Abstract
The BfuABC family is a diverse group of electron bifurcating enzymes that play key roles in anaerobic microbial metabolism. Previous studies have focused almost exclusively on the BfuABC-type hydrogenases but the mechanism and site of electron bifurcation remain unknown. Herein we focus on the Caldicellulosiruptor saccharolyticus (Csac) NfnABC-type Bfu enzyme that catalyzes the oxidation of NADPH and simultaneous reduction of NAD and the redox protein ferredoxin (Fd). Cryo-EM structures determined with and without NAD and Fd reveal seven FeS clusters and one FAD in NfnA, one FeS cluster in NfnC, and three FeS clusters, two Zn ions, and one FMN in NfnB. The Zn ions take the place of FeS clusters previously proposed in other Bfu family members. Csac Nfn for the first time defines the minimum bifurcation site as a flavobicluster consisting of FMN, a [4Fe-4S] (B1) cluster and a [2Fe-2S] (C1) cluster. Binding of NAD to the FMN triggers a series of conformational changes, crucial to the bifurcation of two electron pairs derived from NADPH by the [B1-FMN-C1] flavobicluster into low and high potential electrons that reduce Fd and NAD, respectively. The structures lay the foundation for investigations of the proposed reaction cycle common to all Bfu enzymes.
Subject terms: Cryoelectron microscopy, Oxidoreductases
Structural analysis of a thermophilic bacterial NfnABC complex reveals for the first time that the minimum electron bifurcating site of the Bfu family is a flavobicluster consisting of FMN, a [4Fe-4S] (B1) cluster and a [2Fe-2S] (C1) cluster.
Introduction
Electron bifurcating enzymes couple the endergonic reduction of low-potential substrates with the exergonic reduction of high-potential substrates in one reaction1. Flavin-based electron bifurcation (FBEB) was first discovered in anaerobic bacteria in 20082,3. Since then, FBEB has been found to be widespread in anaerobic microorganisms and has emerged as a key mechanism to enable many thermodynamically unfavorable reactions in the absence of ATP hydrolysis or an ion gradient4. The FBEB enzymes are grouped into four phylogenetically unrelated families4: 1) the electron-transferring flavoprotein (EtfAB) containing complexes, 2) the heterodisulfide reductase (HdrABC) containing complexes, 3) the NADH-dependent ferredoxin NADP reductase (NfnSL), and 4) the NAD(P)- and ferredoxin reducing complexes recently renamed as the BfuABC family5. The first three families all use FAD as the electron bifurcating center, which have been verified by structural characterization, but the fourth family uses FMN instead. The Bfu family is also distinguished by a unique catalytic mechanism involving FMN and multiple iron sulfur (Fe-S) clusters that is distinct from that of the other three families where a single FAD bifurcates electron pairs to high and low potential pathways5.
Our recent bioinformatic and experimental analyses indicated that the electron bifurcating (BF-) BfuABC family is ubiquitous and highly diverse in the microbial world5. These consist of a BF-BfuBC core that reversibly reduces NAD and the redox protein ferredoxin (Fd) with electrons supplied by BfuA (Fig. 1). The BfuB subunit contains multiple Fe-S clusters and FMN while BfuC contains a single cluster. The diversity of this modular system arises from BfuA, which itself reversibly oxidizes a third substrate or a third substrate is oxidized by additional subunits that then feed electrons into BfuA and then to the BfuBC core. Third substrates include H2, CO, NADP, formate or pyruvate, enabling the coupling of electron bifurcation to a wide range of metabolic functions. Based on the subunit composition and Fe-S clusters in BfuA, the Bfu family can be further classified into three types5: Type 1 Bfu enzymes have a catalytic domain to oxidize the third substrate within BfuA which contains four FeS clusters (A1 - A4), Type 2 enzymes contain a catalytic domain in an additional subunit(s) and lack the A3 cluster in BfuA, while Type 3 complexes have additional subunit(s) and contain the A3 cluster.
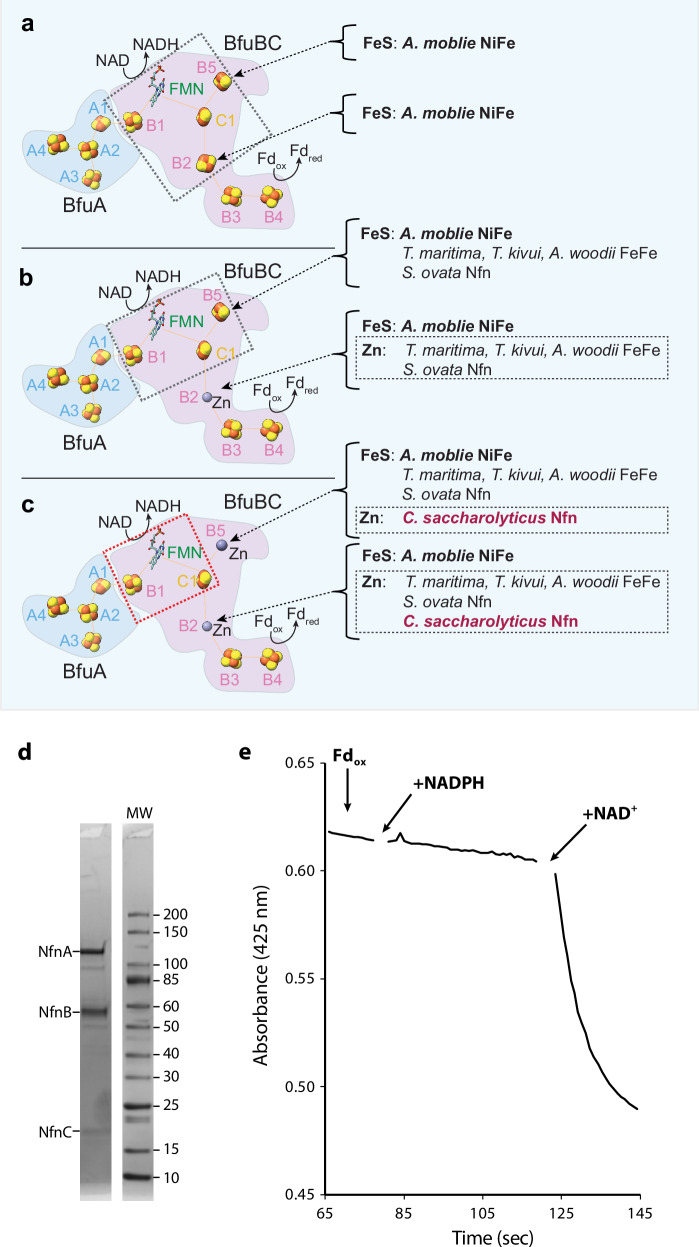
Fig. 1. Current status of the BfuABC family of electron bifurcating enzymes and the bifurcation activity of purified Csac NfnABC.
a In NiFe-ABCSL hydrogenase of A. mobile, five iron sulfur clusters (B1-B5) were modeled in BfuB and the BF-site was considered to be a composite of [B1-FMN-C1-B2-B5]. b In the three subsequent FeFe-ABC hydrogenases (T. maritima, T. kivui and A. woodii) and one NfnABC (S. ovata Stn), only four such iron sulfur clusters (B1, B3, B4 and B5) were mapped in BfuB with the B2 site occupied with a Zn ion and a composite BF-site of [B1-FMN-C1-B5]. c Csac NfnABC has only three iron sulfur clusters in NfnB (B1, B3 and B4) with two Zn ions occupying sites B2 and B5, leaving the flavobicluster [B1-FMN-C1] as the composite BF-site conserved in all resolved BfuABC family members. d SDS-PAGE gel of the purified enzyme showing the presence of all three subunits. e Bifurcating activity of purified NfnABC (~60 µg/ml) in the presence of Csac Fd (~25 µM, purified from Csac), NADPH (0.8 mM), and NAD (1 mM), which were added as indicated. The decrease in the absorbance at 425 nm indicates reduction of Csac Fd, which occurs only in the presence of all three substrates.
