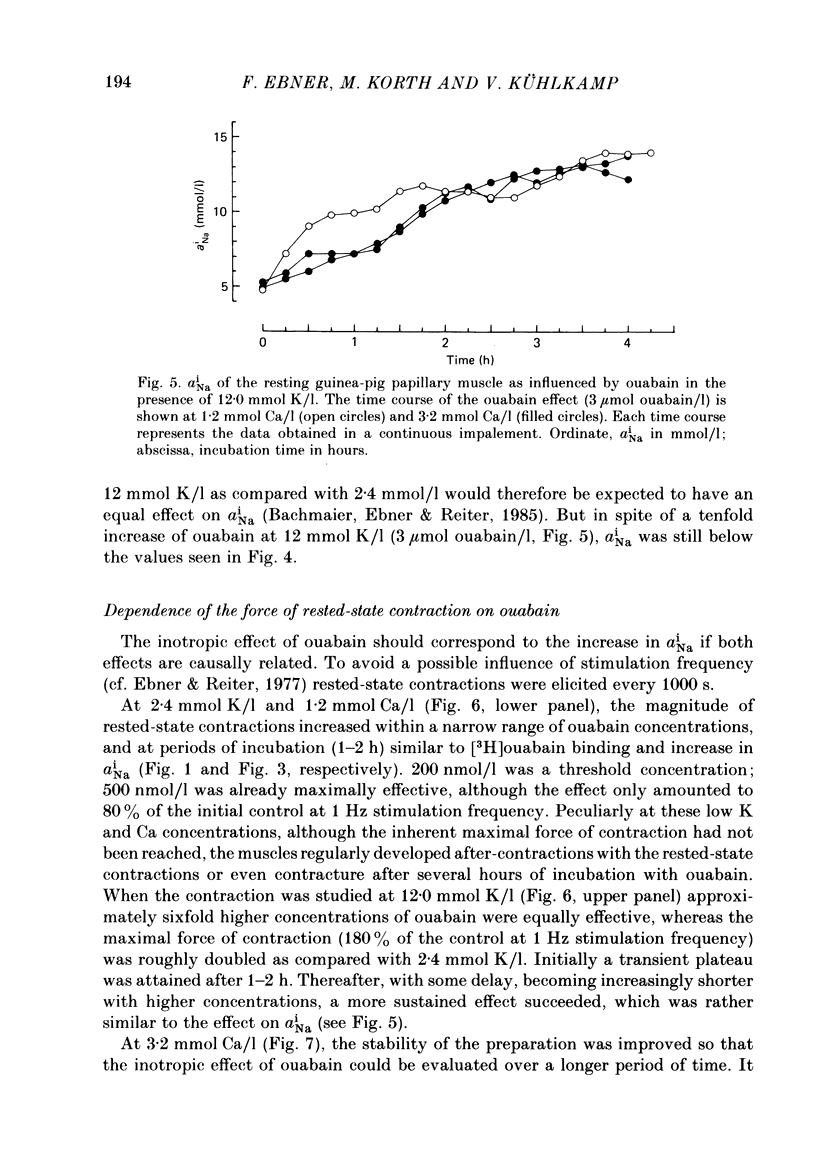
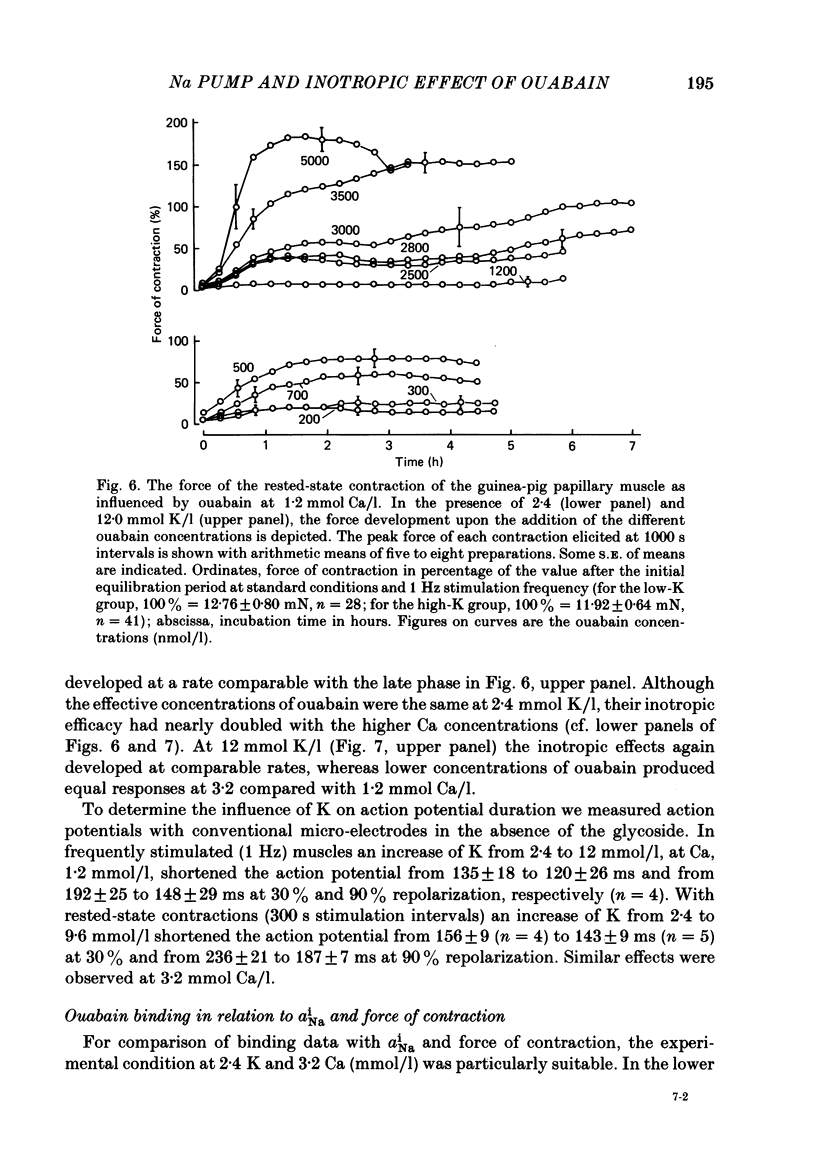
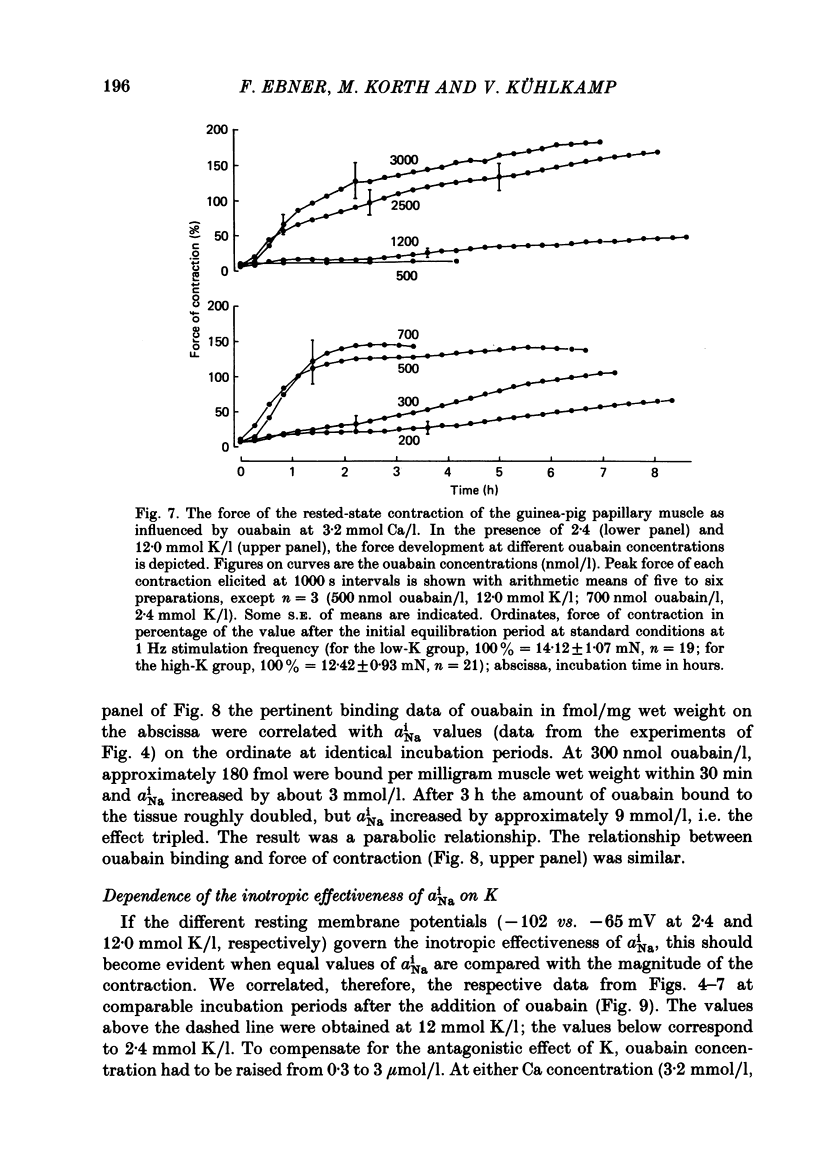
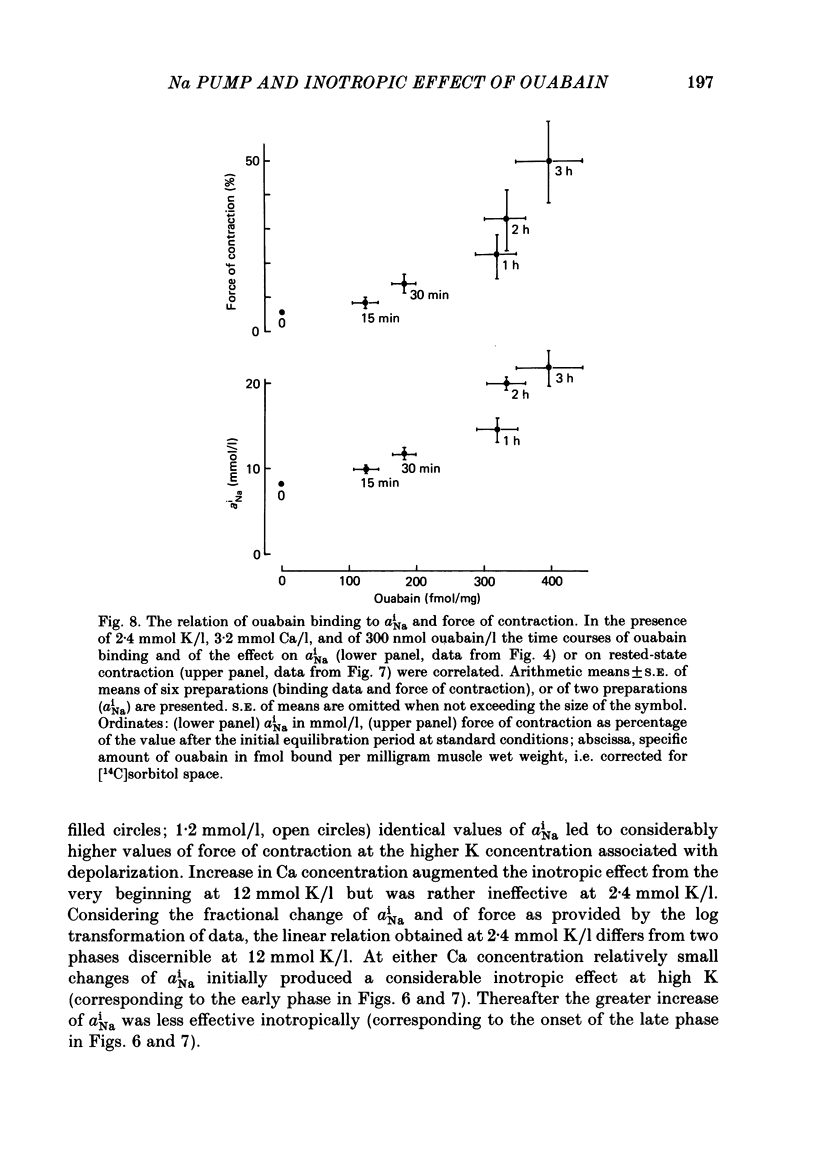
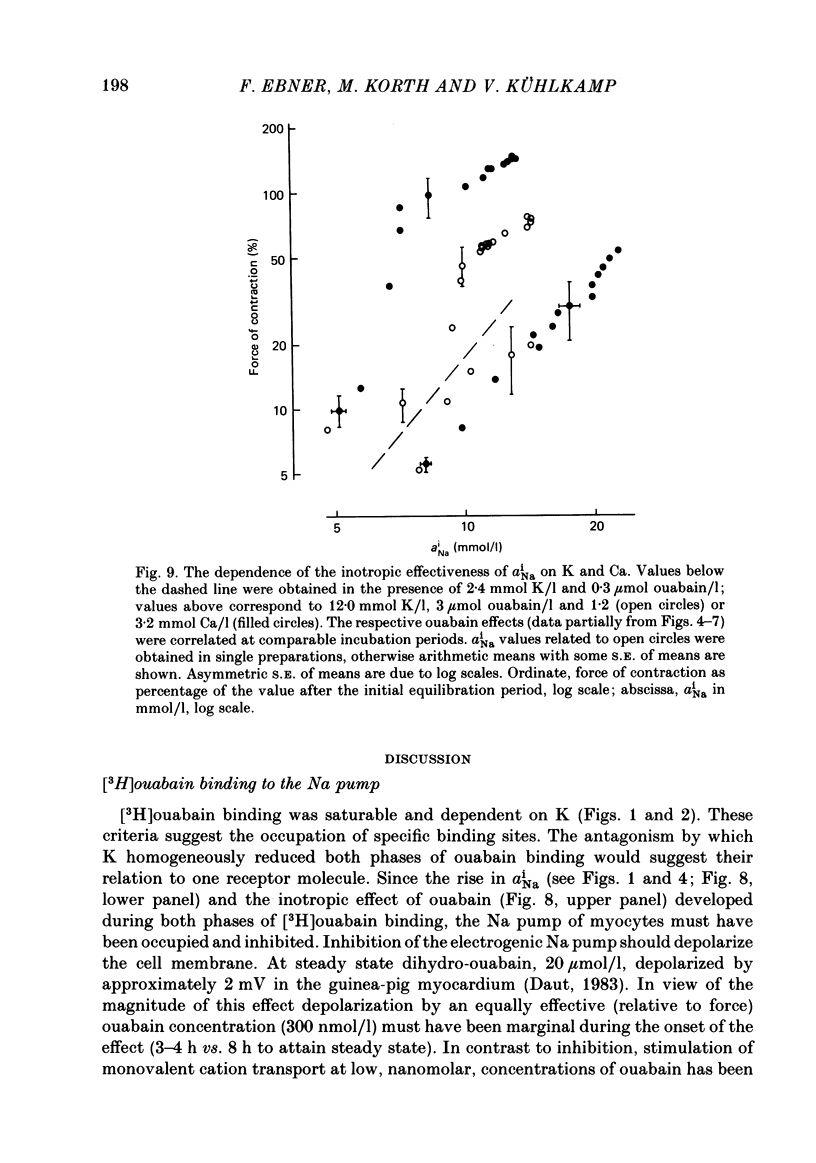
[3H]ouabain binding (1.85-500 nmol/l) was evaluated in resting guinea-pig papillary muscles at 1.2-12 mmol K/l. The time course of binding was biphasic. This finding excluded a homogeneous population of non-interacting binding sites of ouabain, even though the identical susceptibility of both phases to K suggested the occupation of similarly operative receptors. Concomitant with ouabain binding, intracellular Na ion activity (aiNa) increased in the presence of 2.4 or 12 mmol K/l. Occupation of the receptor molecule by ouabain, therefore, conformed to Na-pump inhibition. Although K antagonized both ouabain binding and its effect on aiNa, the antagonistic effect on aiNa was more pronounced. The reduction of passive Na influx with depolarization as well as the stimulation of the Na pump by K presumably contributed to the antagonistic effect of K. The decrease in aiNa from 8 to 5 mmol/l, when in the absence of ouabain K was raised from 2.4 to 12.0 mmol/l, confirmed the relevance of Na fluxes. Simultaneous changes in aiNa and in the force of rested-state contractions were apparent upon addition of ouabain. At 2.4 