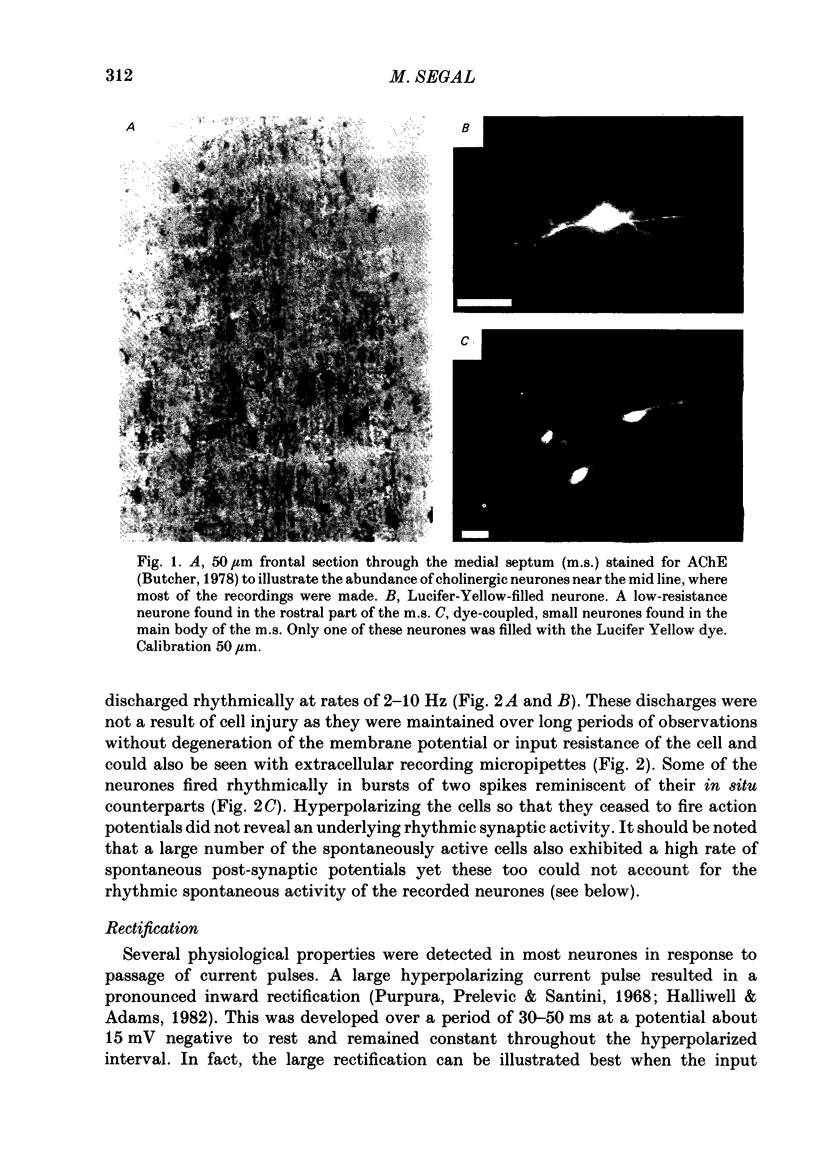
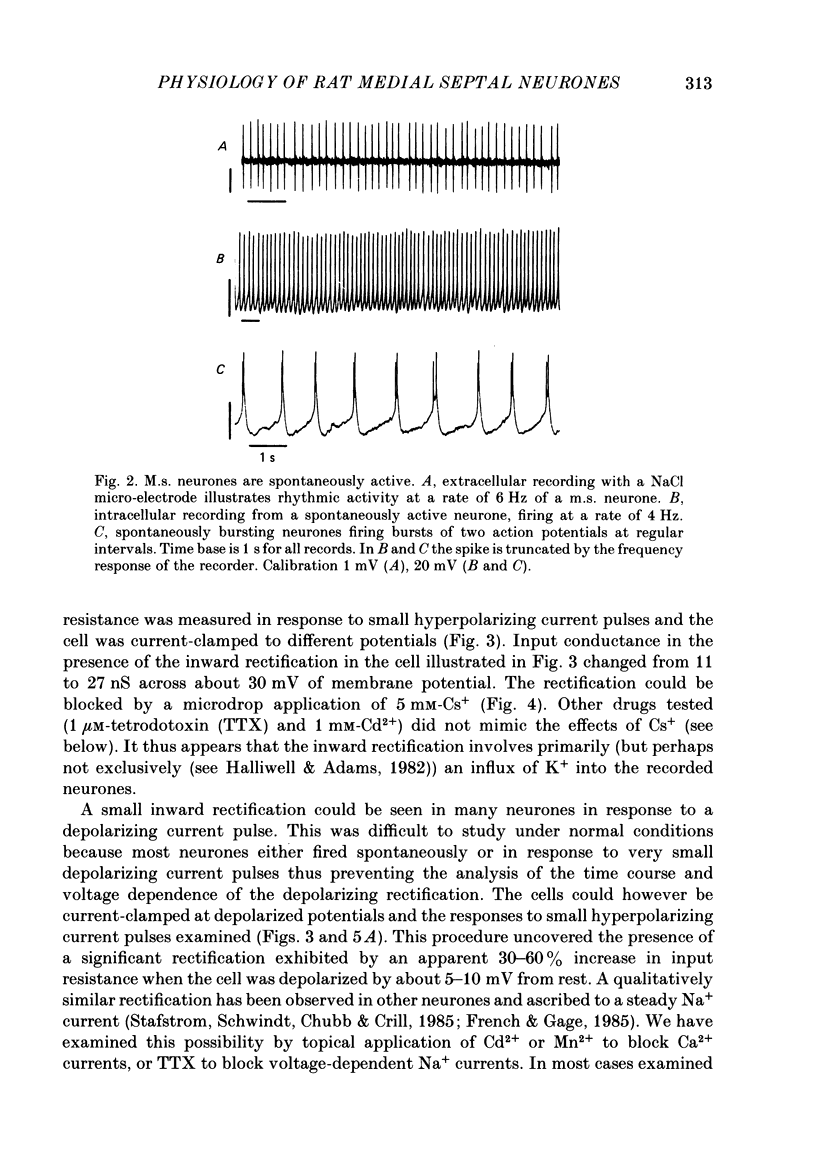
Abstract
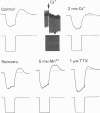
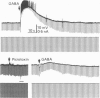
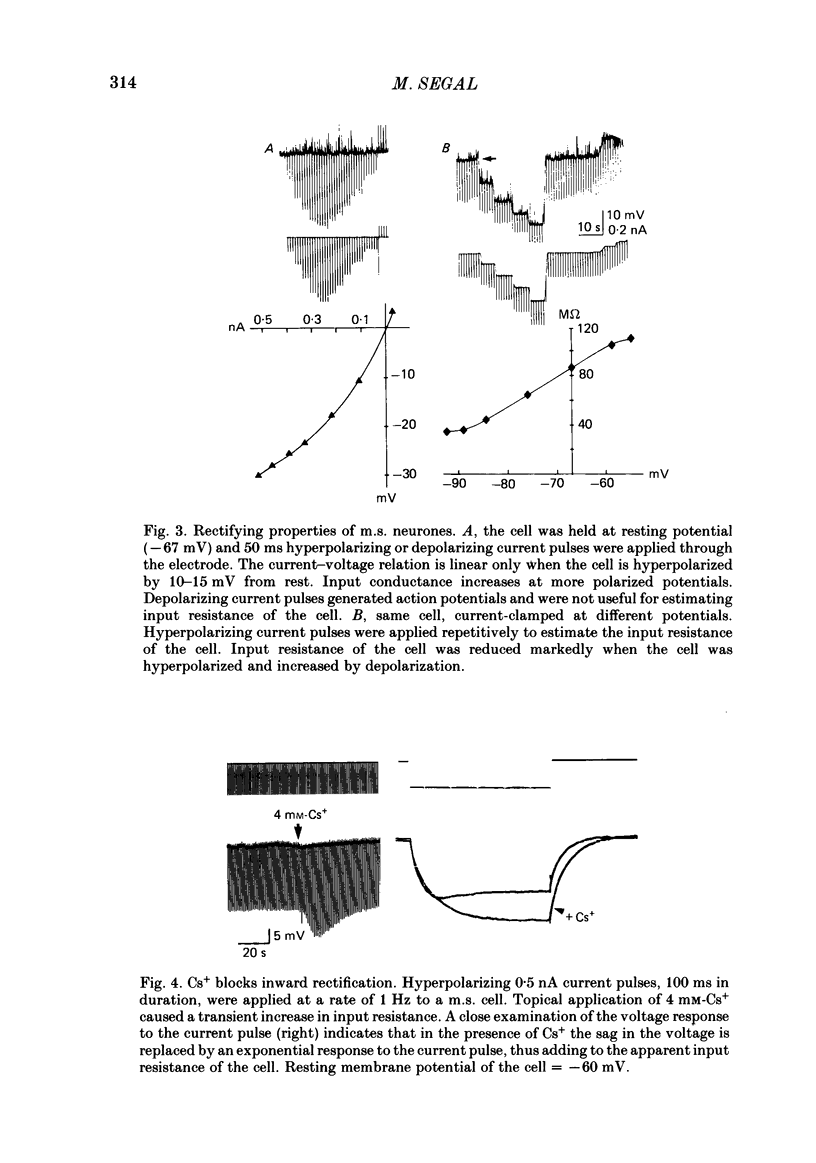
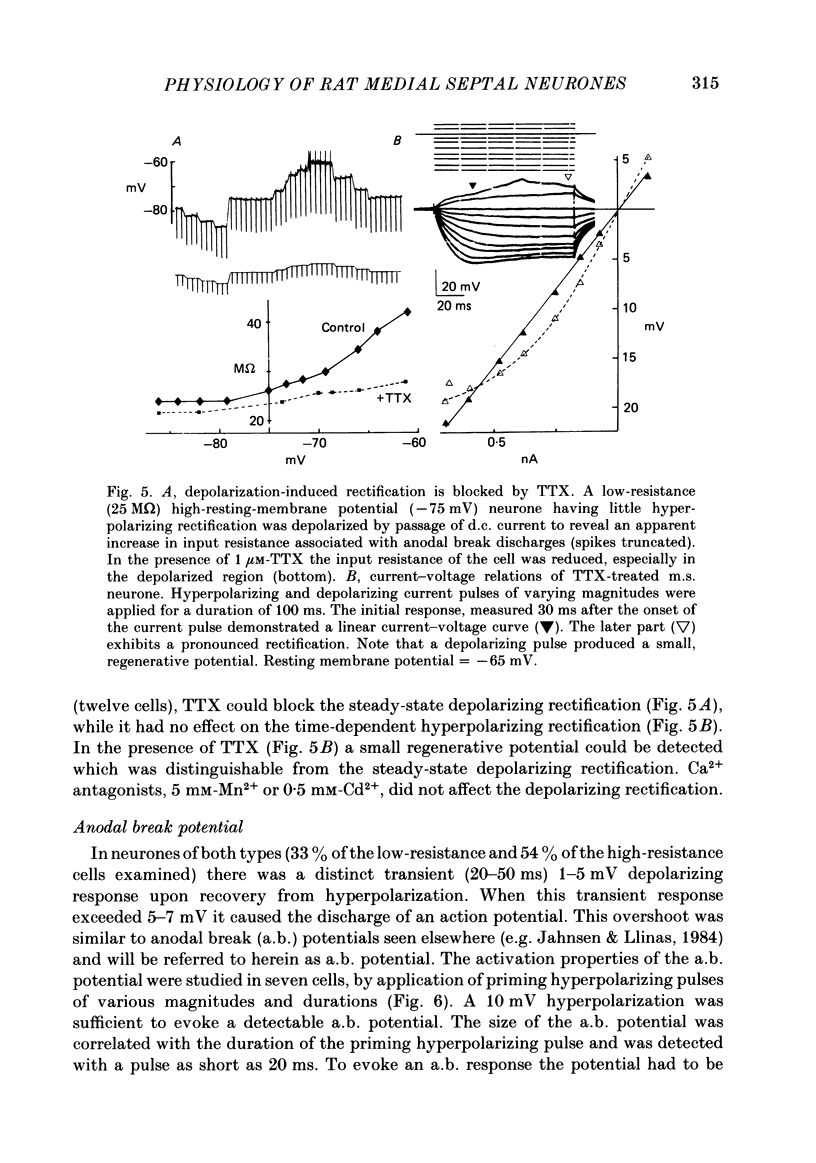
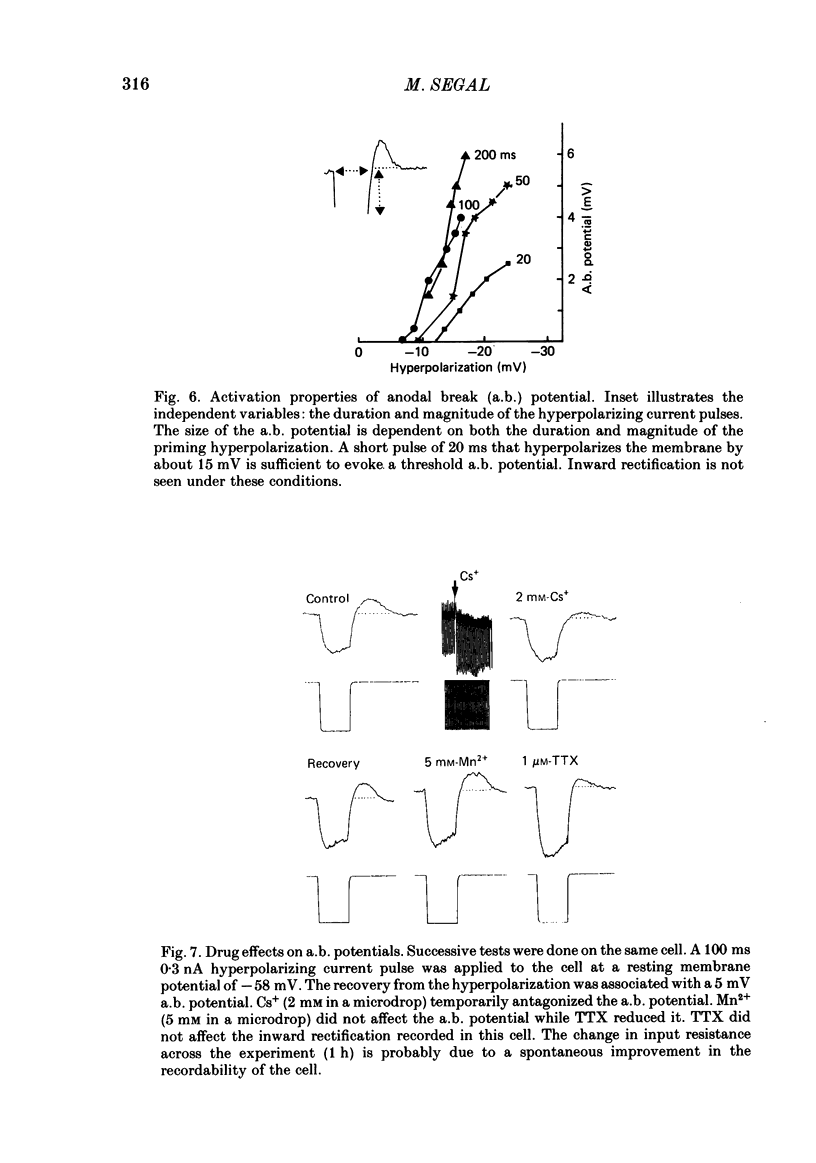
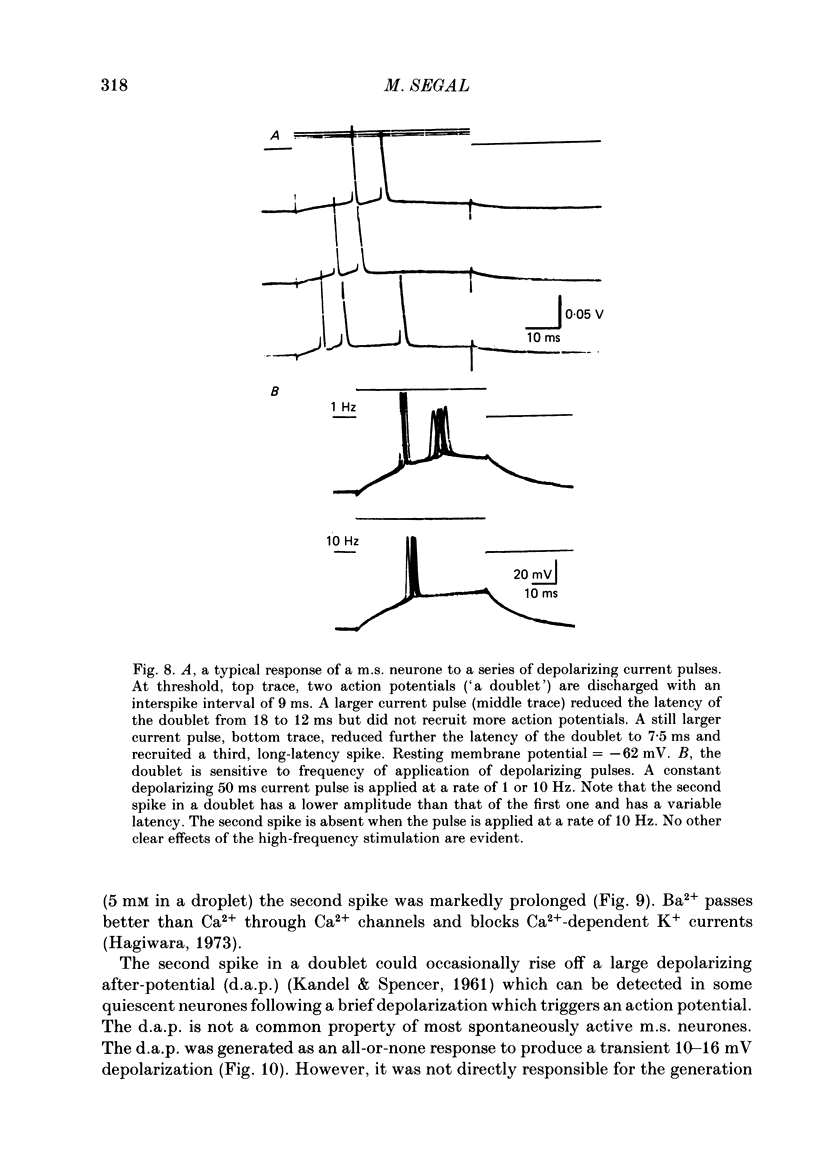
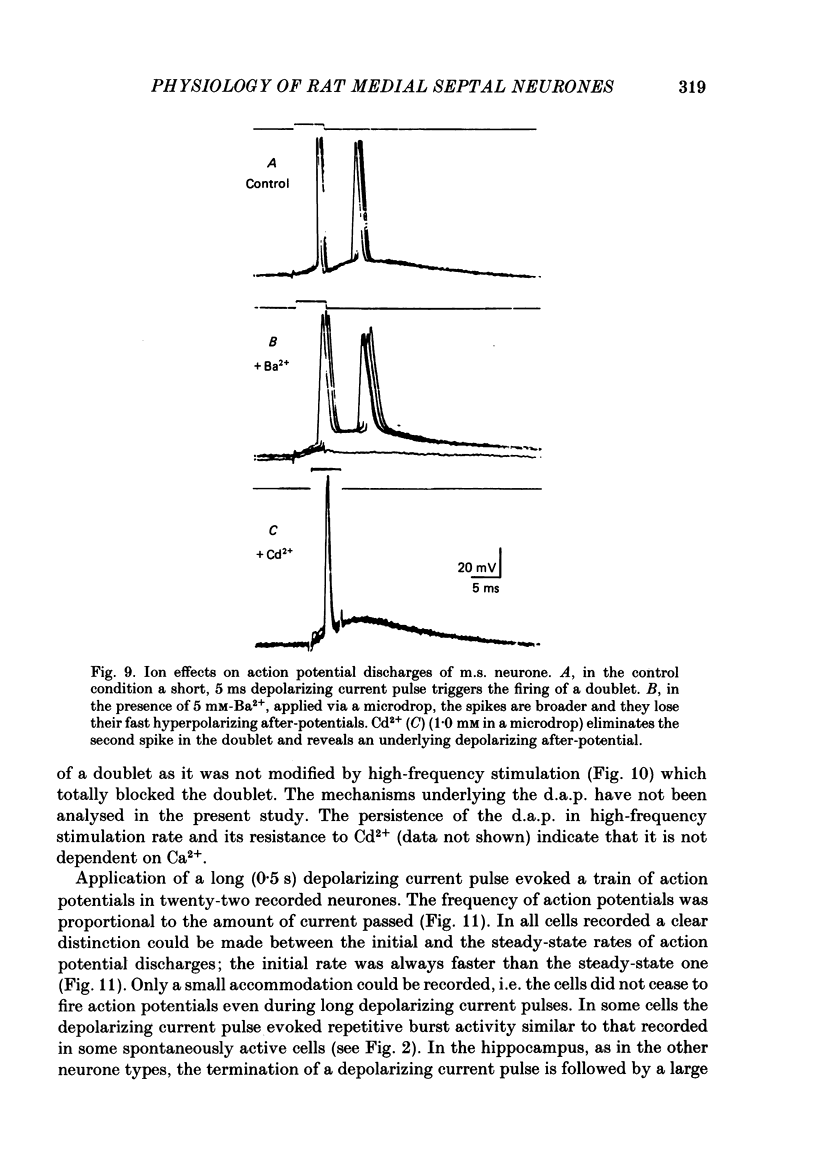
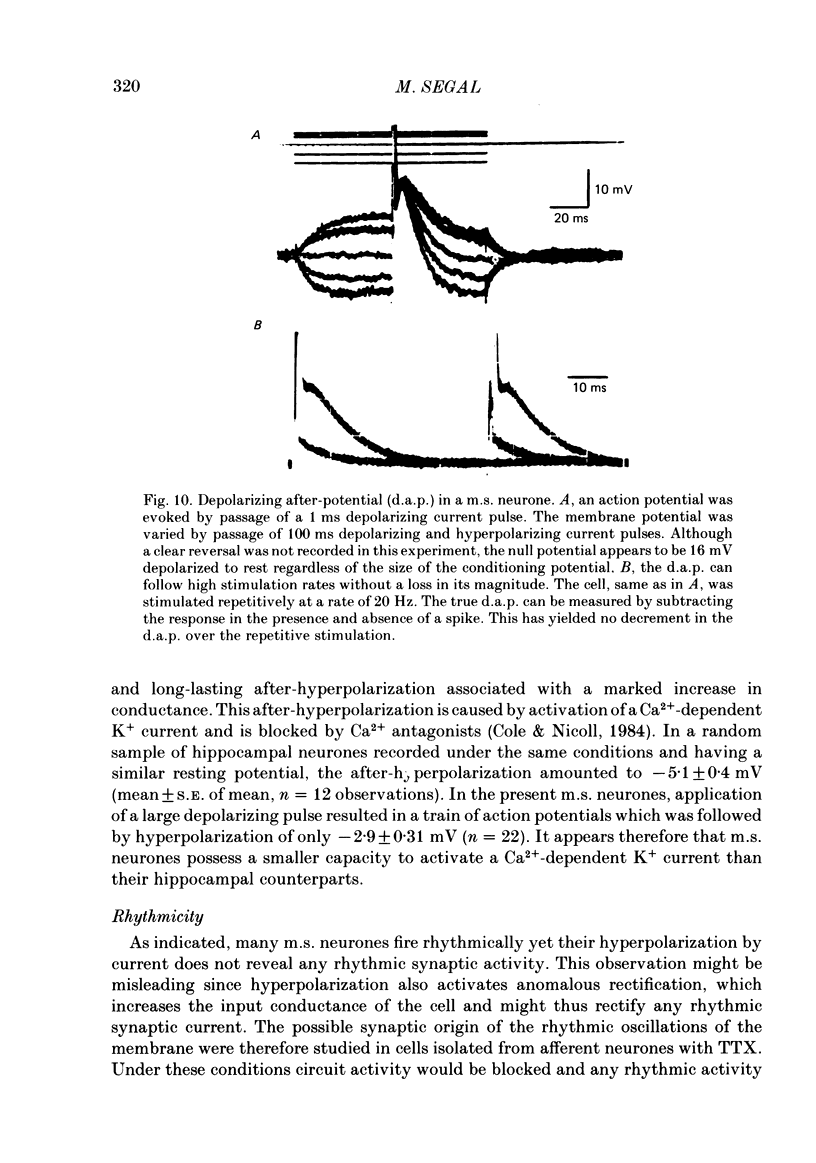
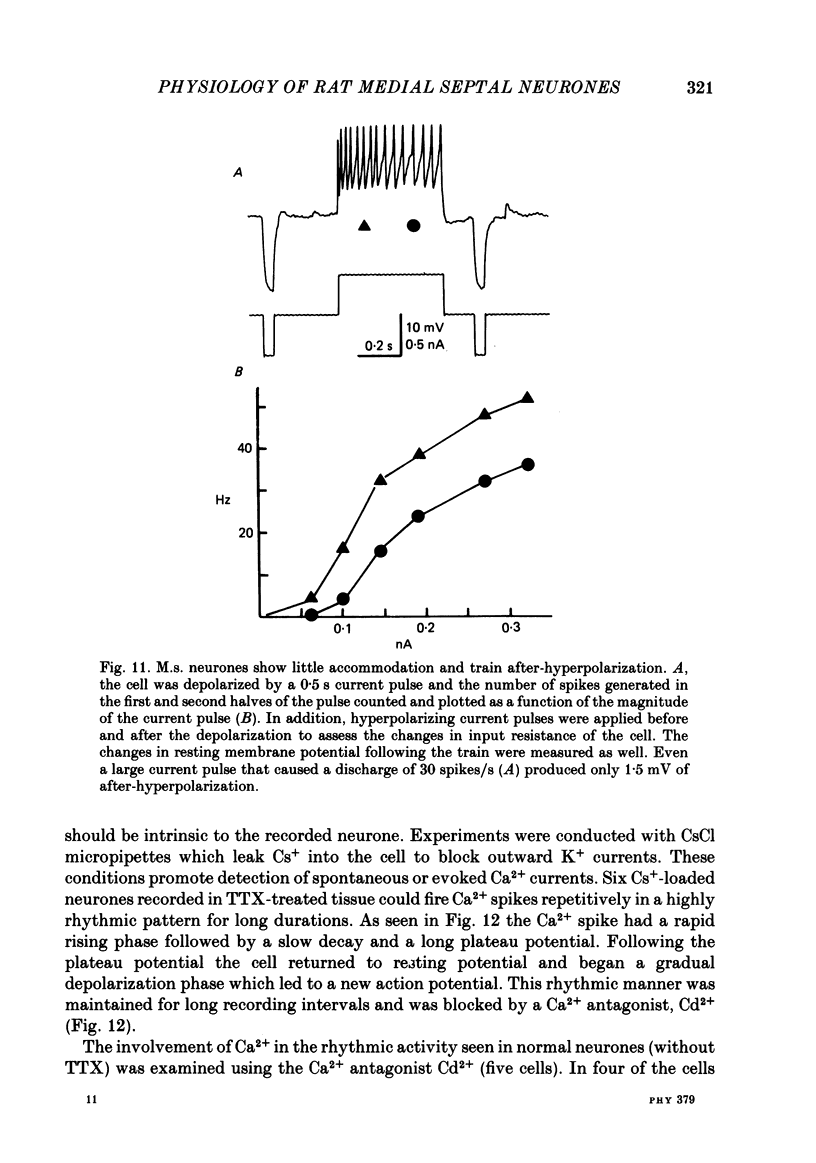
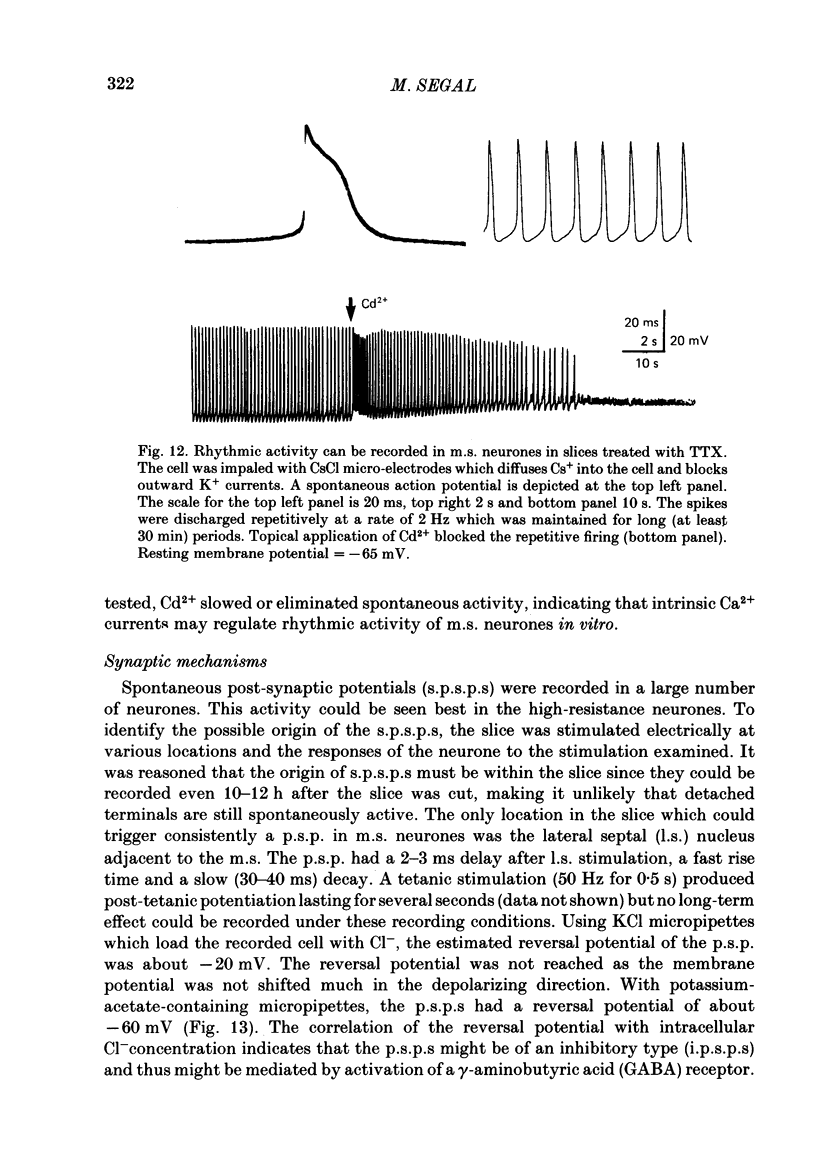
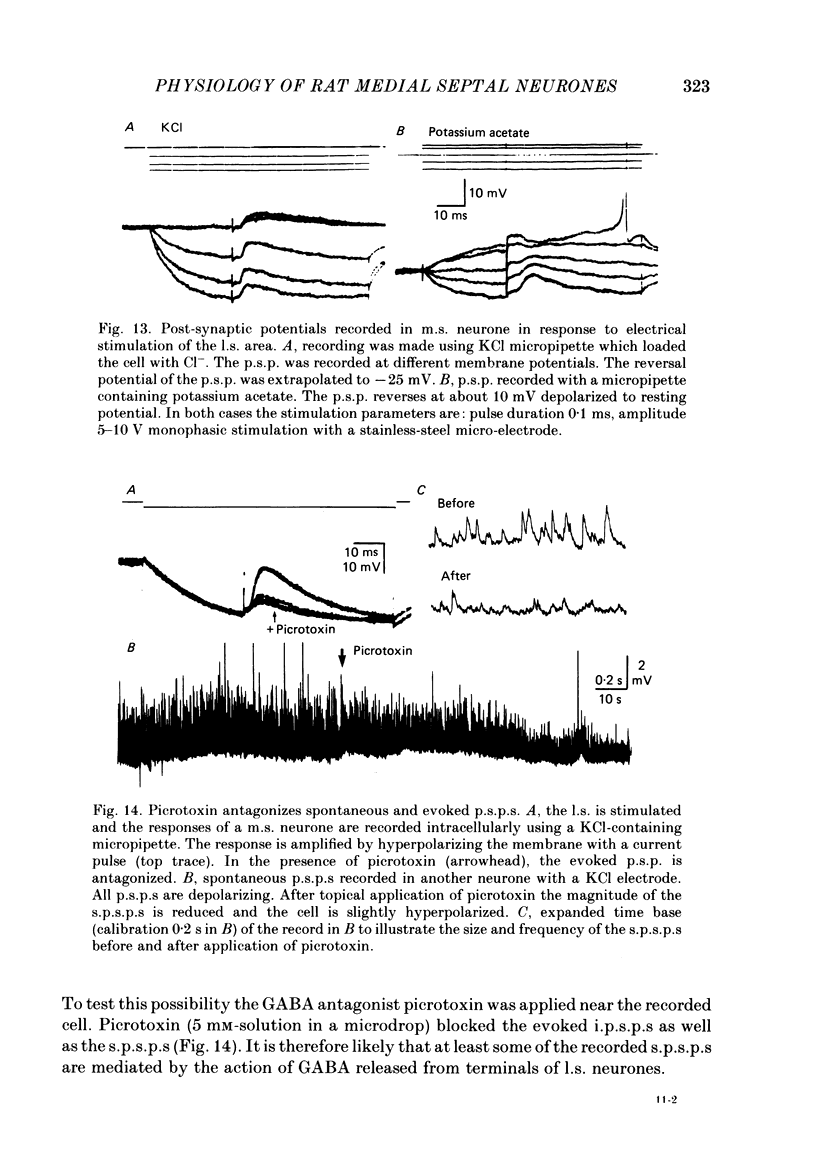
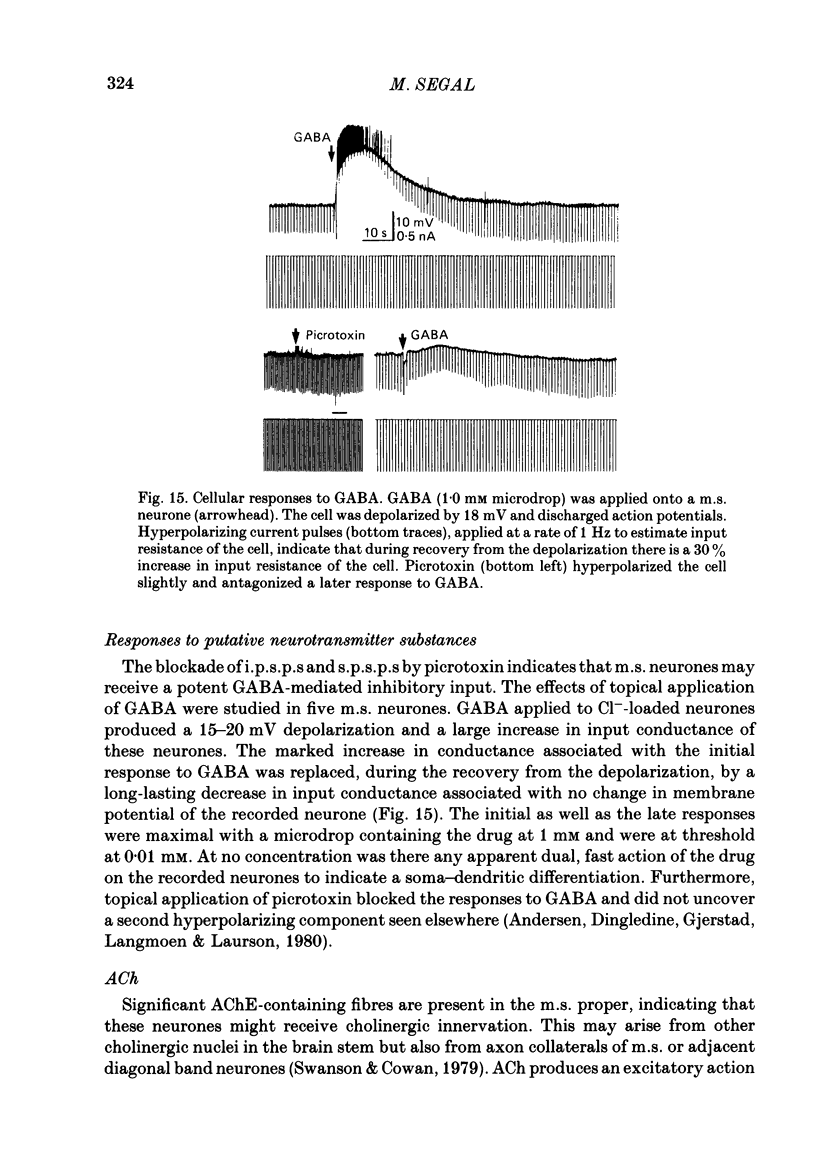
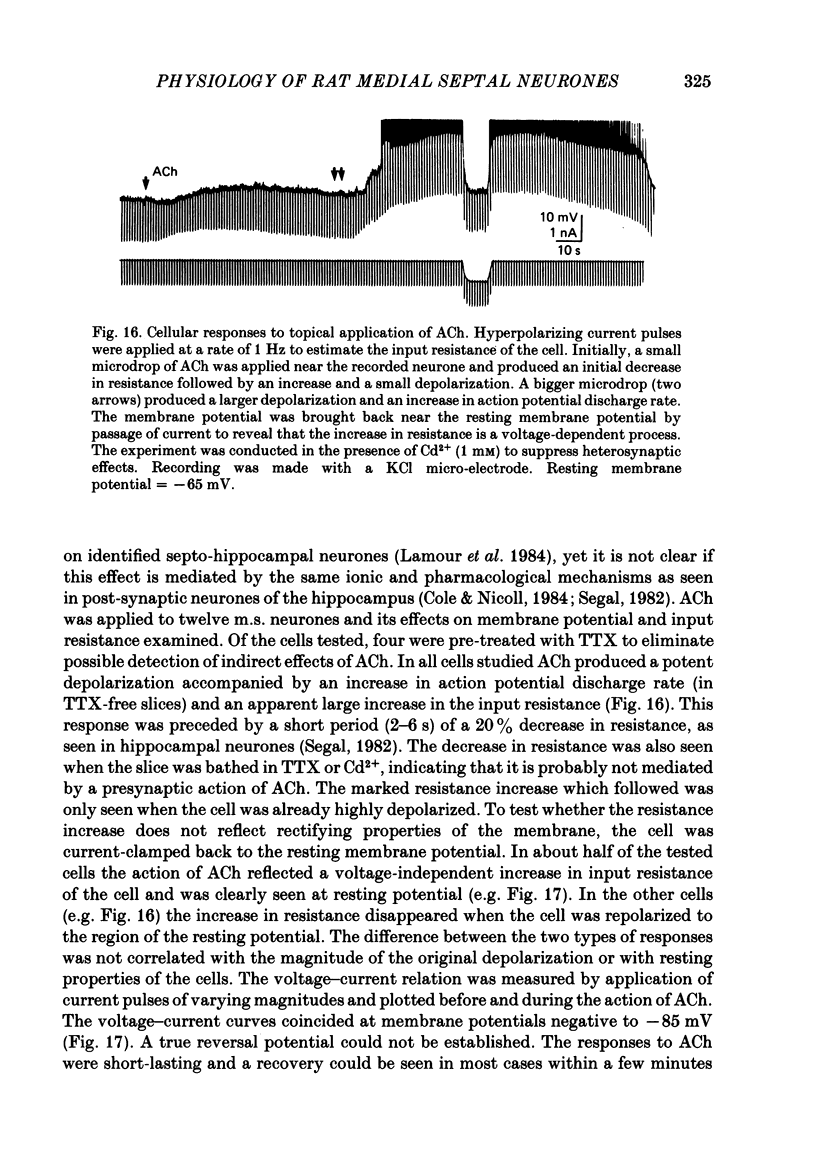
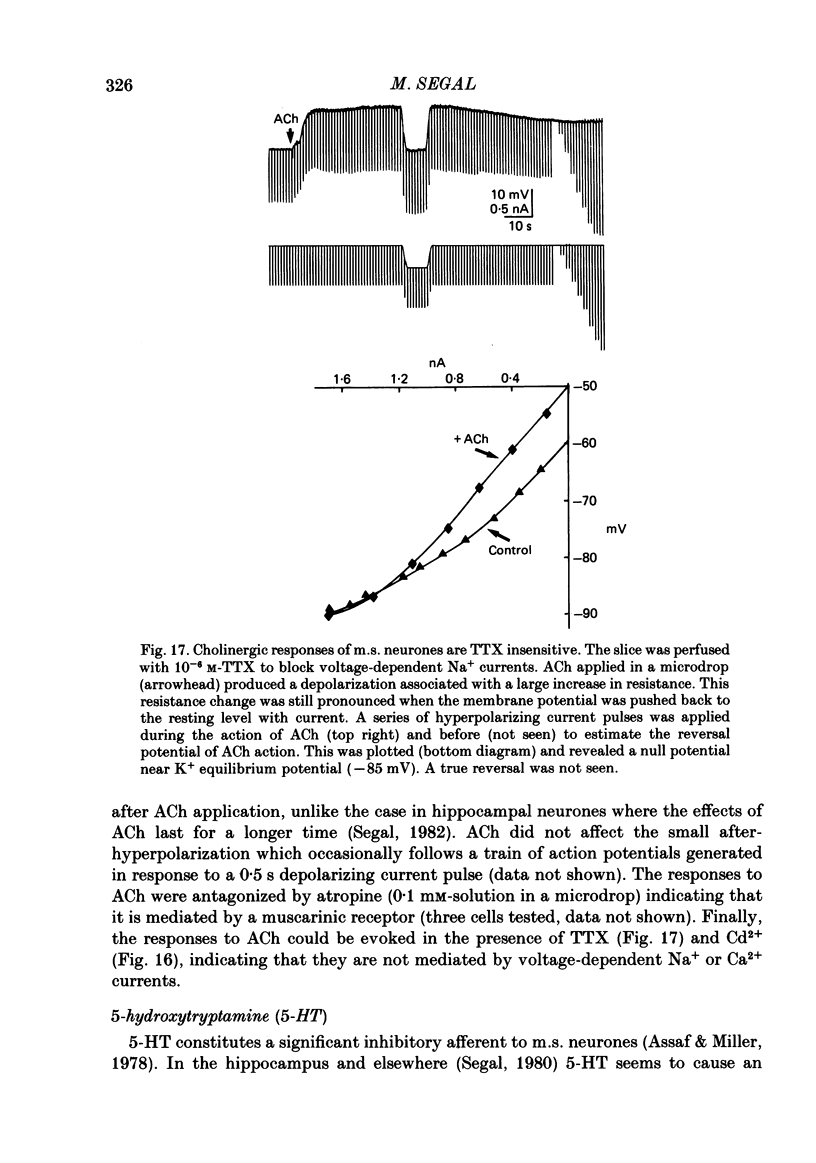
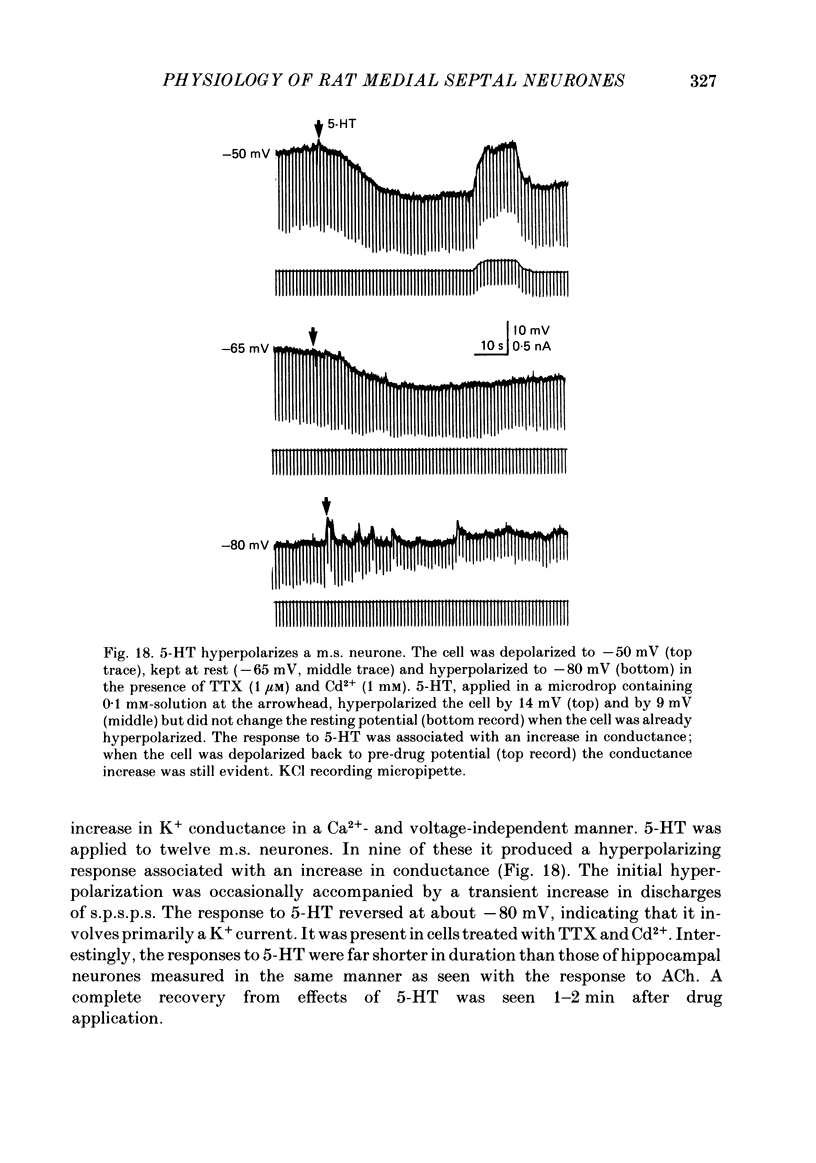
Activity of neurones of the rat medial septal nucleus (m.s.) was recorded in in vitro slice preparations. The recorded population could be divided into low (less than 30 M omega)- and high-input-resistance (greater than 30 M omega) neurones. The high-resistance neurones tended to fire spontaneous action potentials and post-synaptic potentials. Some of the spontaneously active cells fired rhythmically at rates of 2-10 Hz. The rhythmicity disappeared following hyperpolarization of the recorded cell. The cells could fire repetitive Ca2+ spikes in the presence of tetrodotoxin (TTX) and intracellular