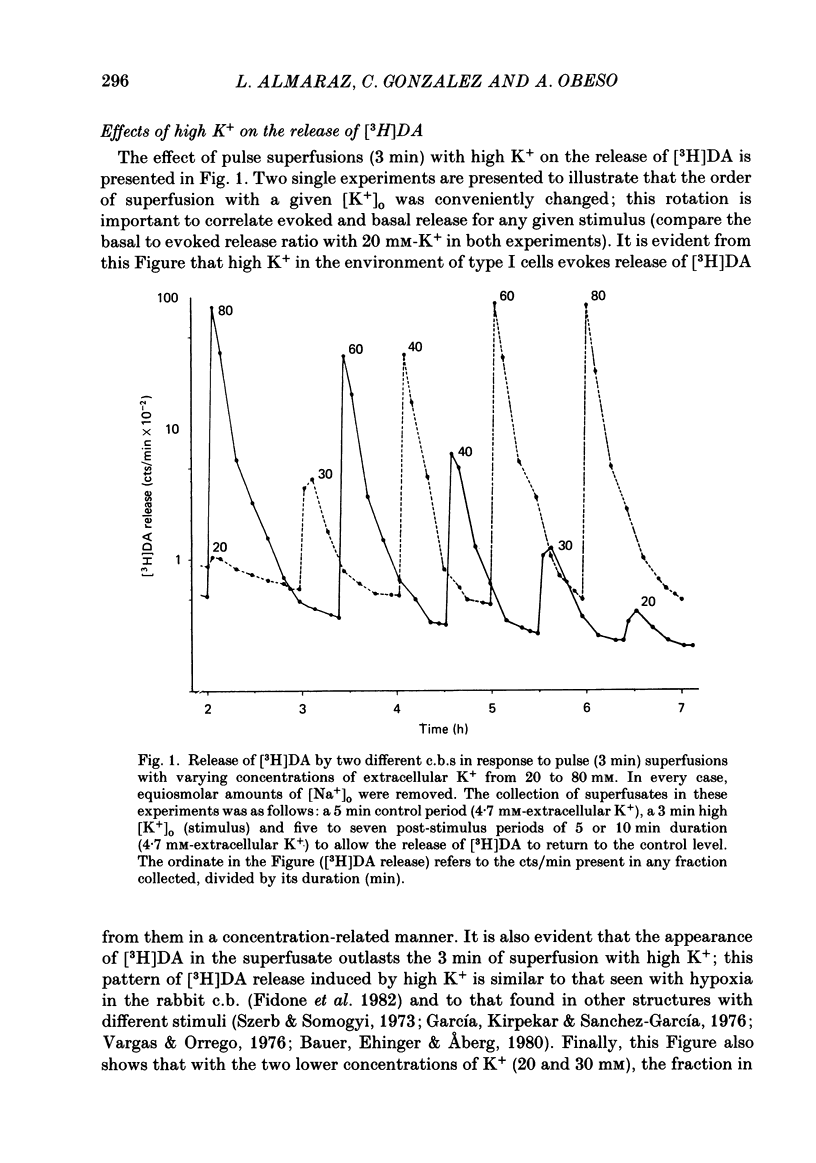
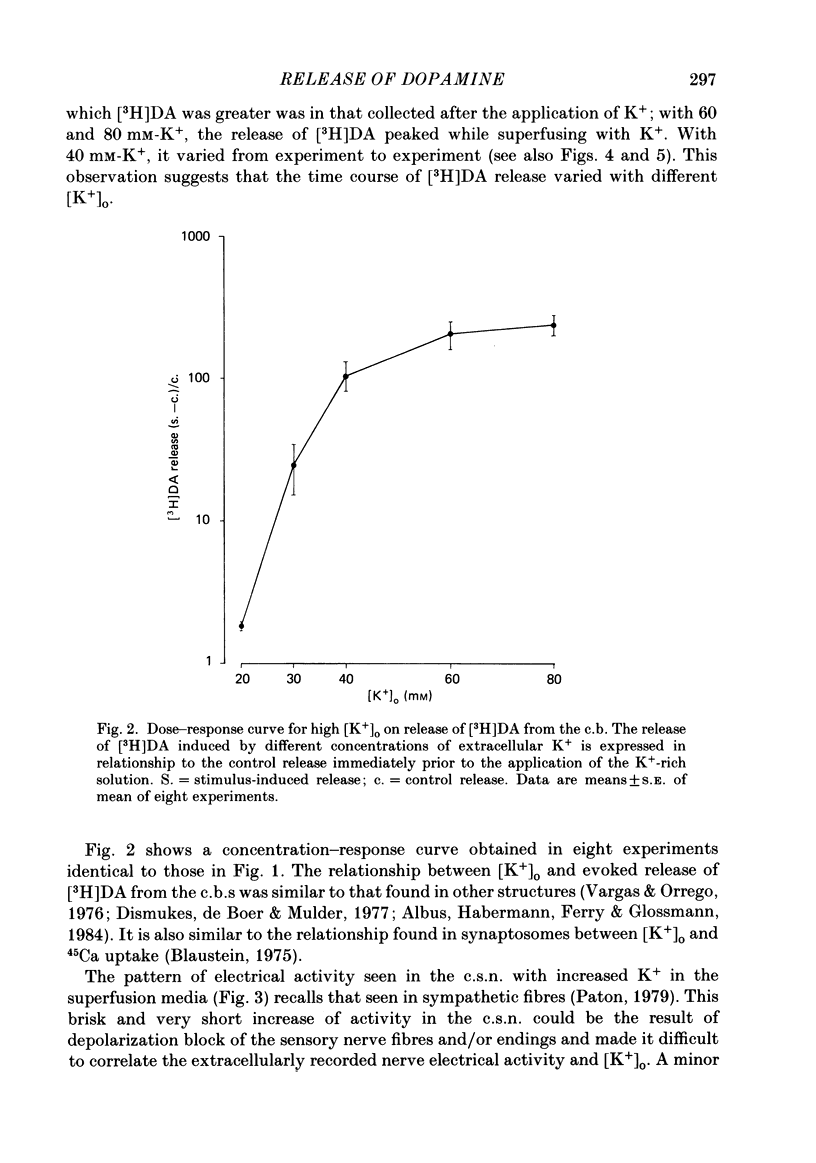
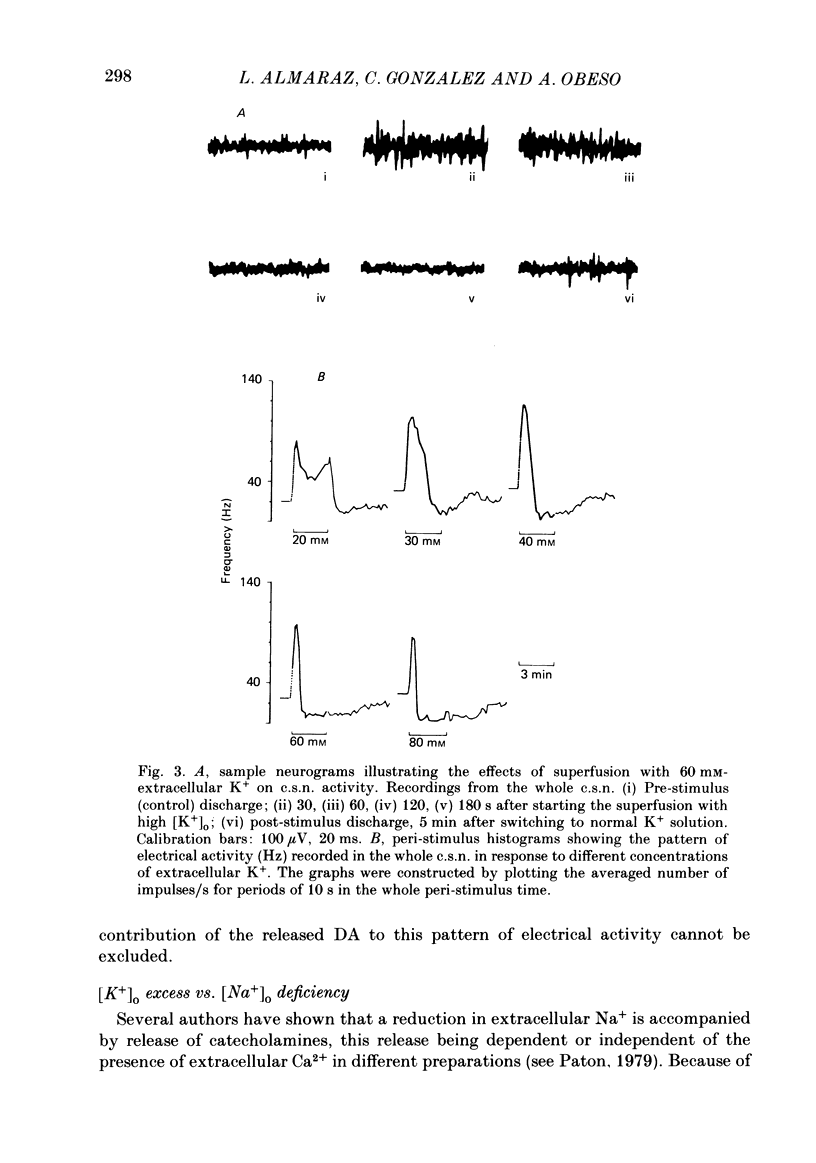
Abstract
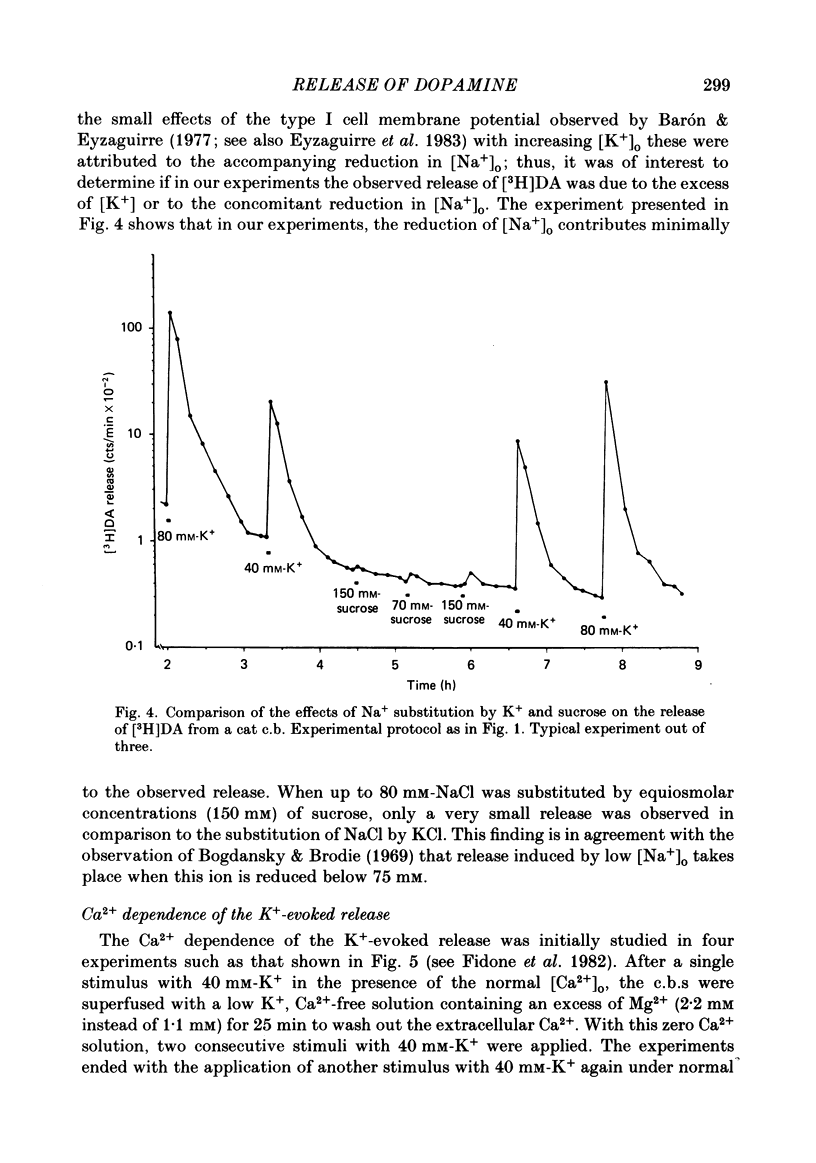
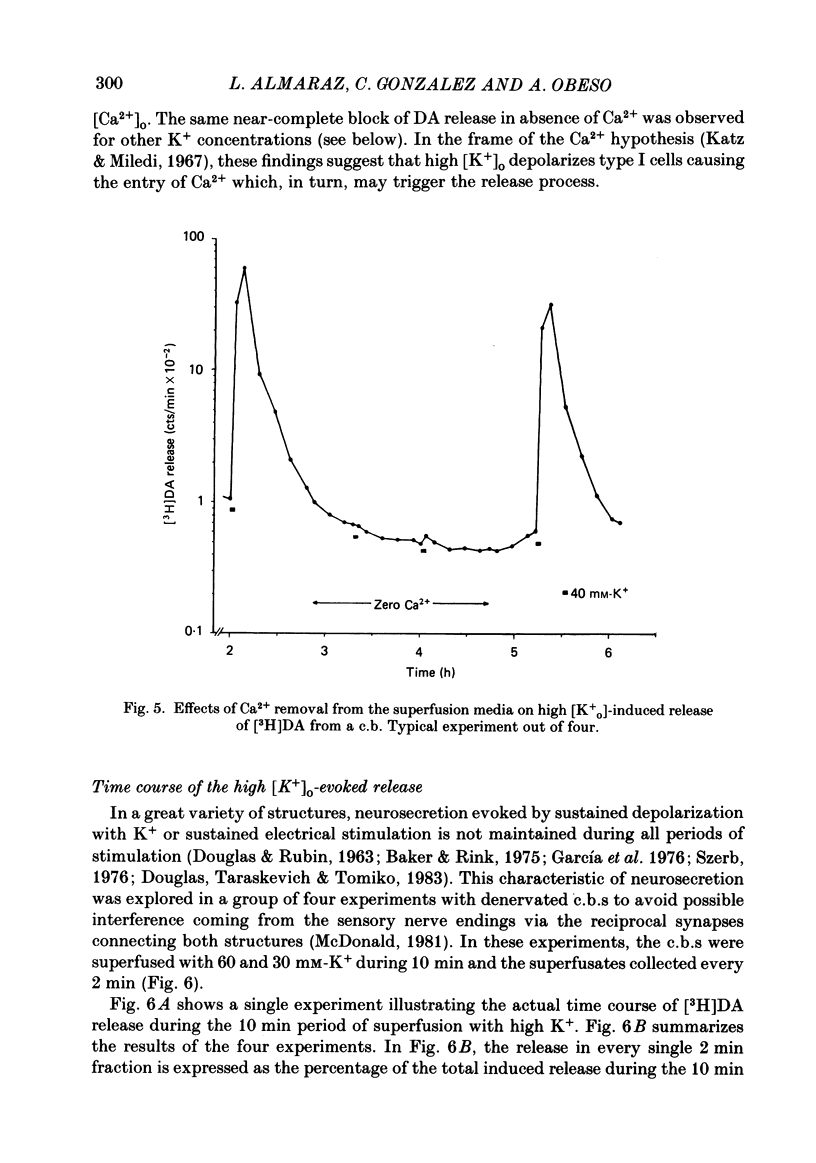
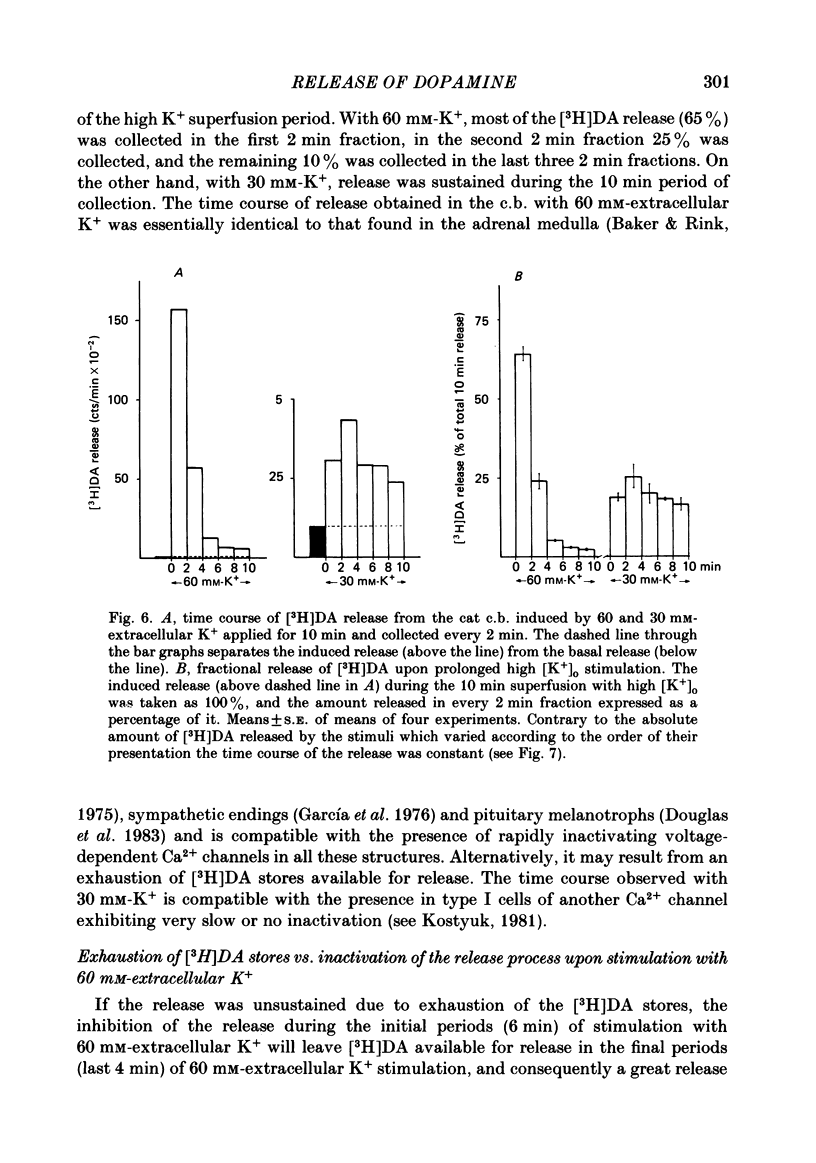
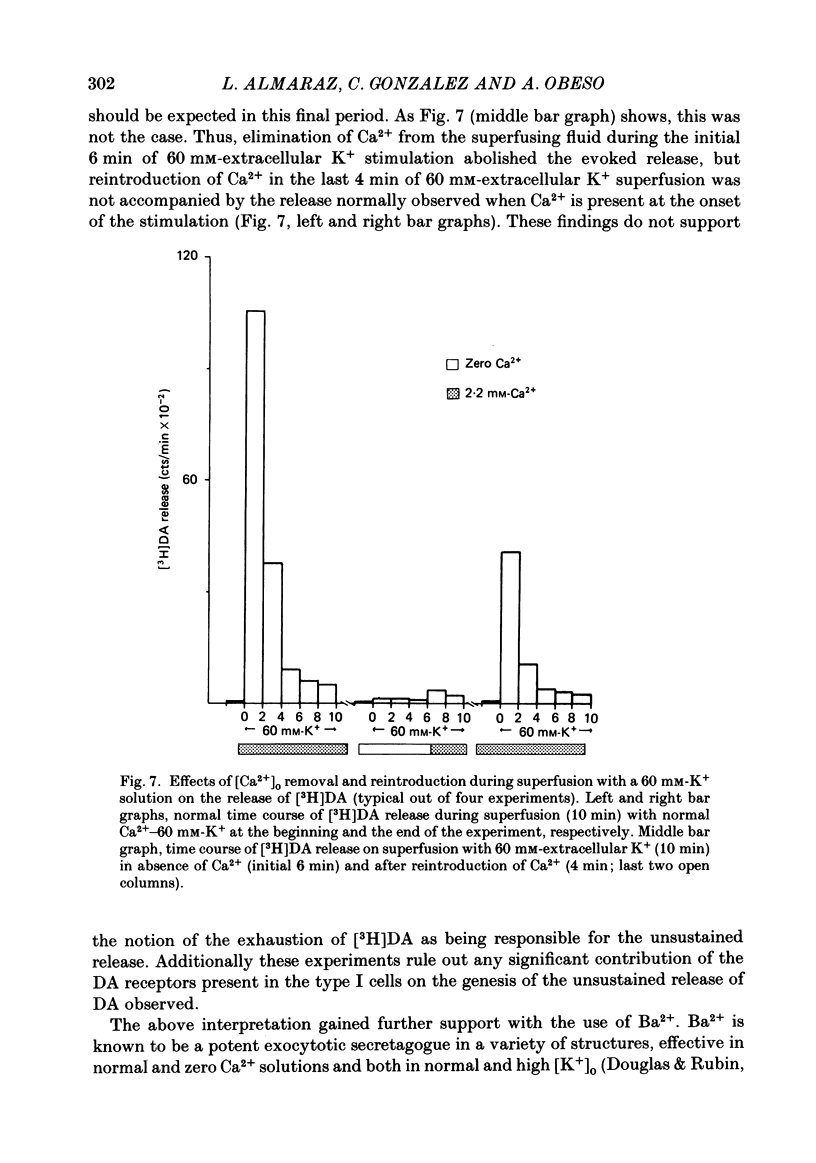
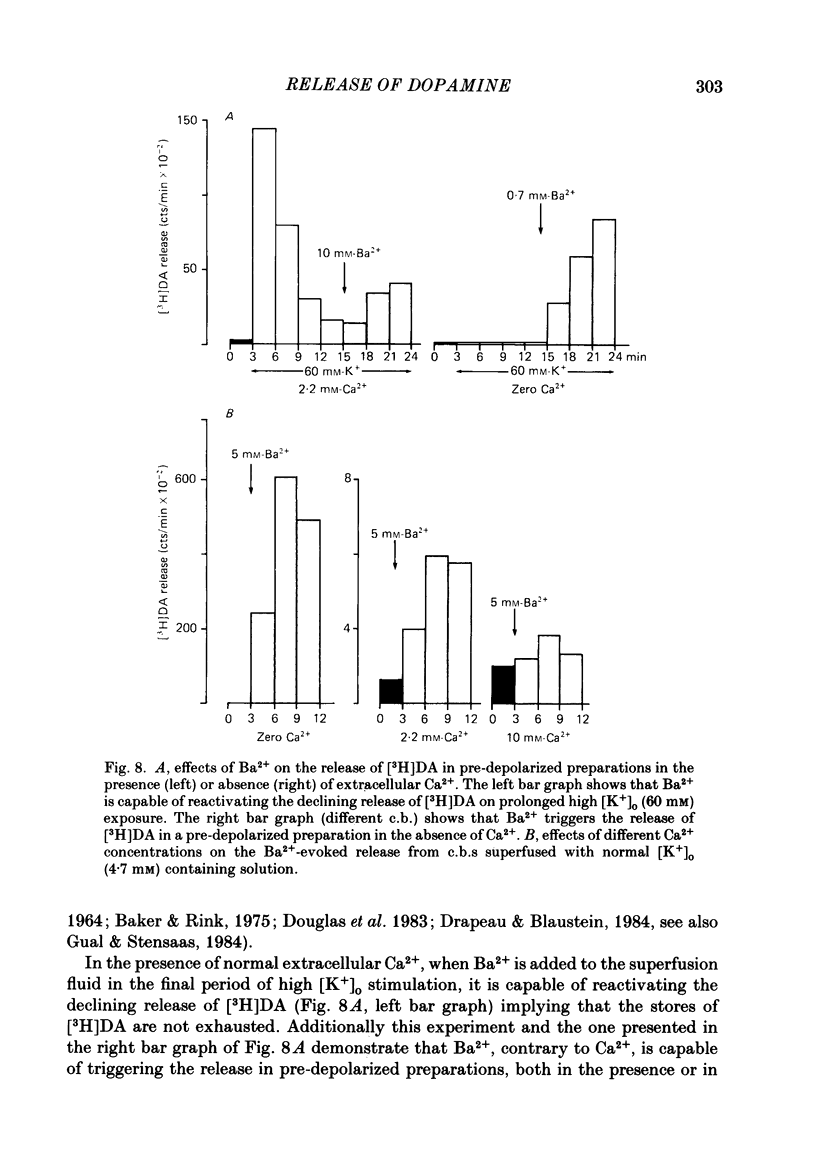
Using an in vitro preparation of the cat carotid body, we have characterized the release of [3H]dopamine (DA) induced by high extracellular K+. Pulse superfusion (3 min) with high K+ Tyrode solution (20-80 mM) evoked a concentration-dependent release of [3H]DA from type I cells with a threshold at about 20 mM-extracellular K+ and a plateau at about 60 mM-extracellular K+. Equivalent low extracellular Na+ concentration ([Na+]o) solutions osmotically balanced with sucrose did not induce release. The high extracellular K+ concentration ([K+]o)-evoked release of [3H]DA by type I cells was dependent on the presence of Ca2+ in the superfusion media. On prolonged (10-14 min) superfusion with high K+ Tyrode solution, the [3H]DA release evoked by 60 mM-extracellular K+ was transient, while that evoked by 30 mM-extracellular K+ was sustained. In preparations