mmol K/l, increase in aiNa raised the force of contraction by constant proportions. At 12 mmol K/l, the inotropic effect produced at comparable values of aiNa was approximately tenfold higher and was susceptible to a change in extracellular Ca concentration. Increase in aiNa, however, was differently effective on force of contraction of low as compared with high values of aiNa. The influence of resting membrane potential on electrogenic Na-Ca exchange is supposed to interfere with the inotropic effectiveness of aiNa after the cell membrane depolarized from -102 mV to -65 mV with the increase of K from 2.4 to 12 mmol/l. In view of the role of both membrane potential and aiNa not just a single mechanism appeared to be involved in the control of force of contraction.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmaier A., Ebner F., Reiter M. Potassium changes the relationship between receptor occupancy and the inotropic effect of cardiac glycosides in guinea-pig myocardium. Br J Pharmacol. 1985 Aug;85(4):755–765. doi: 10.1111/j.1476-5381.1985.tb11073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bers D. M. A simple method for the accurate determination of free [Ca] in Ca-EGTA solutions. Am J Physiol. 1982 May;242(5):C404–C408. doi: 10.1152/ajpcell.1982.242.5.C404. [DOI] [PubMed] [Google Scholar]
- Brown A. M., Lew V. L. The effect of intracellular calcium on the sodium pump of human red cells. J Physiol. 1983 Oct;343:455–493. doi: 10.1113/jphysiol.1983.sp014904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daut J. Inhibition of the sodium pump in guinea-pig ventricular muscle by dihydro-ouabain: effects of external potassium and sodium. J Physiol. 1983 Jun;339:643–662. doi: 10.1113/jphysiol.1983.sp014740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner F., Bachmaier A., Schönsteiner G., Reiter M. Diffusion-controlled receptor occupancy determines the rate of inotropic action of some cardioactive steroids. J Mol Cell Cardiol. 1985 Nov;17(11):1115–1126. doi: 10.1016/s0022-2828(85)80127-9. [DOI] [PubMed] [Google Scholar]
- Ebner F., Schönsteiner G. Inhibition of sarcolemmal Na, K, Mg ATPase from the guinea pig heart is not compatible with a homogeneous population of non-interacting ouabain receptors. Experientia. 1985 Sep 15;41(9):1147–1149. doi: 10.1007/BF01951701. [DOI] [PubMed] [Google Scholar]
- Eisner D. A., Lederer W. J., Vaughan-Jones R. D. The control of tonic tension by membrane potential and intracellular sodium activity in the sheep cardiac Purkinje fibre. J Physiol. 