Cs+. Cd2+ blocked this rhythmicity. Most of the m.s. cells had a non-linear voltage-current relation in both the hyperpolarizing and depolarizing directions. Hyperpolarizing rectification was selectively blocked by extracellular Cs+ whereas depolarizing rectification could be blocked by TTX. A recovery from hyperpolarization was associated in many cells with a transient depolarization (anodal break (a.b.) potential). A 20 ms 15 mV hyperpolarization could trigger an a.b. potential. The a.b. potential was reduced by TTX and Cs+ but not by Cd2+ or Mn2+. Depolarization of quiescent neurones triggered action potential discharges. A common pattern of discharge was a burst of two spikes which kept a fairly constant interspike interval. The second spike in a doublet could not follow a rate of 10 Hz depolarizing current pulses. It was also sensitive to topical application of Cd2+. It is therefore suggested that Ca2+ might be involved in the generation of the doublet. Long depolarizing current pulses produced trains of action potentials, showing little accommodation and little after-hyperpolarization, indicating that these cells possess little Ca2+-dependent K+ current. Many cells emitted spontaneous post-synaptic potentials at high rates. These could be blocked by picrotoxin. Stimulation of the lateral septal (l.s.) nucleus produced a Cl-dependent i.p.s.p. The i.p.s.p. was blocked by picrotoxin. Topical application of gamma-aminobutyric acid (GABA) produced a marked Cl(-)-dependent increase in conductance. It is suggested that l.s. projects a GABA-mediated inhibitory connexion to the m.s. Acetylcholine (ACh) depolarized m.s. neurones and caused an increase in input resistance. The response was present in TTX or Cd2+-containing medium. Atropine blocked responses to ACh. 5-Hydroxytryptamine (5-HT) hyperpolarized m.s. neurones in a manner consistent with an increase in K+ conductance. The effects of 5-HT were seen in TTX- and Cd2+-treated m.s. slices.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen P., Dingledine R., Gjerstad L., Langmoen I. A., Laursen A. M. Two different responses of hippocampal pyramidal cells to application of gamma-amino butyric acid. J Physiol. 1980 Aug;305:279–296. doi: 10.1113/jphysiol.1980.sp013363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostol G., Creutzfeldt O. D. Crosscorrelation between the activity of septal units and hippocampal EEG during arousal. Brain Res. 1974 Feb 15;67(1):65–75. doi: 10.1016/0006-8993(74)90298-4. [DOI] [PubMed] [Google Scholar]
