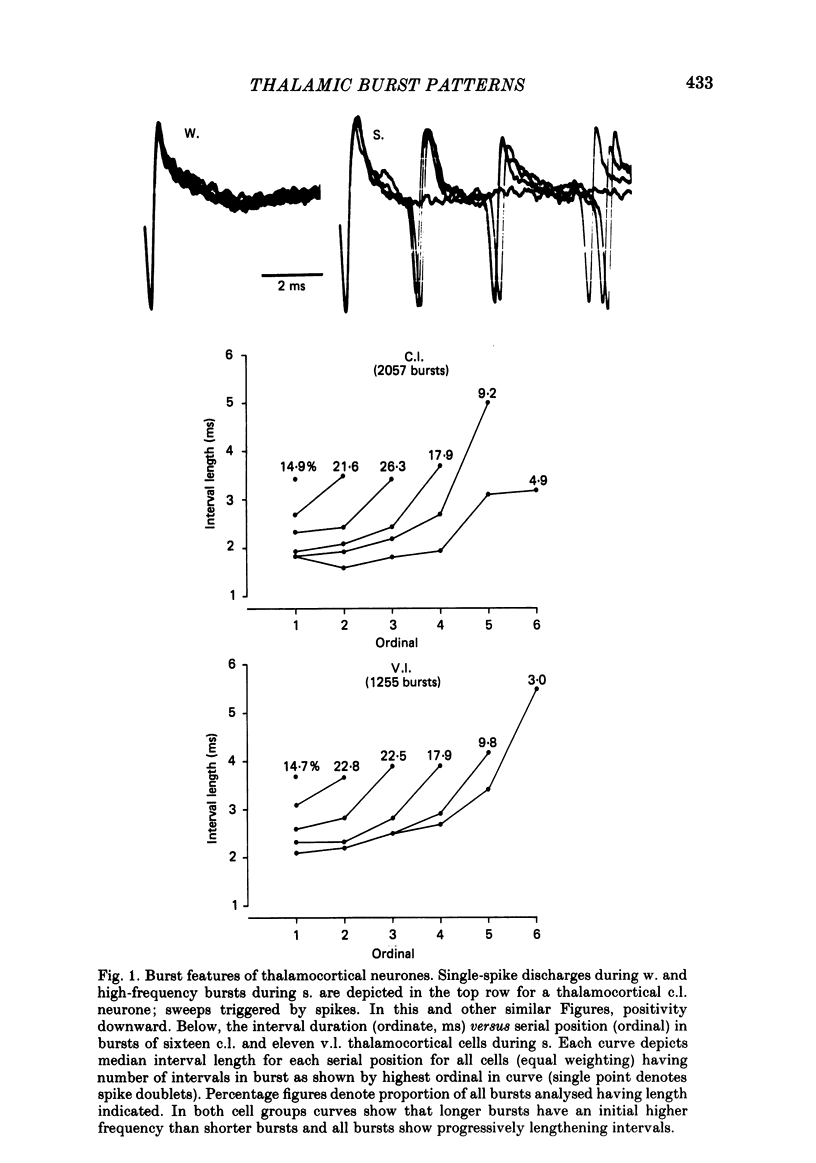