1983 Feb;335:723–743. doi: 10.1113/jphysiol.1983.sp014560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner D. A., Lederer W. J., Vaughan-Jones R. D. The dependence of sodium pumping and tension on intracellular sodium activity in voltage-clamped sheep Purkinje fibres. J Physiol. 1981 Aug;317:163–187. doi: 10.1113/jphysiol.1981.sp013819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner D. A., Lederer W. J., Vaughan-Jones R. D. The effects of rubidium ions and membrane potentials on the intracellular sodium activity of sheep Purkinje fibres. J Physiol. 1981 Aug;317:189–205. doi: 10.1113/jphysiol.1981.sp013820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner D. A., Lederer W. J., Vaughan-Jones R. D. The quantitative relationship between twitch tension and intracellular sodium activity in sheep cardiac Purkinje fibres. J Physiol. 1984 Oct;355:251–266. doi: 10.1113/jphysiol.1984.sp015417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glitsch H. G. Characteristics of active Na transport in intact cardiac cells. Am J Physiol. 1979 Feb;236(2):H189–H199. doi: 10.1152/ajpheart.1979.236.2.H189. [DOI] [PubMed] [Google Scholar]
- Hougen T. J., Spicer N., Smith T. W. Stimulation of monovalent cation active transport by low concentrations of cardiac glycosides. Role of catecholamines. J Clin Invest. 