superfused for 6 min with 60 mM-extracellular K+ and zero extracellular Ca2+ concentration ([Ca2+]o) Tyrode solution, reintroduction of Ca2+ did not elicit a secretory response. Ba2+ was a potent secretagogue of [3H]DA in preparations superfused with normal and zero [Ca2+]o Tyrode solution. Additionally, Ba2+ was capable of eliciting a secretory response from type I cells in preparations previously exposed (6 min) to 60 mM-extracellular K+, whether or not [Ca2+]o was present. With regards to stimulus-secretion coupling, our results indicate that high [K+]o probably depolarizes type I cells. This effect would, in turn, activate voltage-dependent Ca2+ channels, allowing the entrance of this ion to activate the neurosecretory response.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albus U., Habermann E., Ferry D. R., Glossmann H. Novel 1,4-dihydropyridine (Bay K 8644) facilitates calcium-dependent [3H]noradrenaline release from PC 12 cells. J Neurochem. 1984 Apr;42(4):1186–1189. doi: 10.1111/j.1471-4159.1984.tb12729.x. [DOI] [PubMed] [Google Scholar]
- Baker P. F., Rink T. J. Catecholamine release from bovine adrenal medulla in response to maintained depolarization. J Physiol. 1975 Dec;253(2):593–620. doi: 10.1113/jphysiol.1975.sp011209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer B., Ehinger B., Aberg L. [3H]-dopamine release from the rabbit retina. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1980;215(2):71–78. doi: 10.1007/BF00414464. [DOI] [PubMed] [Google Scholar]
- Blaustein M. P. Effects of potassium, veratridine, and scorpion venom on calcium accumulation and transmitter release by nerve terminals in vitro. J Physiol. 1975 Jun;247(3):617–655. doi: 10.1113/jphysiol.1975.sp010950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein M. P., Goldring J. M. Membrane potentials in pinched-off presynaptic nerve ternimals monitored with a fluorescent probe: evidence that synaptosomes have potassium diffusion potentials. J Physiol. 1975 Jun;247(3):589–615. doi: 10.1113/jphysiol.1975.sp010949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdanski D. F., Brodie B. B. The effects of inorganic ions on the storage and uptake of H3-norepinephrine by rat heart slices. J Pharmacol Exp Ther. 1969 Feb;165(2):181–189. [PubMed] [Google Scholar]