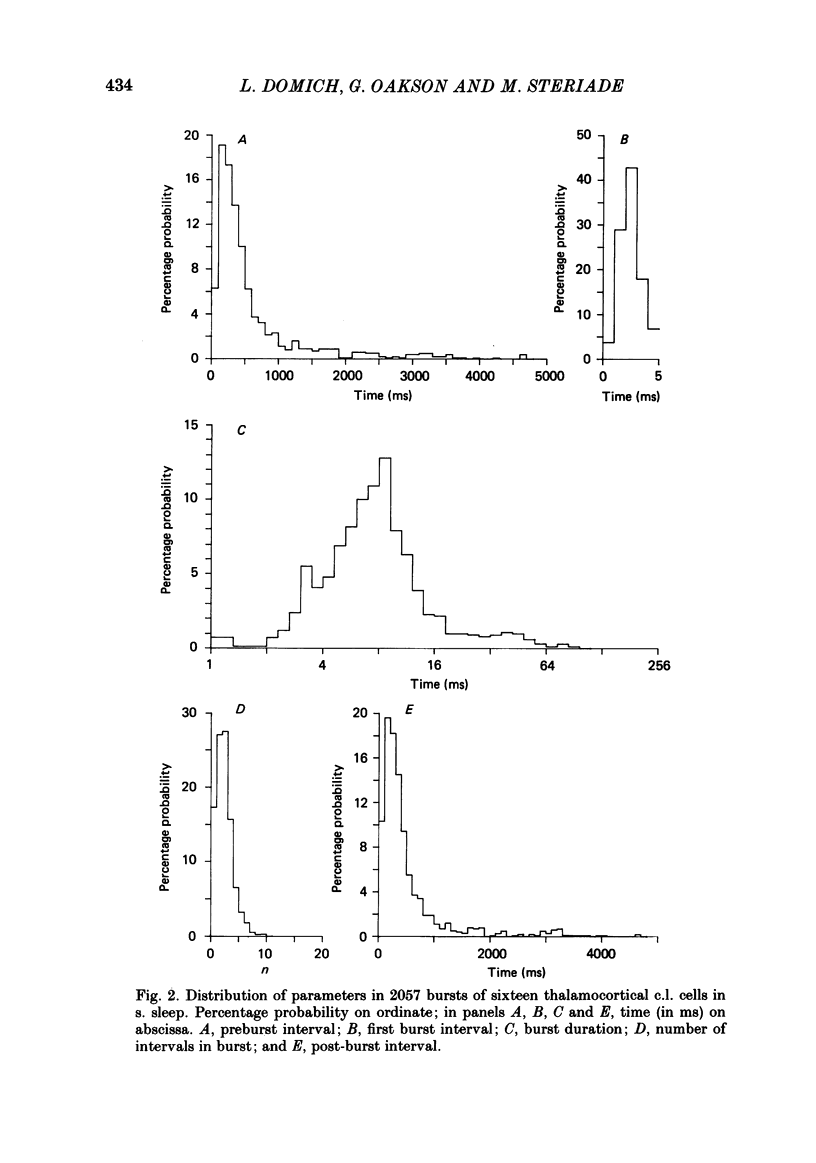