Only a handful of BfuABC complexes have been structurally characterized but these have revealed possible pathways of electron transfer from the third substrate to NAD and Fd via the FMN-based bifurcation site. The first structure to be determined was that of the NiFe-ABCSL hydrogenase of Acetomicrobium mobile6, closely followed by the FeFe-ABC hydrogenase of Thermotoga maritima7. Subsequently, two more FeFe-ABC hydrogenase structures were reported using the enzymes from the bacteria Acetobacterium woodii and Thermoanaerobacter kivui8. In all of these Bfu family enzymes, H2 (H+) is the midpotential donor (acceptor) whose oxidation (reduction) is coupled to the reduction of NAD and Fd. Another type of BfuABC enzyme was discovered that uses NADP/H as the midpotential redox carrier9 and this is a NADH-dependent ferredoxin NADP reductase (Nfn). It catalyzes the same reaction as the phylogenetically-unrelated bifurcating NfnSL referred to above (Eq. 1, where the Fd contains two [4Fe-4S] clusters and is assumed to transfer two electrons). The structure of this Nfn-type of Bfu family member was very recently determined10 using the enzyme from the bacterium Sporomusa ovata (Sov). This was named Stn transhydrogenase10 but herein will be referred to as NfnABC to indicate that it is a member of the Bfu family of bifurcating enzymes and that it is distinct from the NfnSL family.
| 1 |
In general the existing structures of the BfuABC family members have revealed very similar cofactor arrangements but with some very significant differences (Fig. 1a–c). Specifically, all of their BfuA subunits contain a minimum of three [4Fe-4S] clusters (A2, A3 and A4) that, in the bifurcation direction, feed electrons from the midpotenital substrate (H2 or NADPH) to the A1 [2Fe-2S] cluster, which in turn transfers electrons to the B1 [4Fe-4S] cluster of BfuB. In the structure of the A. mobile NiFe-ABCSL hydrogenase, the B1 cluster was proposed to be the entry point into the bifurcation site consisting of FMN and four neighboring iron sulfur clusters: B1 ([4Fe-4S]), B2 ([2Fe-2S]) and B5 ([2Fe-2S] together with C1 ([2Fe-2S]) in BfuC (Fig. 1a)6. It was shown experimentally that NAD binds to the FMN and it was proposed that low potential reductant generated by this novel BF-site reduces the B3 and B4 [4Fe-4S] clusters that then reduce Fd, although the site of Fd binding could not be ascertained experimentally. The role of the ‘isolated’ B5 [2Fe-2S] that is not part of the electron transfer pathway was not at all clear.
Of particular interest in the NiFe-ABCSL hydrogenase structure was the B2 [2Fe-2S] cluster, which had non-standard Cys coordination with one pentacoordinated iron atom. This cluster was thought to be one of the keys to the bifurcation mechanism6. It was therefore of some surprise when the structure of T. maritima FeFe-ABC hydrogenase revealed exactly the same cofactor arrangement in the B subunit as in the NiFe-enzyme except that the [2Fe-2S] cluster at B2 site was replaced by a Zn ion7 (Fig. 1b). This clearly showed that, whatever its role in NiFe-ABCSL, the B2 site is not required for BF. This was subsequently confirmed by the structures of FeFe-ABC hydrogenases from A. woodii and T. kivui8 and of the Sov NfnABC10, all of which contained zinc at the B2 site (Fig. 1b). Hence, from the available structures of Bfu family members, the minimal components responsible for bifurcation are FMN and three Fe-S clusters, B1, B5 and C1 (Fig. 1b). In addition, all structures revealed that a substantial conformation change must occur after the BF-event in which C1 cluster moves away from the FMN and towards the B3/B4 clusters thereby preventing short circuiting of the low potential electron in the C1 cluster to the high potential acceptor via FMN. There were differences in quaternary structures, however, with T. maritima FeFe-ABC7and Sov Nfn-ABC10 existing as tetramers of ABC trimers while A. woodii and T. kivui8 FeFe-ABC were dimers of ABC trimers.
Herein we focus on the NfnABC from Caldicellulosiruptor saccharolyticus (Csac), a thermophilic and strictly anaerobic bacterium. Remarkably, Csac can utilize a wide range of biomass types to produce hydrogen gas at a high yield, close to the theoretical maximum of 4 mol H2 per mol of glucose, and as such, is gaining attention in the bioenergy field11. Key to the bacterium’s extraordinary capability to produce H2 is the NfnABC enzyme. This enzyme uses the same Fd and NADH redox pool as the Csac bifurcating FeFe-ABC hydrogenase that generates hydrogen gas, but instead NfnABC produces NADPH for biosynthesis. In contrast to the electron bifurcating family of NADPH-producing NfnSL enzymes12,13, Csac NfnABC is part of the Bfu family and specifically a Type 1 complex5. To provide insights into its reaction mechanism, we have purified the Csac NfnABC from native cell biomass, confirmed its predicted electron bifurcation properties in vitro and solved two high-resolution structures. One is of the as-purified enzyme and the other has bound substrates NADH and Fd. The high resolution enabled us to unambiguously assign all bound cofactors in the complex. Strikingly, in addition to the B2 site, for the first time we find that the B5 site is also occupied by a Zn ion, and not by the [2Fe-2S] cluster proposed in all other structurally characterized Bfu enzymes (Fig. 1c). The Fd-bound conformation of Csac NfnABC reveals electron transfer pathways in the complex and allows us to propose a potential mechanism of electron bifurcation based on this newly determined minimal composition of the BF-site in Bfu enzymes, namely, a flavobicluster of [B1-FMN-C1].
Results
Cryo-EM analysis of the Csac NfnABC
Csac NfnABC was purified by monitoring the NADPH-dependent reduction of an artificial electron acceptor (benzyl viologen, BV) from native biomass by multistep column chromatography carried out under strictly anaerobic conditions. The purified enzyme contained the expected three subunits (Fig. 1d) and the specific activity in NADPH-dependent BV reduction was 160 units/mg at 70 °C. The electron bifurcation activity of Csac NfnBfu was shown by the reduction of Csac Fd, also purified from native Csac biomass, which only proceeds when the second electron acceptor, NAD, was also present (Figs. 1e, 2b). Csac Fd is a monomeric protein of 6.1 kDa (Supplementary Fig. 1) and contains eight cysteine residues arranged in two conventional motifs that coordinate two [4Fe-4S] clusters. Hence this Fd is of the 8Fe-type and is reduced by two electrons (see Eq. 1).
Fig. 2. Cryo-EM structures of tetrameric Csac NfnABC in the holoenzyme form and in the NADH bound form.
a Domain architecture of NfnA, NfnB and NfnC. b Plausible arrangement of NfnA, NfnB and NfnC and their associated cofactors. The three possible redox reaction sites in the NfnABC complex are also indicated. Two orthogonal views of the EM map (c) and atomic model in cartoons (d) of the tetrameric Csac NfnABC holoenzyme. Two orthogonal views of the EM map (e) and atomic model in cartoons (f) of the tetrameric Csac NfnABC bound to both FMN and NAD. The locations of two invisible C-term domains of NfnB and NfnC are marked by two shaded areas. In panels c-f, NfnA is colored in peach, gold, beige, and wheat in protomers 1 to 4, respectively. NfnB, NfnC, FMN, NAD, and FAD are in different colors.
For cryo-EM structural analysis, 2D class averages of the raw particle images revealed the presence of D2 symmetry, indicating a dimer-of-dimers architecture with four copies of the NfnABC complex (Supplementary Fig. 2b). Following 3D classification and reconstruction, we obtained a 3D EM map at 2.9 Å (Supplementary Fig. 2c). This map has a solid core but is flexible with partial densities at the periphery. Further refinement by imposing the D2 symmetry improved the overall map resolution to 2.7 Å with a much better resolved core region (Supplementary Fig. 2c, d, f). We next improved the more flexible peripheral regions to 3.2 Å via symmetry expansion, followed by signal subtraction and focused 3D classification and local refinement (Supplementary Fig. 2c, e, g). Finally, we superimposed the focused map into the D2 symmetric consensus map to generate a composite map of the intact complex (Fig. 2c, Supplementary Fig. 2c). We used AlphaFold predicted initial models to facilitate manual model building (Table 1). Because of the good overall resolution, the main chains of the entire NfnABC sequences, except for the N-terminal 21 residues of NfnC, could be traced clearly, and most sidechains were built with high confidence (Fig. 2d, Supplementary Movie 1, Supplementary Fig. 3). To provide insights into the bifurcating mechanism, we added both substrates NADH and Fd into the holoenzyme solution and derived a composite EM map by combining a D2 symmetric 3.0-Å resolution consensus NfnABC map with a focus-refined local NfnBC core map at 3.3 Å resolution (Fig. 2e, f, Supplementary Fig. 4).
Table 1.