- Chiocchio S. R., Biscardi A. M., Tramezzani J. H. Catecholamines in the carotid body of the cat. Nature. 1966 Nov 19;212(5064):834–835. doi: 10.1038/212834a0. [DOI] [PubMed] [Google Scholar]
- DOUGLAS W. W., RUBIN R. P. STIMULANT ACTION OF BARIUM ON THE ADRENAL MEDULLA. Nature. 1964 Jul 18;203:305–307. doi: 10.1038/203305a0. [DOI] [PubMed] [Google Scholar]
- Dismukes K., de Boer A. A., Mulder A. H. On the mechanism of alpha-receptor mediated modulation of 3H-noradrenaline release from slices of rat brain neocortex. Naunyn Schmiedebergs Arch Pharmacol. 1977 Sep;299(2):115–122. doi: 10.1007/BF00498553. [DOI] [PubMed] [Google Scholar]
- Douglas W. W., Rubin R. P. The mechanism of catecholamine release from the adrenal medulla and the role of calcium in stimulus-secretion coupling. J Physiol. 1963 Jul;167(2):288–310. doi: 10.1113/jphysiol.1963.sp007150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W., Taraskevich P. S., Tomiko S. A. Secretagogue effect of barium on output of melanocyte-stimulating hormone from pars intermedia of the mouse pituitary. J Physiol. 1983 May;338:243–257. doi: 10.1113/jphysiol.1983.sp014671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drapeau P., Blaustein M. P. Initial release of [3H]dopamine from rat striatal synaptosomes: correlation with calcium entry. J Neurosci. 