- Assaf S. Y., Miller J. J. The role of a raphe serotonin system in the control of septal unit activity and hippocampal desynchronization. Neuroscience. 1978;3(6):539–550. doi: 10.1016/0306-4522(78)90018-0. [DOI] [PubMed] [Google Scholar]
- Baisden R. H., Woodruff M. L., Hoover D. B. Cholinergic and non-cholinergic septo-hippocampal projections: a double-label horseradish peroxidase-acetylcholinesterase study in the rabbit. Brain Res. 1984 Jan 2;290(1):146–151. doi: 10.1016/0006-8993(84)90745-5. [DOI] [PubMed] [Google Scholar]
- Cole A. E., Nicoll R. A. Characterization of a slow cholinergic post-synaptic potential recorded in vitro from rat hippocampal pyramidal cells. J Physiol. 1984 Jul;352:173–188. doi: 10.1113/jphysiol.1984.sp015285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J. A., Stevens C. F. Voltage clamp studies of a transient outward membrane current in gastropod neural somata. J Physiol. 1971 Feb;213(1):21–30. doi: 10.1113/jphysiol.1971.sp009365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutar P., Lamour Y., Jobert A. Septohippocampal neurons in the rat: an in vivo intracellular study. Brain Res. 1985 Aug 5;340(1):135–142. doi: 10.1016/0006-8993(85)90782-6. [DOI] [PubMed] [Google Scholar]
- French C. R., Gage P. W. A threshold sodium current in pyramidal cells in rat hippocampus. Neurosci Lett. 1985 May 23;56(3):289–293. doi: 10.1016/0304-3940(85)90257-5. [DOI] [PubMed] [Google Scholar]