Data collection, processing, model refinement and validation statistics
| NfnABC holoenzyme (Composite map3, EMD-44761, PDB ID 9BP5) | NfnABC bound to NADH (Composite map6, EMD-44751, PDB ID 9BOV) | |
|---|---|---|
| Data collection and processing | ||
| Microscope | Titan Krios (FEI) | Titan Krios (FEI) |
| Magnification | 105,000 | 105,000 |
| Voltage (kV) | 300 | 300 |
| Electron exposure (e-/Å2) | 60 | 60 |
| Defocus rang (μm) | 1.0–1.6 | 1.0–1.6 |
| Pixel size (Å) | 0.828 | 0.828 |
| Symmetry imposed | D2 for consensus map, C1 for protomer | D2 for consensus map, C1 for protomer |
| Initial particle images (No.) | 279,815 | 463,896 |
| Final particle images (No.) | 108,700 (consensus map) 104,777 (NfnBC core) | 78,470 (consensus map) 223,634 (NfnBC core) |
| Map resolution (Å) | 2.7 (consensus, map1) 3.2 (NfnBC core, map2) | 3.0 (consensus, map4) 3.3 (NfnBC core, map5) |
| FSC threshold | 0.143 | 0.143 |
| Map resolution range (Å) | 2.2–5.0 | 2.5–5.0 |
| Refinement | ||
| Model resolution (Å) | 2.7 | 3.1 |
| FSC threshold | 0.5 | 0.5 |
| Model resolution range (Å) | 2.2–5.0 | 2.5–5.0 |
| Map sharpening B factor (Å2) | 88.7 | 97.0 |
| Model composition | ||
| Non-hydrogen atoms | 59,656 | 55,356 |
| Protein residues | 7,668 | 7,088 |
| Ligands | 60 | 48 |
| B factors (Å2) | ||
| Protein | 52.1 | 54.9 |
| Ligand | 46.4 | 44.9 |
| R.m.s. deviations | ||
| Bond lengths (Å) | 0.006 | 0.005 |
| Bond angles (°) | 0.576 | 0.534 |
| Validation | ||
| Molprobity score | 1.48 | 1.44 |
| Clash score | 6.07 | 5.72 |
| Poor rotamers (%) | 0.45 | 0.25 |
| Ramachandran plot | ||
| Favored (%) | 97.17 | 97.31 |
| Allowed (%) | 2.83 | 2.69 |
| Disallowed (%) | 0 | 0 |
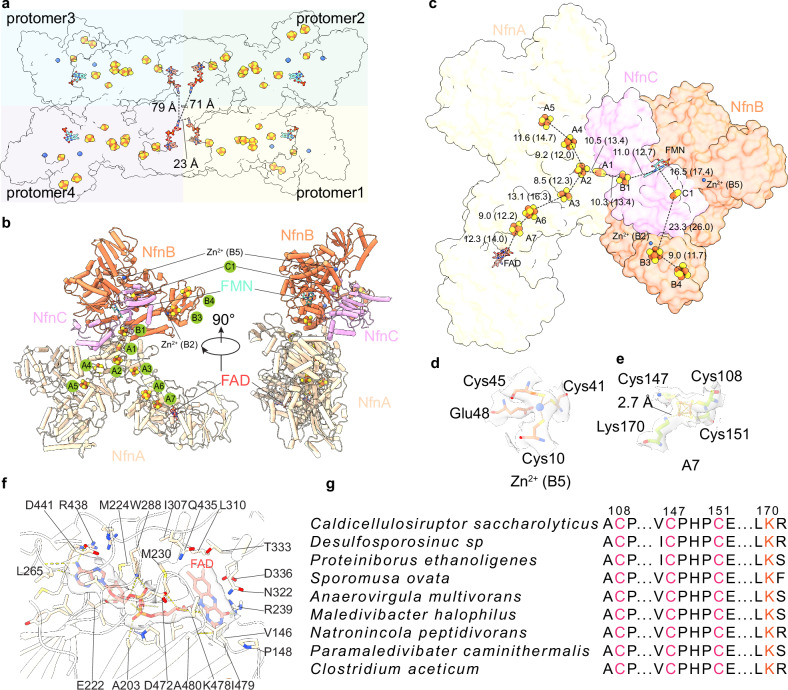
The tetrameric architecture of the Csac NfnABC heterotrimer
From gene sequence analysis, Csac NfnABC is predicted to be a heterotrimer with NfnA (130 kDa), NfnB (64 kDa), and NfnC (20 kDa), with an estimated total mass of 214 kDa. NfnA is predicted to have three domains, an N-terminal (NTD), an NfnL-like (homologous to large subunit of the unrelated NfnSL) and an Fdh/NuoG-like. NfnB is a multi-domain protein, predicted to have a thioredoxin fold, a Rossmann fold, a ubiquitin-like domain, a four-helix bundle, and a C-terminal (CTD) domain, while NfnC has a NTD and a CTD thioredoxin domain (Fig. 2a). The structural model confirms the predicted domain architecture of NfnABC (Fig. 2a, c, d). The holoenzyme is a tetramer of the NfnABC heterotrimer. Tetramerization of the NfnABC is exclusively mediated by interactions among the four NfnA subunits in the middle, with the four NfnBC cores at the four corners of the tetramer (Fig. 2c, d, Supplementary Fig. 5). In the presence of NADH and Fd, the holoenzyme retains the same tetrameric architecture. However, the CTDs of both NfnB and NfnC become flexible and are not observed, and Fd is also missing in the consensus map at higher resolution (Fig. 2e, f).
Within each NfnABC heterotrimer structure, the three subunits bind extensively via a combination of hydrophilic and hydrophobic interactions (Supplementary Fig. 6b, c). The protomer of Csac NfnABC resembles a trefoil, in which NfnL domain and Fdh-like domain of NfnA contribute one lobe separately and NfnB and NfnC together constitute the third lobe (Fig. 3b, c). The NTD of NfnC embeds in a cleft formed by the Fdh-like domain of NfnA and Rossmann fold of NfnB (Fig. 3b, c, Supplementary Fig. 6b). These extensive interactions tightly anchor NfnC to the protomer complex. In addition to the Fdh-like domain and Rossmann fold, the NTD of NfnA and four-helix bundle of NfnB also contribute to the trefoil shape stability via considerable interactions (Fig. 3b, Supplementary Fig. 6c).
Fig. 3. The distribution of electron transporting cofactors indicates that the individual protomer complex is functional.
a Cofactor organization in the tetramer of the Csac NfnABC complex. The nearest distances of cofactors between neighboring protomers are indicated. b Atomic model of one protomer of the NfnABC complex is shown in the cartoon with Fe-S clusters and zinc ion in spheres, and FMN and FAD in sticks. c Arrangement of cofactors in one NfnABC complex protomer. The distances in Å of both edge-to-edge and center-to-center (in parentheses) between two nearby cofactors are labeled. Superposition of the EM density of Zn2+ at the B5 site (d) and the 4Fe-4S cluster at the A7 site (e). EM map is shown as gray surface with 60% transparency, the coordinating residues are shown in sticks. f Detailed interactions in the FAD binding pocket in NfnA. FAD and residues within 4 Å are shown as sticks. The EM density of FAD is superimposed and shown in gray with 60% transparency. g Sequence alignment of NfnA reveals conserved Cys and Lys residues coordinating the A7 [4Fe-4S].
Independently functional Csac NfnABC protomers
The four Csac NfnABC sub-complexes pack together tightly to form the tetrameric architecture, raising the question of whether electron transfer across the protomer boundary, as proposed for another Bfu family member, the FeFe-ABC hydrogenase of T. maritima7, or does each protomer function independently? We examined the cofactor distances between protomers and found that the four FAD molecules in the four NfnA are the closest cofactors among the four NfnABC protomers, and the shortest distance is 23 Å (Fig. 3a). This distance is much longer than 15 Å, the distance generally accepted as the upper limit for electron transfer. Because the four NfnA proteins are centrally located and mediate tetramerization, they are unlikely to undergo large conformational changes during catalytic cycle to shorten the closest FAD-FAD distance. We suggest that there is no electron transfer across protomer boundary and NfnABC protomers function individually. The independently functioning Csac NfnABC is consistent with the homologous Sov NfnABC10. Other than perhaps for the structural stability, it is currently unclear why these enzymes assemble into tetrameric super complexes.
Three rather than four or five iron-sulfur clusters in the Csac NfnB
As expected, the Csac NfnABC holoenzyme harbors many redox cofactors as electron carriers (Fig. 3a–f, Supplementary Fig. 3). NfnA contains one [2Fe-2S] cluster (A1), six [4Fe-4S] clusters (A2-A7), and one FAD in the middle NfnB-like domain that, curiously, is homologous to the FAD-binding domain of the phylogenetically-unrelated NfnSL (Fig. 3b, c, f, Supplementary Fig. 6d). Csac NfnC contains one [2Fe-2S] cluster (C1) in the CTD, as found in the C subunit of all other Bfu family members (Supplementary Figs. 3a and 6a). In NfnB, the FMN binds in a conserved cleft surrounded by the Rossmann domain, the ubiquitin-like fold, and the four-helix bundle (Fig. 3b, c, Supplementary Fig. 6a). Interestingly, the Csac NfnB contains three [4Fe-4S] clusters (B1, B3, and B4), rather than five such clusters in HydB of the NiFe-ABCSL hydrogenase6 or four such clusters in the most recently reported structures of Sov NfnB (of NfnABC)10 and FeFe-HydB (of FeFe-ABC)8 (Fig. 1). The B1 cluster is adjacent to FMN and is coordinated by the end of the four-helix bundle, and the B3 and B4 clusters are coordinated by the CTD (Fig. 3b, c, Supplementary Fig. 6a). Csac NfnB contains a Zn2+ ion at B2, as do all other BfuABC family members so far characterized with the notable exception of the NiFe-HydB6 (Fig. 1, Supplementary Fig. 3b). The previous assignment of the B2 [2Fe-2S] in NiFe-ABCSL was based on the relatively large EM density of the B2 site and the then-available metal analyses indicating the absence of zinc in the native FeFe-HydABC14. But most recent analyses have detected zinc in FeFe-HydABC8, indicating that the B2 site should be a zinc in NiFe-HydABCSL as well.