1983 Apr;3(4):703–713. doi: 10.1523/JNEUROSCI.03-04-00703.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyzaguirre C., Koyano H., Taylor J. R. Presence of acetylcholine and transmitter release from carotid body chemoreceptors. J Physiol. 1965 Jun;178(3):463–476. doi: 10.1113/jphysiol.1965.sp007637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming R. M., Clark W. G. Quantitative thin-layer chromatographic estimation of labeled dopamine and norepinephrine, their precursors and metabolites. J Chromatogr. 1970 Oct 21;52(2):305–312. doi: 10.1016/s0021-9673(01)96577-x. [DOI] [PubMed] [Google Scholar]
- Garcia A. G., Kirpekar S. M., Sanchez-Garcia P. Release of noradrenaline from the cat spleen by nerve stimulation and potassium. J Physiol. 1976 Oct;261(2):301–317. doi: 10.1113/jphysiol.1976.sp011560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman N. W., McCloskey D. I. Intracellular potentials in the carotid body. Brain Res. 1972 Apr 28;39(2):501–504. doi: 10.1016/0006-8993(72)90452-0. [DOI] [PubMed] [Google Scholar]
- Grönblad M., Akerman K. E., Eränkö O. Electron-dense precipitates in glomus cells of rat carotid body after fixation in glutaraldehyde and pyroantimonate-osmium tetroxide mixture as possible indicators of calcium localization. Cell Tissue Res. 1981;217(1):93–104. doi: 10.1007/BF00233829. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Byerly L. Calcium channel. Annu Rev Neurosci. 1981;4:69–125. doi: 10.1146/annurev.ne.04.030181.000441. [DOI] [PubMed] [Google Scholar]
- Hanbauer I., Hellstrom S. The regulation of dopamine and noradrenaline in the rat carotid body and its modification by denervation and by hypoxia. J Physiol. 1978 Sep;282:21–34. doi: 10.1113/jphysiol.1978.sp012445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashida Y., Eyzaguirre C. Voltage noise of carotid body type I cells. Brain Res. 1979 May 5;167(1):189–194. doi: 10.1016/0006-8993(79)90277-4. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. A study of synaptic transmission in the absence of nerve impulses. J Physiol. 1967 Sep;192(2):407–436. doi: 10.1113/jphysiol.1967.sp008307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo H., Iwanaga T., Nakajima T. Immunocytochemical study on the localization of neuron-specific enolase and S-100 protein in the carotid body of rats. Cell Tissue Res. 1982;227(2):291–295. doi: 10.1007/BF00210887. [DOI] [PubMed] [Google Scholar]