- Gogolák G., Stumpf C., Petsche H., Sterc J. The firing pattern of septal neurons and the form of the hippocampal theta wave. Brain Res. 1968 Feb;7(2):201–207. doi: 10.1016/0006-8993(68)90098-x. [DOI] [PubMed] [Google Scholar]
- Gray J. A., McNaughton N. Comparison between the behavioural effects of septal and hippocampal lesions: a review. Neurosci Biobehav Rev. 1983 Summer;7(2):119–188. doi: 10.1016/0149-7634(83)90014-3. [DOI] [PubMed] [Google Scholar]
- Hagiwara S. Ca spike. Adv Biophys. 1973;4:71–102. [PubMed] [Google Scholar]
- Halliwell J. V., Adams P. R. Voltage-clamp analysis of muscarinic excitation in hippocampal neurons. Brain Res. 1982 Oct 28;250(1):71–92. doi: 10.1016/0006-8993(82)90954-4. [DOI] [PubMed] [Google Scholar]
- Jahnsen H., Llinás R. Ionic basis for the electro-responsiveness and oscillatory properties of guinea-pig thalamic neurones in vitro. J Physiol. 1984 Apr;349:227–247. doi: 10.1113/jphysiol.1984.sp015154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KANDEL E. R., SPENCER W. A. Electrophysiology of hippocampal neurons. II. After-potentials and repetitive firing. J Neurophysiol. 1961 May;24:243–259. doi: 10.1152/jn.1961.24.3.243. [DOI] [PubMed] [Google Scholar]