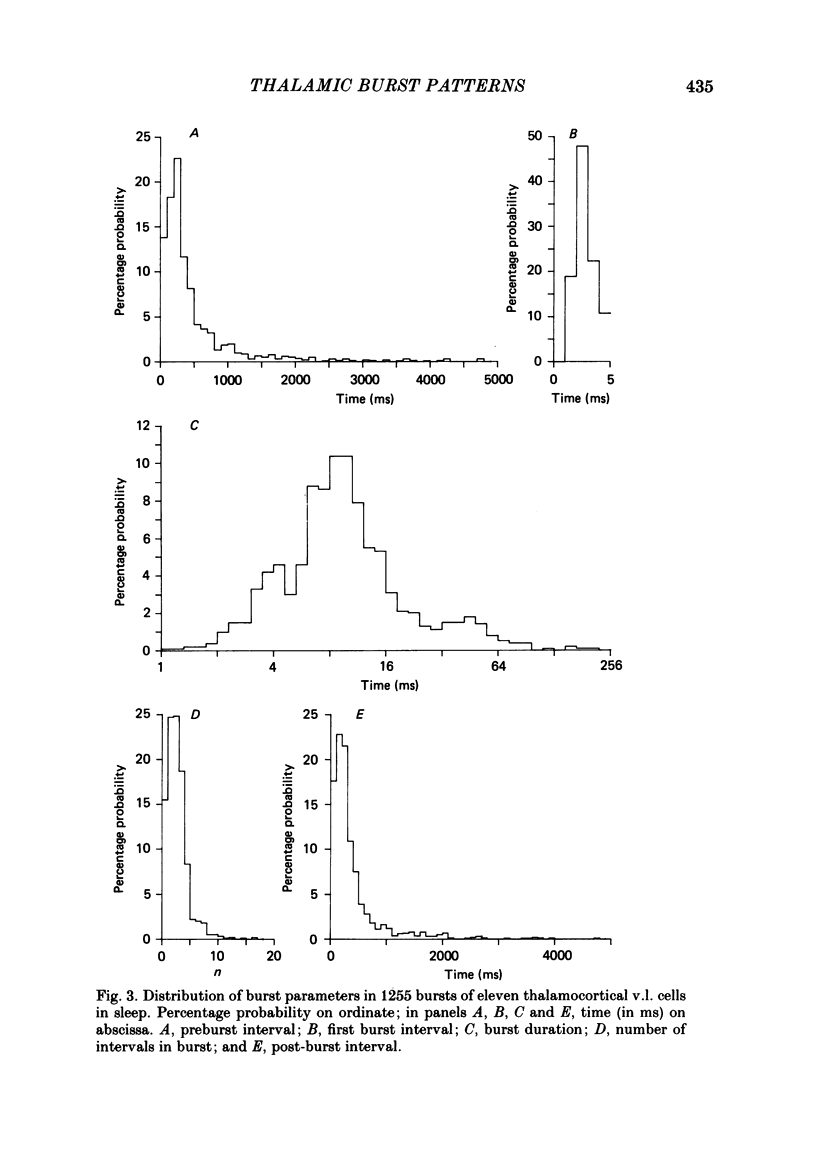
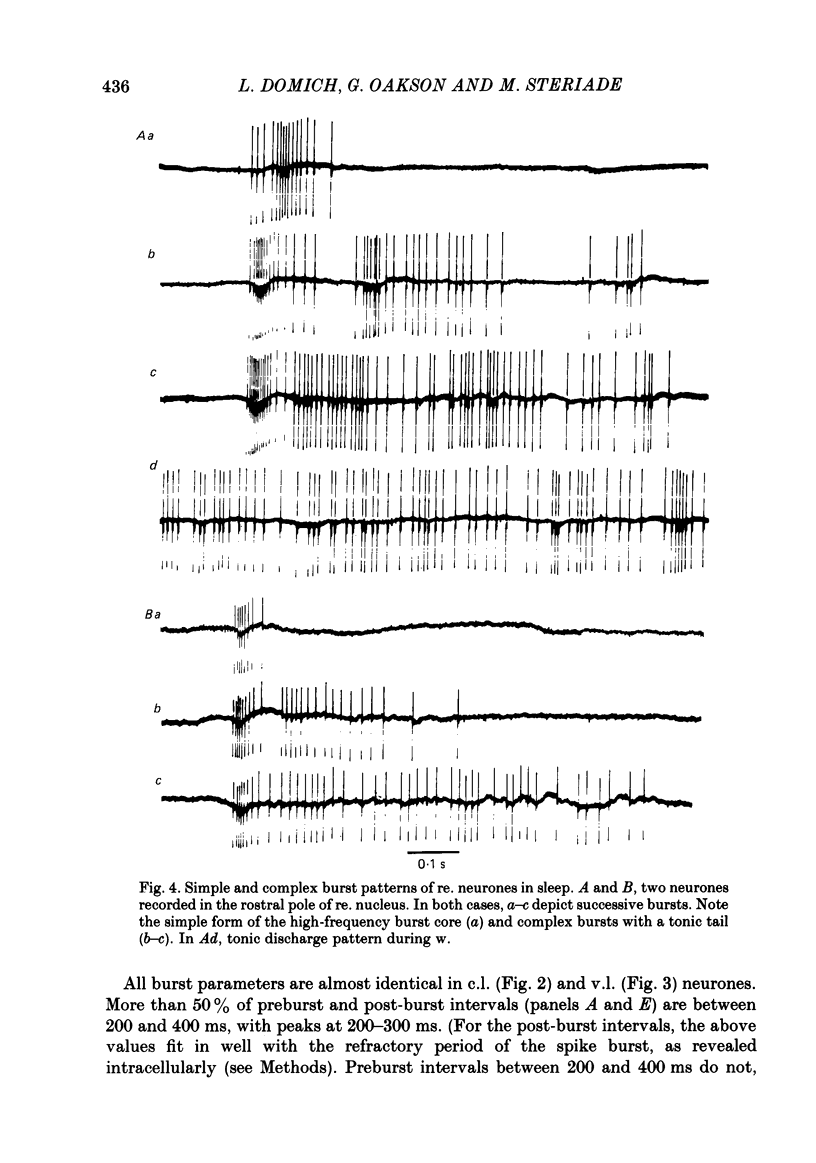