1981 Nov;68(5):1207–1214. doi: 10.1172/JCI110366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- January C. T., Fozzard H. A. The effects of membrane potential, extracellular potassium, and tetrodotoxin on the intracellular sodium ion activity of sheep cardiac muscle. Circ Res. 1984 Jun;54(6):652–665. doi: 10.1161/01.res.54.6.652. [DOI] [PubMed] [Google Scholar]
- Kameyama M., Kakei M., Sato R., Shibasaki T., Matsuda H., Irisawa H. Intracellular Na+ activates a K+ channel in mammalian cardiac cells. Nature. 1984 May 24;309(5966):354–356. doi: 10.1038/309354a0. [DOI] [PubMed] [Google Scholar]
- Kazazoglou T., Renaud J. F., Rossi B., Lazdunski M. Two classes of ouabain receptors in chick ventricular cardiac cells and their relation to (Na+,K+)-ATPase inhibition, intracellular Na+ accumulation, Ca2+ influx, and cardiotonic effect. J Biol Chem. 1983 Oct 25;258(20):12163–12170. [PubMed] [Google Scholar]
- Lechat P., Malloy C. R., Smith T. W. Active transport and inotropic state in guinea pig left atrium. Circ Res. 1983 Apr;52(4):411–422. doi: 10.1161/01.res.52.4.411. [DOI] [PubMed] [Google Scholar]
- Lee C. O., Kang D. H., Sokol J. H., Lee K. S. Relation between intracellular Na ion activity and tension of sheep cardiac Purkinje fibers exposed to dihydro-ouabain. Biophys J. 1980 Feb;29(2):315–330. doi: 10.1016/S0006-3495(80)85135-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz A., Lindenmayer G. E., Allen J. C. The sodium-potassium adenosine triphosphatase: pharmacological, physiological and biochemical aspects. Pharmacol Rev. 1975 Mar;27(01):3–134. [PubMed] [Google Scholar]