- Kostyuk P. G. Calcium channels in the neuronal membrane. Biochim Biophys Acta. 1981 Dec;650(2-3):128–150. doi: 10.1016/0304-4157(81)90003-4. [DOI] [PubMed] [Google Scholar]
- LEVER J. D., BOYD J. D. Osmiophile granules in the glomus cells of the rabbit carotid body. Nature. 1957 May 25;179(4569):1082–1083. doi: 10.1038/1791082b0. [DOI] [PubMed] [Google Scholar]
- Nachshen D. A., Blaustein M. P. Some properties of potassium-stimulated calcium influx in presynaptic nerve endings. J Gen Physiol. 1980 Dec;76(6):709–728. doi: 10.1085/jgp.76.6.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeso A., Almaraz L., Gonzalez C. Correlation between adenosine triphosphate levels, dopamine release and electrical activity in the carotid body: support for the metabolic hypothesis of chemoreception. Brain Res. 1985 Nov 25;348(1):64–68. doi: 10.1016/0006-8993(85)90360-9. [DOI] [PubMed] [Google Scholar]
- Rigual R., Gonzalez E., Fidone S., Gonzalez C. Effects of low pH on synthesis and release of catecholamines in the cat carotid body in vitro. Brain Res. 1984 Aug 20;309(1):178–181. doi: 10.1016/0006-8993(84)91026-6. [DOI] [PubMed] [Google Scholar]