Importantly, we have found the first example of where the [2Fe-2S] cluster at the B5 position in Sov NfnB, NiFe-HydB and FeFe-HydB is replaced by a second Zn2+ ion in Csac NfnB (Fig. 3d, Supplementary Figs. 3b, c and 6a). To confirm, we have performed ICP-MS analysis of purified Csac NfnABC and found that iron and zinc are the only metals present in significant amounts. The Fe:Zn ratio was 13.5, which corresponds to 40 Fe atoms per mole (based on nine [4Fe-4S] and two [2Fe-2S]) and 3.0 Zn atom/mole. Although the Zn content is more than expected, the monomeric metal ion at the B5 site can only be Zn or Fe. If Fe, then this would correspond to a Fe(Cys)4 rubredoxin-like site, which has a characteristic visible absorption peak near 490 nm. However, that was not observed with Csac NfnABC (Supplementary Fig. 1c). We therefore conclude that the monomeric metal site is Zn.
NADH binding next to FMN in the NfnB core
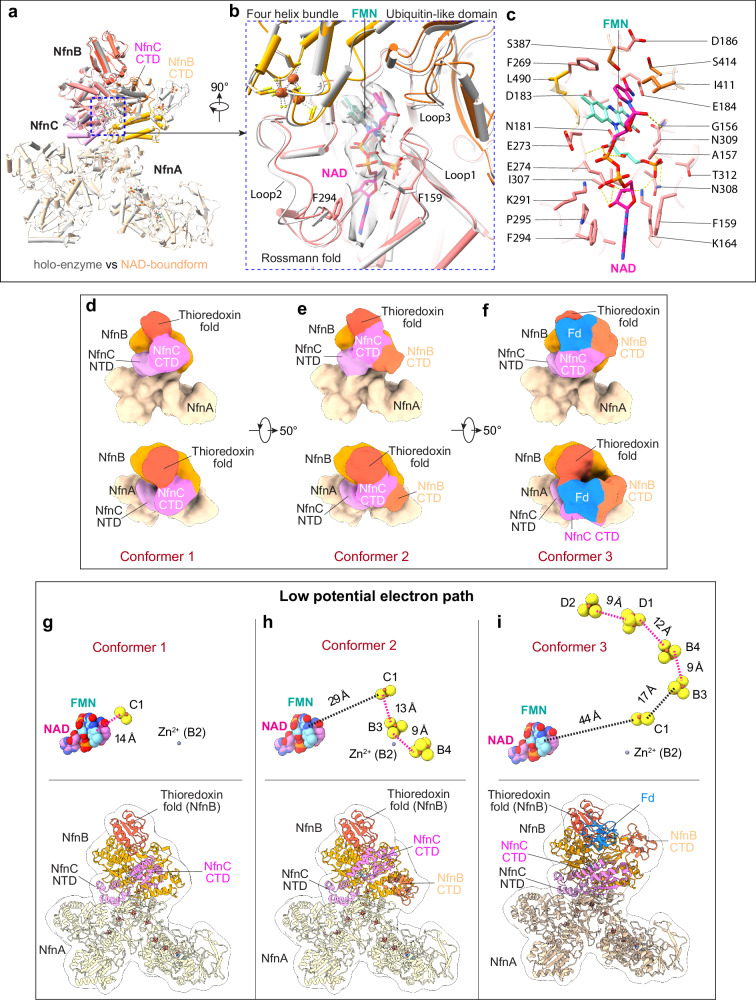
In the substrate-bound structure, the NADH has well-defined densities in Csac NfnB (Fig. 4a, b). As expected, the NADH is positioned next to the FMN, and the NADH nicotinamide moiety forms a π-π interaction with the isoalloxazine ring of FMN to enable rapid electron transfer between the two cofactors (Fig. 4b, c). We found that NADH binding results in a series of conformational changes in NfnB. Specifically, the NADH nicotinamide moiety interacts with loop 3 (408TGAIMGSGG416) of the NfnB ubiquitin-like domain and pulls the domain slightly towards to NADH (Fig. 4a–c). The adenine moiety of NADH pulls two loops (loop 1: 150GLRGRGGAGFP160, and loop 2: aa 283-299) of the NfnB Rossman fold domain towards itself and becomes sandwiched between and forms π-π interactions with the loop 1 residue Phe-159 and the loop 2 residue Phe-294. The NADH is further stabilized by a hydrogen bond network, including its nicotinamide ribose forming an H-bond with the loop 1 Gly-156, its adenine ribose forming two H-bonds with the NfnB Lys-164 and Glu-274, and its phosphate moieties forming two H-bonds with the FMN ribitol group. Interestingly, loop 1 and loop 3 are short and glycine rich, a feature that facilitates the entry of NADH for electron donation and subsequent departure of NAD by lowering the energy barrier for the NADH/NAD exchange15. Therefore, the NADH binding pocket in Csac NfnB appears to have evolved to precisely bind and orient the NADH with respect to the FMN while also evolving features to allow facile NAD dissociation.
Fig. 4. Conformational changes triggered by NAD binding.
a Superposition of the NAD bound form with the Csac NfnABC holoenzyme. In the NAD bound form, the two CTDs of NfnB and NfnC are highly mobile. b Closeup of the FMN binding pocket. The NAD nicotinamide forms a pi-pi interaction with the FMN isoalloxazine ring to facilitate electron transfer, and the NAD adenine is trapped between and stabilized by Phe-159 and Phe-294. The EM densities of FMN and NAD are shown in gray surface with 60% transparency. c Detailed side chain interactions with FMN and NAD. FMN, NAD, and the protein interacting residues (within 4 Å) are shown as sticks. Conformers 1 (d), 2 (e), and 3 (f) captured by 3DVA analysis are shown in two views. The three key domains affected by NAD binding, NfnB CTD, NfnC CTD and the NfnB thioredoxin fold, are labeled in each conformer. The corresponding model of conformers 1 (g), 2 (h), and 3 (i) are shown in cartoons and superimposed on the EM maps rendered in transparent surfaces. Key cofactors are shown as insets on top. D1 and D2 are two [4Fe-4S] clusters in the Csac Fd. The edge-to-edge distances in Å between cofactors are labeled.
In addition to these local conformational changes in the NADH binding pocket, NADH binding destabilizes the NfnBC core (Supplementary Fig. 7). The B2 Zn2+ is coordinated by three cysteine and one histidine residue (i.e., 3C1H), while the B5 Zn2+ is coordinated by three cysteine and one glutamate residue (i.e., 3C1E). The NfnB thioredoxin domain appears to have undergone a subtle conformational change upon NADH binding, as the EM density of this domain becomes weaker, and the EM density of the B5 Zn2+ observed in the holoenzyme is now missing (Supplementary Figs. 3b, d and 7). The B2 Zn2+-containing helical bundle domain and the linker to the NfnB CTD remain stable with defined EM densities, but the NfnB CTD is invisible and becomes highly mobile (Supplementary Figs. 3 and 7). Meanwhile, the NfnC CTD is also destabilized but is less mobile than the NfnB CTD (Supplementary Fig. 7).
Potential electron transfer path through B1-FMN-C1 electron bifurcation site
The structure of the Csac NfnBC core is similar to those of other characterized Bfu family members, e.g., with a R.M.S.D. of 1.1 Å compared to the BC core of the FeFe-ABC hydrogenase from T. kivui8 (Supplementary Fig. 6e). The FMN binding mode and its neighboring arrangement of iron-sulfur clusters (B1 and C1) are also conserved, suggesting that Csac NfnABC utilizes the same electron bifurcation mechanism involving what is now a composite flavobicluster of B1-FMN-C1 as the BF-site (Fig. 1).