- Lamour Y., Dutar P., Jobert A. Septo-hippocampal and other medial septum-diagonal band neurons: electrophysiological and pharmacological properties. Brain Res. 1984 Sep 10;309(2):227–239. doi: 10.1016/0006-8993(84)90588-2. [DOI] [PubMed] [Google Scholar]
- Llinás R., Yarom Y. Electrophysiology of mammalian inferior olivary neurones in vitro. Different types of voltage-dependent ionic conductances. J Physiol. 1981 Jun;315:549–567. doi: 10.1113/jphysiol.1981.sp013763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macadar O., Roig J. A., Monti J. M., Budelli R. The functional relationship between septal and hippocampal unit activity and hippocampal theta rhythm. Physiol Behav. 1970 Dec;5(12):1443–1449. doi: 10.1016/0031-9384(70)90134-4. [DOI] [PubMed] [Google Scholar]
- McLennan H., Miller J. J. The hippocampal control of neuronal discharges in the septum of the rat. J Physiol. 1974 Mar;237(3):607–624. doi: 10.1113/jphysiol.1974.sp010500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETSCHE H., STUMPF C., GOGOLAK G. [The significance of the rabbit's septum as a relay station between the midbrain and the hippocampus. I. The control of hippocampus arousal activity by the septum cells]. Electroencephalogr Clin Neurophysiol. 1962 Apr;14:202–211. doi: 10.1016/0013-4694(62)90030-5. [DOI] [PubMed] [Google Scholar]