- Sheu S. S., Fozzard H. A. Transmembrane Na+ and Ca2+ electrochemical gradients in cardiac muscle and their relationship to force development. J Gen Physiol. 1982 Sep;80(3):325–351. doi: 10.1085/jgp.80.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi K., Iida S. Two apparently different ouabain binding sites of (Na + -K + )-ATPase. Biochim Biophys Acta. 1972 Oct 23;288(1):98–102. doi: 10.1016/0005-2736(72)90226-x. [DOI] [PubMed] [Google Scholar]
- Temma K., Akera T. Enhancement of cardiac actions of ouabain and its binding to Na+, K+-adenosine triphosphatase by increased sodium influx in isolated guinea-pig heart. J Pharmacol Exp Ther. 1982 Nov;223(2):490–496. [PubMed] [Google Scholar]
- Vaughan-Jones R. D., Lederer W. J., Eisner D. A. Ca2+ ions can affect intracellular pH in mammalian cardiac muscle. Nature. 1983 Feb 10;301(5900):522–524. doi: 10.1038/301522a0. [DOI] [PubMed] [Google Scholar]
- Wasserstrom J. A., Schwartz D. J., Fozzard H. A. Relation between intracellular sodium and twitch tension in sheep cardiac Purkinje strands exposed to cardiac glycosides. Circ Res. 1983 Jun;52(6):697–705. doi: 10.1161/01.res.52.6.697. [DOI] [PubMed] [Google Scholar]