Based on the edge-to-edge distance between two adjacent cofactors, we identify a potential electron transfer pathway that originates from FAD in NfnA, goes through a chain of five iron-sulfur clusters (A7-A6-A3-A2-A1) to reach the BF-flavobicluster of [B1-FMN-C1] that further extends through B3 to end at B4 (Fig. 3c). In this pathway, most connecting cofactors are within 15 Å distance required for electron transfer, except for C1 which is 16.5 Å from the upstream FMN and 23.3 Å from the downstream B3. Indeed, in all prior structural analyses of Bfu family members, the C1 [2Fe-2S] cluster has been proposed to be a gating cluster that via conformational changes prevents backflow of high energy low potential electrons from C1 back to the flavin10.
For NfnA, one conserved feature found so far in all Bfu family members is the non-standard coordination of the A7 [4Fe-4S] cluster, which is liganded by three cysteine residues (Cys-108, Cys-147 and Cys-151) and one lysine (Lys-170) (Fig. 3d, g). While the underlying reason for such a configuration requires further investigation, A7 is the first iron-sulfur cluster in the electron transfer path to accept electrons from NADPH oxidation via FAD, and the presence of the positively charged lysine may modify the redox potential of this [4Fe-4S] cluster to facilitate electron transfer from FAD to A7. Indeed, mutating the corresponding Lys residue in the Sov NfnABC enzyme to Arg and to Cys reduced the bifurcating activity by approximately 50% and 100%, espectively10.
However, in the NfnA subunit, the A4 and A5 sites each occupied by a [4Fe-4S] cluster are not included in the electron transfer path from NADPH to the [B1-FMN-C1] BF-site. A4 and A5 are connected to the A2 cluster in the NfnA Fdh-like domain but form a dead-end side branch. A protein homolog structural search by DALI16 shows that NfnAB resembles the W-containing formate hydrogenase (FDH-1) from Methylorubrum extorquens17 especially in the middle NfnL domain of NfnA with an R.M.S.D. of only 1.3 Å (Supplementary Fig. 6f). In addition, the Mo-containing formate dehydrogenase from Cupriavidus necator (FdsABG) shows homology to Csac NfnA and its three iron-sulfur clusters, corresponding to A2-A4-A5 in NfnA, are functional in electron transfer18. However, the corresponding pterin binding site in Csac NfnA is incomplete, and as expected there is no pterin density in the EM map of Csac NfnA (Supplementary Fig. 6g, h). Therefore, the A2-A4-A5 side branch in Csac NfnA is likely nonfunctional and an evolution remnant. However, there are three aromatic Tyr (Y733, Y834, and Y1178) within 14 Å of the A5 cluster (Supplementary Fig. 6i), raising the possibility that this branch may function as an overflow electron pool.
Three conformations of the NfnBC core in the presence of NADH and Fd
Csac NfnABC becomes more flexible in the presence of substrates NADH and Fd and particularly in the NfnBC core region, which is most important for understanding the electron bifurcation mechanism. We therefore performed a three-dimensional variation analysis (3DVA) in CryoSPARC19 focusing on one NfnABC protomer. We down-sampled the particle images by a factor of 4 and low-pass filtered the particle dataset to 15 Å. 3DVA analysis revealed that most regions of the protomer complex are stable, except for the NfnB thioredoxin domain that has undergone a modest conformational change, and the CTDs of both NfnB and NfnC that contain the B3/B4 and C1 clusters, respectively, that are subjected to substantial rearrangement (Supplementary Movie 2). Based on the number of resolved flexible domains, the structural variation can be sorted into three discrete conformations, referred to as conformers 1, 2, and 3 (Fig. 4d–f). The CTDs of both NfnB and NfnC and of Fd were assigned based on their flexibility observed in the high-resolution map (Supplementary Fig. 7). The atomic models of NfnB CTD, NfnC CTD and Fd were rigid-docked into the assigned EM densities of the three conformers (Fig. 4g–i). Conformer 1 contains only one flexible domain, the CTD of NfnC, conformer 2 resolves the two partially flexible CTDs of both NfnB and NfnC, and conformer 3 resolves both CTDs plus the bound Fd. We note that conformer 3 is the only example so far in all of the structural analyses of BF-enzymes, not just of BfuABC family members, in which the binding site of the electron acceptor Fd is revealed, with the notable exception of NfnSL12. The fact that Fd density is present only in one conformer and the resolution of all three conformers are quite low suggests that Fd only transiently binds to the Csac NfnABC complex. In any event, the location of Fd enables us to better understand the electron transfer mechanism, as described below.
Proposed mechanistic insights
Csac NfnABC contains one FAD and one FMN, but the structures have shown that the FAD in NfnA is located at the end of electron transfer chain that is incompatible with a direct role in electron bifurcation, in contrast to all other types of flavin-based BF-enzymes. On the other hand, the FMN in NfnB is at the junction of electron transfer routes between NADPH and Fd and must be directly involved in bifurcation, in agreement with several other characterized FMN mediated bifurcation complexes10. However, this FMN directly transfers two high potential electrons in the form of a single hydride to NAD, as well as being involved in generating two low potential single electrons to reduce Fd. This ‘conflict of function’ for FMN can only be resolved by invoking iron-sulfur clusters as part of the site of electron bifurcation, as discussed below. NADPH binds to Csac NfnA adjacent to the FAD, as revealed by the homologous structure of NADPH-bound Sov NfnABC published recently10. Importantly, NADPH binding did not induce any conformational changes in Sov NfnABC10. We assume that the electron bifurcation process in Csac NfnABC begins with NADPH binding to and donating the first electron pair to the NfnA FAD, which relays the electrons one at a time via the A7-A6-A3-A2-A1 pathway to reach the B1 cluster, also presumably without any conformational change (Figs. 3c and 5a). Note that two NADPH must be oxidized to complete the overall catalytic reaction in which NAD and Fd are each reduced two electrons (Eq. 1). It is possible that electrons from two or even three NADPH could reside within the NfnA subunit distributed between clusters A7, A6, A3, A2 and A1, with ‘overflow’ to A4 and A5, prior to bifurcation reaction. Clearly, spectroscopic analyses are needed to investigate this aspect.
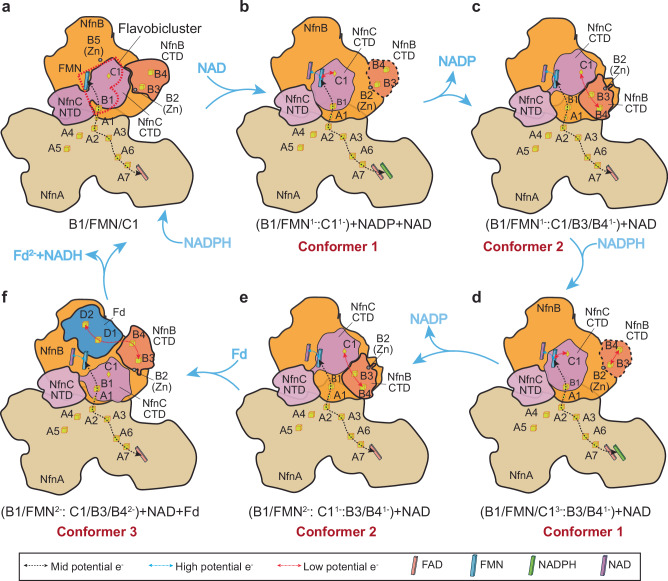
Fig. 5. NAD binding is proposed to trigger two conformation-change-coupled electron bifurcation processes.
The red heart shape in the resting oxidized enzyme (a) encircles the flavobicluster [B1-FMN-C1] that is proposed to mediate electron bifurcation. For the complete reaction cycle (Eq. 1) two NADPH are oxidized and the number of electrons on the various clusters and combinations of clusters are indicated. The first NADPH binds to NfnA and donates two electrons to FAD and the mid potential electron path (a). NAD then binds to FMN in NfnB and triggers a series conformational changes in the NfnBC core that enable the first of two electron bifurcation processes. In the first, the flavobicluster generates a high-potential electron that remains within the BF-site (B1/FMN1-, Conformer 1, b), while the low potential electron on C1 (C1-) after a conformational change reduces B3/B4 (B3/B41-, Conformer 2, c). C1 returns to form the [B1-FMN-C1] flavobicluster and NAD still remains bound to FMN. A second NADPH then binds and donates two more electrons to the bicluster (B1/FMN/C11-) to give a bicluster with three electrons (B1/FMN/C13-, d). A second bifurcation event then occurs and reduced C1 dissocates once more (e) to generate a site containing a second low potential electron (C1/B3/B42-). This state enables NfnB CTD, NfnC CTD and the NfnB thioredoxin domain to form a tripod, which expands to bind Fd (Conformer 3; (C1/B3/B42-, f). Two electron transfer events then occur (shown here simultaneously). Bound Fd is reduced by two low potential electrons and dissociates, and the two high potential electrons on the B1/FMN sub-site reduce NAD to generate NADH. NADH dissociates to generate the resting oxidized holoenzyme (a).