- Purpura D. P., Prelevic S., Santini M. Hyperpolarizing increase in membrane conductance in hippocampal neurons. Brain Res. 1968 Feb;7(2):310–312. doi: 10.1016/0006-8993(68)90109-1. [DOI] [PubMed] [Google Scholar]
- Segal M. A potent transient outward current regulates excitability of dorsal raphe neurons. Brain Res. 1985 Dec 16;359(1-2):347–350. doi: 10.1016/0006-8993(85)91448-9. [DOI] [PubMed] [Google Scholar]
- Segal M. Brain stem afferents to the rat medial septum. J Physiol. 1976 Oct;261(3):617–631. doi: 10.1113/jphysiol.1976.sp011577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal M. Flow of conditioned responses in limbic telencephalic system of the rat. J Neurophysiol. 1973 Sep;36(5):840–854. doi: 10.1152/jn.1973.36.5.840. [DOI] [PubMed] [Google Scholar]
- Segal M. Multiple action of acetylcholine at a muscarinic receptor studied in the rat hippocampal slice. Brain Res. 1982 Aug 19;246(1):77–87. doi: 10.1016/0006-8993(82)90144-5. [DOI] [PubMed] [Google Scholar]
- Segal M. The action of serotonin in the rat hippocampal slice preparation. J Physiol. 1980 Jun;303:423–439. doi: 10.1113/jphysiol.1980.sp013297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafstrom C. E., Schwindt P. C., Chubb M. C., Crill W. E. Properties of persistent sodium conductance and calcium conductance of layer V neurons from cat sensorimotor cortex in vitro. J Neurophysiol. 1985 Jan;53(1):153–170. doi: 10.1152/jn.1985.53.1.153. [DOI] [PubMed] [Google Scholar]