- Szerb J. C., Somogyi G. T. Depression of acetylcholine release from cerebral cortical slices by cholinesterase inhibition and by oxotremorine. Nat New Biol. 1973 Jan 24;241(108):121–122. doi: 10.1038/newbio241121a0. [DOI] [PubMed] [Google Scholar]
- Szerb J. C. Storage and release of labelled acetylcholine in the myenteric plexus of the guinea-pig ileum. Can J Physiol Pharmacol. 1976 Feb;54(1):12–22. doi: 10.1139/y76-003. [DOI] [PubMed] [Google Scholar]
- Vargas O., Orrego F. Elevated extracellular potassium as a stimulus for releasing [EH] norepinephrine and [14C] alpha-amino isobutyrate from neocortical slice. Specificity and calcium dependency of the process. J Neurochem. 1976 Jan;26(1):31–34. doi: 10.1111/j.1471-4159.1976.tb04431.x. [DOI] [PubMed] [Google Scholar]
- Verna A. Ulstrastructure of the carotid body in the mammals. Int Rev Cytol. 1979;60:271–330. doi: 10.1016/s0074-7696(08)61265-6. [DOI] [PubMed] [Google Scholar]
- Weil-Malherbe H. The chemical estimation of catecholamines and their metabolites in body fluids and tissue extracts. Methods Biochem Anal. 1971;(Suppl):119–152. doi: 10.1002/9780470110409.ch5. [DOI] [PubMed] [Google Scholar]