For the first time, the minimal cofactor content of the BF-site of a Bfu family member has now been established, namely, it is the flavobicluster [B1-FMN-C1]. The fundamental issue is how this BF site receives four midpotential electrons from NADPH, and generates two high potential electrons in the form of an hydride to reduce NAD to NADH and simultaneously generates two low potential electrons that one a time reduce Fd. Clearly, it is not chemically possible for a) the same FMN to carry out both transformations and b) for an intermediate NAD semiquinone radical to exist. Previous attempts to rationalize a BF-mechanism for BfuABC family members have not taken this into account7,8,10. It is presumably not a coincidence that the flavobicluster [B1-FMN-C1] can accommodate four electrons but how it releases them two at one time to reduce NAD via FMN and two one at a time to ultimately reduce Fd via C1 is not known. Fd-bound NfnB can potentially accomodate four (low potential) electrons sequentially from C1 (Fig. 5), two reducing the 8Fe-Fd and two reducing the B3/B4 clusters.
Transfer of the two low-potential electrons from the flavobicluster via C1 to Fd is clearly complex, because the transfer route is not preformed like that in NfnA and instead is configured on-demand involving a substantial conformational change and transient Fd binding to the enzyme. Furthermore, we have shown that electron bifurcation by Csac NfnABC occurs only in the presence of NAD, Fd and NADPH (Fig. 1e), but a structure with all three substrates bound is still lacking. Therefore, mechanistic insights can only be inferred based on the three observed NAD/Fd bound conformers (Fig. 4d–i). We propose the following six-step reaction that transits two low potential electrons from the BF-flavobicluster site to Fd (Fig. 5a-f). The enzyme initially exists in the resting fully oxidized state in the absence of substrates. Upon NADPH binding and oxidation by the NfnA FAD, electron transfer to the BF-site via the A1 cluster is poised to occur (Fig. 5a). The binding of NAD must trigger orchestrated conformational changes in the NfnBC core, in this case accompanied by bifurcation and generation of both high and low potential electrons within the flavobicluster [B1-FMN-C1] site. This potentially leads to movement of the cluster-containing CTDs of both NfnB (containing B3 and B4) and NfnC (containing C1) (Figs. 4d and 5b). Hence, in contrast to what is seen in the resting state (Fig. 5a), the C1 cluster must be close enough (≤15 Å) to FMN to allow electron transfer, as shown in conformer 1 (Fig. 4g). Because the NfnC CTD is internal and blocked by the NfnB CTD, it is likely that NfnB CTD moves first, followed by movement of the NfnC CTD containing the reduced C1 cluster (conformers 1 and 2) (Fig. 4g, h). Because of these movements, the highly bent NfnC configuration can now stretch and relax, leading to an increased distance between the C1 [2Fe-2S] and the FMN. The larger distance (>28 Å) prevents reverse electron transfer from C1 to the FMN. Meanwhile, the fully mobile NfnB CTD recovers partial rigidity to interact with NfnC CTD again, enabling electron transfer from C1 to B3/B4 clusters. In essence, a one-electron reduced tricluster [C1/B3/B41-] is transiently formed while the high potential electron generated by electron bifurcation must remain with the flavobicluster site, now containing only one cluster ([B1-FMN1-], Fig. 5c).
We assume that completion of the reaction cycle (Eq. 1) is initiated by the binding of a second NADPH to NfnA, although, as noted above, two or even three NADPH could be oxidized prior to the occurrence of the first bifurcation step. The arrival of the two electrons from the second NADPH at the BF-site is assumed to occur after reduction of B3/B4 and the reforming of the now singly reduced flavobicluster ([B1/FMN/C11-]. Reduction of the bifurcation site by these two electrons generates triply reduced [B1/FMN/C13-] (Fig. 5d) and initiates a second electron bifurcation event that again reduces the C1 cluster (Fig. 5e). This leads to a second conformational change whereby C1 reforms a transient doubly reduced tricluster ([C1/B3/B42-] that is poised to reduce Fd (Fig. 5f). This step is largely supported by the conformer 3 structure (Fig. 4i), in which the two displaced CTDs of NfnB and NfnC along with the NfnB thioredoxin domain form a tripod to recruit Fd, and Fd binding in the tripod enables electron transfer from ([C1/B3/B42-] to the D1/D2 clusters of Fd (Figs. 4f, i and 5f). This shares the same path as what has been previously proposed for Bfu family members where all putative mechanisms show direct electron transfer from C1 to B3/B4 and reduction of Fd by the B4 cluster10.
In any event, the results presented here show that the low potential electron transfer branch (post electron bifurcation) involves a C1-B3-B4-Fd interation and that this branch is configured on-demand via a series of domain movements and refolding. Shown to occur simulateously in Fig. 5f, when bound Fd is reduced by two low potential electrons and dissociates, two high potential electrons on the [B1/FMN2-] subsite reduce NAD by a hydride transfer from FMN to generate NADH, which then dissociates to generate the resting oxidized holoenzyme (Fig. 5a). The essence of this overall catalytic mechanism is that the C1 cluster transfers low potential electrons from the bifurcating [B1/FMN/C1] flavobicluster via the NfnC CTD conformational change to generate a tricluster [C1/B3/B4] that reduces Fd, while the remaining [B1/FMN] subsite reduces NAD. Clearly, the essential feature of this mechanism is the dissocation of C1 from the flavobicluster [B1/FMN/C1] to form the tricluster [C1/B3/B4] and this charge separation prevents low potential electrons from reducing NAD as the tricluster reduces Fd (conformer 3, Fig. 4i)
Discussion
The fourth electron bifurcating family of enzymes—the BfuABC family—is the latest identified and is the least well understood. A number of structures of this family of enzymes have been reported, and among the available structures, the assignment of certain cofactors is controversial or inconsistent due to lack of resolution in the relevant region of the experimental EM maps8,10–12. In our current study of Csac NfnABC, we have achieved a high-resolution EM map that has enabled the model building of all domains in the complex, and more importantly, the accurate assignment of all factors, including one FAD, one FMN, one NADH, nine [4Fe-4S] clusters, two [2Fe-2S] clusters and, for the first time, two zinc ions (Fig. 3, Supplementary Fig. 3) and Fd (Fig. 4f, i). Accurate identification of electron transport cofactors lays the foundation for deciphering the electron transfer path(s) and ultimately, the electron bifurcating mechanism.
The BfuABC family of enzymes share the conserved electron bifurcating BC core, with varied metabolic functions specified either by NfnA or additional subunit(s) attached to NfnA5. We have shown that the conserved NfnBC core contains a stable platform composed of the thioredoxin fold, the Rossmann fold, the ubiquitin-like domain, and the four-helix bundle of NfnB and the NTD of NfnC. Within this stable platform, the proposed flavobicluster [B1-FMN-C1] bifurcation site functions as a hub between its preceding A1 [2Fe-2S] cluster bridging the electron transfer from NfnA to the NfnBC core and the NfnB CTD containing two B3/B4 [4Fe-4S] clusters that facilitate electron transfer to Fd. We have further shown that NAD binds adjacent to the FMN in a large pocket formed by the NfnB Rossmann fold, ubiquitin-like domain, and four-helix bundle, and that NAD binding is the key event in the electron bifurcation process, triggering long-range and substantial conformational changes of the two CTDs of NfnB and NfnC. This enables Fd binding and subsequent acceptance of low potential electrons from the flavobicluster site via as the C1-containing CTD moves towards Fd. Although we propose a minimal bifurcating core, [B1-FMN-C1], the other clusters are also required for bifurcation functionality (Figs. 1 and 5). For example, homologs of the BfuBC subunits containing FMN and the B1 and C1 clusters are found in non-bifurcating enzymes such as NAD(H)-dependent NiFe hydrogenase (PDB ID 5XF9), NAD(H)-dependent formate dehydrogenase (PDB ID 6TGA) and in respiratory complex I (PDB ID 3IAM). However, their BfuB homologs lack the B2, B3, B4 and B5 binding sites20–22. In addition, the essential requirement of the B3 and B4 clusters was shown in the FeFe hydrogenase (HydABC) from T. kivui where their deletion abolished bifurcating activity8.
We have revealed what enables Fd binding is the formation of the tripod-shaped trap via the rearrangement of the NfnB thioredoxin and CTD domains and the NfnC CTD. Because this trap assembles only transiently and Fd binding is short-lived, we were unable to determine the Fd-binding pose to a high resolution. Therefore, the detailed interactions between Fd and the tripod trap require further investigation. Nevertheless, our work underscores the substantial conformational changes of the two CTDs of NfnB and NfnC as triggered by NAD(H) binding and their critical role in configuring the low potential electron transfer path “on-demand” to enable electron bifurcation by the flavobicluster of Csac NfnABC. Given the absolute structural conservation of the BC core within the Bfu family, the observed conformational changes in Csac NfnABC complex are proposed to occur and play essential role in the BF-mechanism of all of the Bfu family enzymes.