- Swanson L. W., Cowan W. M. The connections of the septal region in the rat. J Comp Neurol. 1979 Aug 15;186(4):621–655. doi: 10.1002/cne.901860408. [DOI] [PubMed] [Google Scholar]
- Vandermaelen C. P., Aghajanian G. K. Electrophysiological and pharmacological characterization of serotonergic dorsal raphe neurons recorded extracellularly and intracellularly in rat brain slices. Brain Res. 1983 Dec 19;289(1-2):109–119. doi: 10.1016/0006-8993(83)90011-2. [DOI] [PubMed] [Google Scholar]
- Vinogradova O. S., Brazhnik E. S., Karanov A. M., Zhadina S. D. Neuronal activity of the septum following various types of deafferentation. Brain Res. 1980 Apr 14;187(2):353–368. doi: 10.1016/0006-8993(80)90208-5. [DOI] [PubMed] [Google Scholar]
- Wainer B. H., Levey A. I., Rye D. B., Mesulam M. M., Mufson E. J. Cholinergic and non-cholinergic septohippocampal pathways. Neurosci Lett. 1985 Feb 28;54(1):45–52. doi: 10.1016/s0304-3940(85)80116-6. [DOI] [PubMed] [Google Scholar]
- Wong R. K., Prince D. A. Participation of calcium spikes during intrinsic burst firing in hippocampal neurons. Brain Res. 1978 Dec 29;159(2):385–390. doi: 10.1016/0006-8993(78)90544-9. [DOI] [PubMed] [Google Scholar]