Structural analysis reveals conformational changes and the cofactors most likely involved in electron transfer paths, but such analysis does not provide the redox potentials of individual cofactors nor how the redox potential changes upon conformational changes. Therefore, the exact electron bifurcation mechanism within the [B1-FMN-C1] flavobicluster is not at all clear, and particularly how that the potential of the C1 cluster changes depending upon its location, specifically, as part of the BF-site or adjacent to the B3/B4 clusters and Csac Fd. How the BfuABC enzymes coordinate both electronically and conformationally the bifurcation of four electrons will require study by a range of biophysical tools, using the results of the current study as a framework to move forward.
Materials and Methods
Growth of microorganism
Caldicellulosiruptor saccharolyticus (Csac) was grown in a medium containing glucose (5 g/liter) and yeast extract (0.5 g/l) as carbon sources, as described before23. It was routinely grown anaerobically in 100-ml serum bottles with 50 ml of medium and a headspace of 20% CO2 and 80% N2 at 70 °C with shaking (120 rpm). When grown in a 20-liter fermenter, additional yeast extract (5 g/l) was added to the medium. The fermenter was stirred at 150 rpm and was sparged with 20% CO2 and 80% N2 at a rate of 1.5 l/min. Cells were harvested by centrifugation (10,000 g in a continuous-flow Sharples centrifuge) and were subsequently frozen in liquid N2 and stored at −80 °C
Purification NfnABC and Fd from Csac
All the following protein purification steps were performed anaerobically either in a Coy anaerobic chamber (95% Ar, 5% H2) or by using degassed buffers with Ar positive pressure with a constant flow of Ar. Cells (15 g wet weight) were resuspended in 50 ml of buffer (50 mM HEPES, pH 7.5, 5% glycerol, 5% trehalose, 1 mM cysteine, 0.1 mM phenylmethylsulfonyl fluoride, and deoxyribonuclease, 50 mg/l). Csac cells were broken on ice by sonication (30-s intervals, amplitude 80; Qsonica, model Q55). An extract of soluble proteins was prepared by ultracentrifugation at 100,000 g for 1 hour. Anion exchange chromatography was carried out using a 50-ml QHP custom column (XK 26/20 Cytiva, Marlborough, MA) equilibrated with 25 mM HEPES (pH 7.5), containing 1% trehalose and 1 mM cysteine. Bound proteins were eluted with a linear gradient for 0 to 500 mM NaCl in the same buffer; the Nfn-ABC activity was eluted as a single peak when ~300 mM NaCl was applied. Size exclusion chromatography was carried out using a HiLoad Superdex 200 prepgrade XK 26/60 column (Cytiva, Marlborough, MA) with a running buffer of 25 mM HEPES (pH 7.5), with 300 mM NaCl, and 1% trehalose at a flow rate of 2.6 ml/min. Csac Nfn-ABC eluted at high molecular weight (~700 kDa). NfnABC fractions were diluted 5-fold with 25 mM HEPES (pH 7.5), containing 1% trehalose, and 1 mM cysteine and loaded on a 12 ml custom Resource Q column (16/20 Cytiva, Marlborough, MA). Proteins were eluted with a linear gradient of 100-500 mM NaCl in the same buffer, and Nfn-Bfu was eluted when ~260 mM NaCl was applied. All fractions were collected in Ar-flushed serum vials sealed with butyl rubber stoppers and were stored at 4 °C or frozen at −80 °C. About 2 mg of NfnABC was obtained from 15 g of cell paste. Csac Fd was purified from the same batch of cells by following its visible absorbance at 390 nm. The protein eluted from the anion exchange column as single brown peak when 250 mM NaCl was applied. It subsequently eluted from a HiLoad Superdex S75 16/60 column corresponding to a mass of ~ 6 kDa (Supplemental Fig. 1a). The UV-visible spectra of the purified Csac Fd and NfnABC were measured using a Agilent Cary 3500 spectrophotometer and are shown in Supplementary Fig. 1b and 1c, respectively.
Enzyme assays
For purification purposes, NfnABC was followed by the reduction of benzyl viologen by NADPH at 600 nm (ɛ = 7.4 mM−1 cm−1) at 70 °C. NfnABC bifurcating activity was demonstrated by following the NAD dependent reduction of Csac Fd at 425 nm (ɛ = 13 mM−1 cm−1) by NADPH. The reaction mixture contained 0.8 mM NADPH, 1 mM NAD+ and 25 μM Csac Fd.
Cryo-EM grids preparation and data collection
Purified Nfn-BfuABC samples at a concentration of 1.2 mg/ml were sealed in stoppered vials. FMN (final concentration 20 µM) was added to replace any that have been lost during purification. Where indicated, NADH (50 µM) and Csac Fd (10 μM) were added to the indicated final concentrations. For cryo-EM analysis, 4 μL samples were added by gastight syringe and applied onto freshly glow-discharged EM grids (Quantifoil R2/1 Copper, 300 mesh) in an FEI Vitrobot Mark IV with chamber humidity set to 100% and temperature set to 9 °C. Extra solution was blotted using a piece of filter paper with the blotting force set to 3 and blotting time set to 3 s. The blotted grids were vitrified by plunging into liquid ethane cooled by liquid nitrogen. The grids were stored in liquid nitrogen until data collection.
We first screened the EM grids in a TFS Arctica microscope operated at 200 kV. EM grids with good particle distribution and thin ice were chosen and loaded into the Titan Krios microscope operated at 300 kV for high resolution data recording. The cryo-EM data sets were recorded on a Gatan K3 camera at a microscope magnification of 105,000x using the SerialEM automatic data collection software. Under this scope magnification, each image pixel corresponds to 0.414 Å at the specimen level. Fifty-frame movies were recorded with a total dose of 60 e-/Å and an exposure time of 1 s. The defocus value varied from −1.0 μm to −1.6 μm.
Image processing
We collected two data sets, one for the holo-enzyme (with added FMN) and the other to which NADH and Fd had also been added. These two datasets were processed similarly using CryoSPARC_v4.124. In brief, the movie stacks were aligned and averaged by patch motion correction, following by CTF parameter estimation and CTF effect corrections using patch CTF estimation. Blob picking was used to pick a small set of particles that was used for automatic particle picking by TOPAZ25. Two rounds of 2D classification were carried out to remove junk particles. The retained particles were subjected to ab initio and heterogeneous refinement. The good particles in the best 3D class were used for non-uniform refinement by imposing the D2 symmetry. Then focused 3D classification, followed by D2 symmetry expansion and particle subtraction was performed to resolve partially mobile asymmetric features. Another focused refinement on the NfnB and NfnC was carried out to improve the local resolution. Eventually, the focus refined maps were combined with the consensus D2-symmetric map to generate the final composite map.
For the dataset of the Csac NfnABC holoenzyme, 279,815 particles were selected from 6,332 images by TOPAZ picking25. After 2D classification, 3D classification, and duplicates removal, a total of 108,700 particles were used for non-uniform refinement with D2 symmetry, leading to a consensus map at an average resolution of 2.7 Å. The NfnBC core was refined to 3.2 Å through focused 3D classification and local refinement using 104,777 particles.
For the dataset of the Csac NfnABC with NADH and Fd added, 16,457 movies were recorded, and 13,780 micrographs were retained by manual inspection after CTF estimation. A total of 463,890 particles were selected using TOPAZ picking25. A total of 78,470 particles were retained after a series of polishing, 2D classification, 3D classification, and duplicates removal. A D2 symmetric consensus map at 3.0 Å was generated by non-uniform refinement. The NfnBC core was refined to 3.3 Å via focused 3D classification and local refinement using 223,634 particles.
Model building, refinement, and validation
For the Csac NfnABC holoenzyme, the AlphaFold26 generated models of individual subunits NfnA, NfnB and NfnC were docked into the EM map as an initial model using UCSF ChimeraX27. FAD, FMN, [4Fe-4S] clusters, [2Fe-2S] clusters and Zn2+ were manually built into the EM map using Coot28. The completed model was subjected to real-space refinement in PHENIX29 and manually adjusted in Coot28. For the Csac NfnABC complexed with NADH and Fd, the coordinates of holoenzyme were used as an initial model and docked into the 3D EM map using ChimeraX27. The model was manually adjusted in Coot28 and the NADH was manually docked in the EM density. The final model was refined in real space using PHENIX29. Finally, the model was validated by MolProbity30. UCSF ChimeraX27 were used to prepare figures.
Protein dynamics analysis
To investigate the conformational change in the dataset of the Csac NfnABC complex with NADH and Fd, the dataset composed of 223,634 particles used for protomer local refinement was lowpass filtered to 15 Å and subjected to 3-dimensional variability analysis (3DVA) in CryoSPARC_v4.119. 20 EM maps were generated, which showed discrete heterogeneity and domain movement. Three representative maps were selected for further analysis. The stable platform (NfnA and NfnBC core minus the C-term domains of both NfnB and NfnC), the C-term domain of NfnB, the C-term domain of NfnC, and an Fd model generated by AlphaFold26 were rigid-body docked into the two maps to generate corresponding structural models. Figures and movies were generated using UCSF ChimeraX27.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
Cryo-EM images were collected at the David Van Andel Advanced Cryo-Electron Microscopy Suite at the Van Andel Institute. We thank G. Zhao and X. Meng for help with data collection. This work was funded by grants from the Division of Chemical Sciences, Geosciences and Biosciences, Office of Basic Energy Sciences of the U.S. Department of Energy (DE-SC0020085 to Huilin Li and DE-FG02-95ER20175 to M.W.W.A.) and Van Andel Institute (to Huilin Li).
Author contributions
M.W.W.A. and Huilin Li conceived and designed experiments. Hua Li, G.J.S. and X.F. performed experiments. Hua Li, G.J.S., X.F., M.W.W.A. and Huilin Li analyzed the data and wrote the manuscript.
Peer review
Peer review information
Communications Biology thanks James Blaza and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Christina Karlsson Rosenthal.
Data availability
The atomic coordinates of the Csac NfnABC holoenzyme and the enzyme bound with NAD reported in this paper have been deposited in the Protein Data Bank with accession code 9BP5 and 9BOV, respectively. The composite EM map, consensus EM map, and the NfnBC-focus refined EM map of the Csac NfnABC holoenzyme can be accessed by EM Data Bank (EMDB) codes EMD-44761, EMD-44749, and EMD-44750, respevtively. And the composite EM map, consensus EM map, and the NfnBC-focus refined EM map of the NADH-bound enzyme complex can be accessed by EMDB codes EMD-44751, EMD-44752, and EMD-44753, respectively. Source data and all other data are available from the corresponding authors on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Michael W. W. Adams, Email: adamsm@uga.edu
Huilin Li, Email: Huilin.Li@vai.org.
Supplementary information
The online version contains supplementary material available at 10.1038/s42003-025-07706-8.
References
- 1.Peters, J. W. et al. A new era for electron bifurcation. Curr Opin Chem Biol47, 32–38 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herrmann, G., Jayamani, E., Mai, G. & Buckel, W. Energy conservation via electron-transferring flavoprotein in anaerobic bacteria. J Bacteriol190, 784–791 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li, F. et al. Coupled ferredoxin and crotonyl coenzyme A (CoA) reduction with NADH catalyzed by the butyryl-CoA dehydrogenase/Etf complex from Clostridium kluyveri. J Bacteriol190, 843–850 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buckel, W. & Thauer, R. K. Flavin-Based Electron Bifurcation, A New Mechanism of Biological Energy Coupling. Chem Rev118, 3862–3886 (2018). [DOI] [PubMed] [Google Scholar]
- 5.Schut, G. J. et al. An Abundant and Diverse New Family of Electron Bifurcating Enzymes With a Non-canonical Catalytic Mechanism. Front Microbiol13, 946711 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feng, X., Schut, G. J., Haja, D. K., Adams, M. W. W. & Li, H. Structure and electron transfer pathways of an electron-bifurcating NiFe-hydrogenase. Sci Adv8, eabm7546 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Furlan, C. et al. Structural insight on the mechanism of an electron-bifurcating [FeFe] hydrogenase. Elife11, e79361 (2022). [DOI] [PMC free article] [PubMed]
- 8.Katsyv, A. et al. Molecular Basis of the Electron Bifurcation Mechanism in the [FeFe]-Hydrogenase Complex HydABC. J Am Chem Soc145, 5696–5709 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kremp, F., Roth, J. & Muller, V. The Sporomusa type Nfn is a novel type of electron-bifurcating transhydrogenase that links the redox pools in acetogenic bacteria. Sci Rep10, 14872 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar, A. et al. Molecular architecture and electron transfer pathway of the Stn family transhydrogenase. Nat Commun14, 5484 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kadar, Z. et al. Yields from glucose, xylose, and paper sludge hydrolysate during hydrogen production by the extreme thermophile Caldicellulosiruptor saccharolyticus. Appl Biochem Biotechnol113-116, 497–508 (2004). [DOI] [PubMed] [Google Scholar]
- 12.Lubner, C. E. et al. Mechanistic insights into energy conservation by flavin-based electron bifurcation. Nat Chem Biol13, 655–659 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang, S., Huang, H., Moll, J. & Thauer, R. K. NADP+ reduction with reduced ferredoxin and NADP+ reduction with NADH are coupled via an electron-bifurcating enzyme complex in Clostridium kluyveri. J Bacteriol192, 5115–5123 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verhagen, M. F., O’Rourke, T. & Adams, M. W. The hyperthermophilic bacterium, Thermotoga maritima, contains an unusually complex iron-hydrogenase: amino acid sequence analyses versus biochemical characterization. Biochim Biophys Acta1412, 212–229 (1999). [DOI] [PubMed] [Google Scholar]
- 15.Krieger, F., Moglich, A. & Kiefhaber, T. Effect of proline and glycine residues on dynamics and barriers of loop formation in polypeptide chains. J Am Chem Soc127, 3346–3352 (2005). [DOI] [PubMed] [Google Scholar]
- 16.Holm, L., Laiho, A., Toronen, P. & Salgado, M. DALI shines a light on remote homologs: One hundred discoveries. Protein Sci32, e4519 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoshikawa, T. et al. Multiple electron transfer pathways of tungsten-containing formate dehydrogenase in direct electron transfer-type bioelectrocatalysis. Chem Commun (Camb)58, 6478–6481 (2022). [DOI] [PubMed] [Google Scholar]
- 18.Young, T. et al. Crystallographic and kinetic analyses of the FdsBG subcomplex of the cytosolic formate dehydrogenase FdsABG from Cupriavidus necator. J Biol Chem295, 6570–6585 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Punjani, A. & Fleet, D. J. 3D variability analysis: Resolving continuous flexibility and discrete heterogeneity from single particle cryo-EM. J Struct Biol213, 107702 (2021). [DOI] [PubMed] [Google Scholar]
- 20.Shomura, Y. et al. Structural basis of the redox switches in the NAD(+)-reducing soluble [NiFe]-hydrogenase. Science357, 928–932 (2017). [DOI] [PubMed] [Google Scholar]
- 21.Radon, C. et al. Cryo-EM structures reveal intricate Fe-S cluster arrangement and charging in Rhodobacter capsulatus formate dehydrogenase. Nat Commun11, 1912 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berrisford, J. M. & Sazanov, L. A. Structural basis for the mechanism of respiratory complex I. J Biol Chem284, 29773–29783 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang, S. J. et al. Classification of ‘Anaerocellum thermophilum’ strain DSM 6725 as Caldicellulosiruptor bescii sp. nov. Int J Syst Evol Microbiol60, 2011–2015 (2010). [DOI] [PubMed] [Google Scholar]
- 24.Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat Methods14, 290–296 (2017). [DOI] [PubMed] [Google Scholar]
- 25.Bepler, T. et al. Positive-unlabeled convolutional neural networks for particle picking in cryo-electron micrographs. Nat Methods16, 1153–1160 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature596, 583–589 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goddard, T. D. et al. UCSF ChimeraX: Meeting modern challenges in visualization and analysis. Protein Sci27, 14–25 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr D Biol Crystallogr66, 486–501 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liebschner, D. et al. Macromolecular structure determination using X-rays, neutrons and electrons: recent developments in Phenix. Acta Crystallogr D Struct Biol75, 861–877 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen, V. B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr D Biol Crystallogr66, 12–21 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The atomic coordinates of the Csac NfnABC holoenzyme and the enzyme bound with NAD reported in this paper have been deposited in the Protein Data Bank with accession code 9BP5 and 9BOV, respectively. The composite EM map, consensus EM map, and the NfnBC-focus refined EM map of the Csac NfnABC holoenzyme can be accessed by EM Data Bank (EMDB) codes EMD-44761, EMD-44749, and EMD-44750, respevtively. And the composite EM map, consensus EM map, and the NfnBC-focus refined EM map of the NADH-bound enzyme complex can be accessed by EMDB codes EMD-44751, EMD-44752, and EMD-44753, respectively. Source data and all other data are available from the corresponding authors on reasonable request.