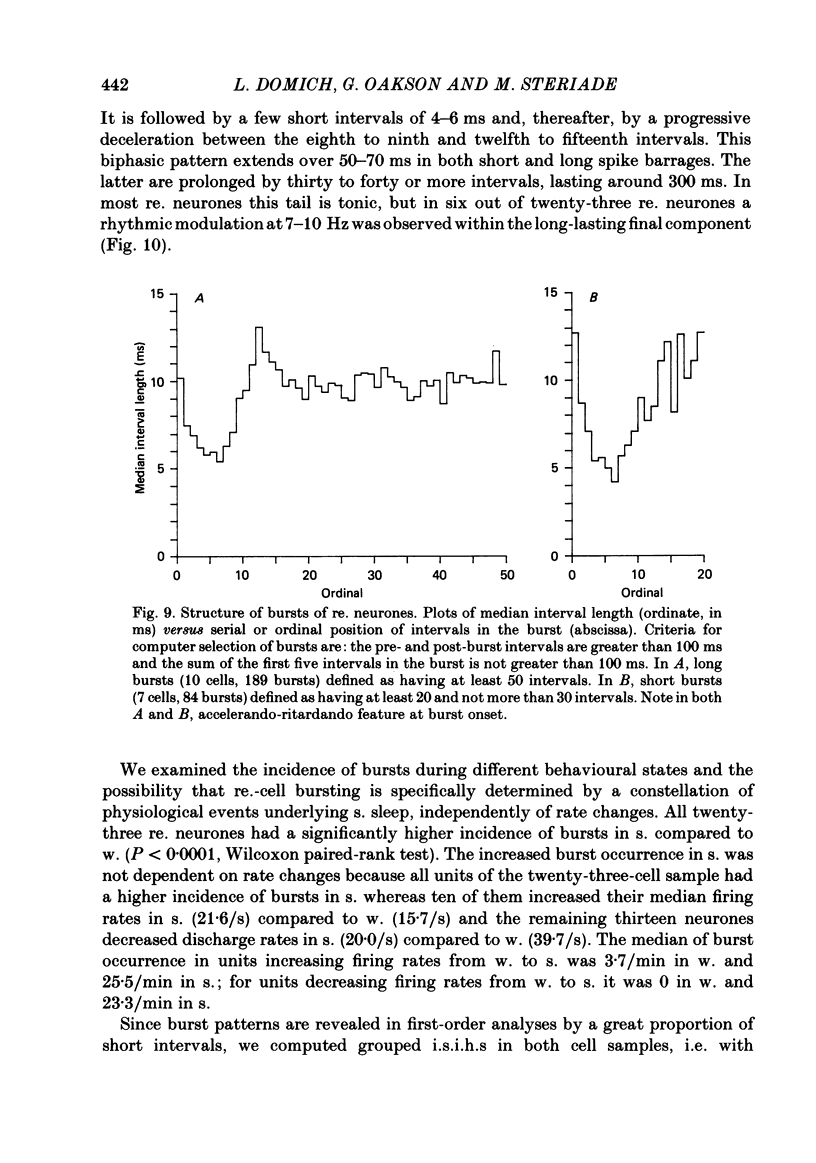
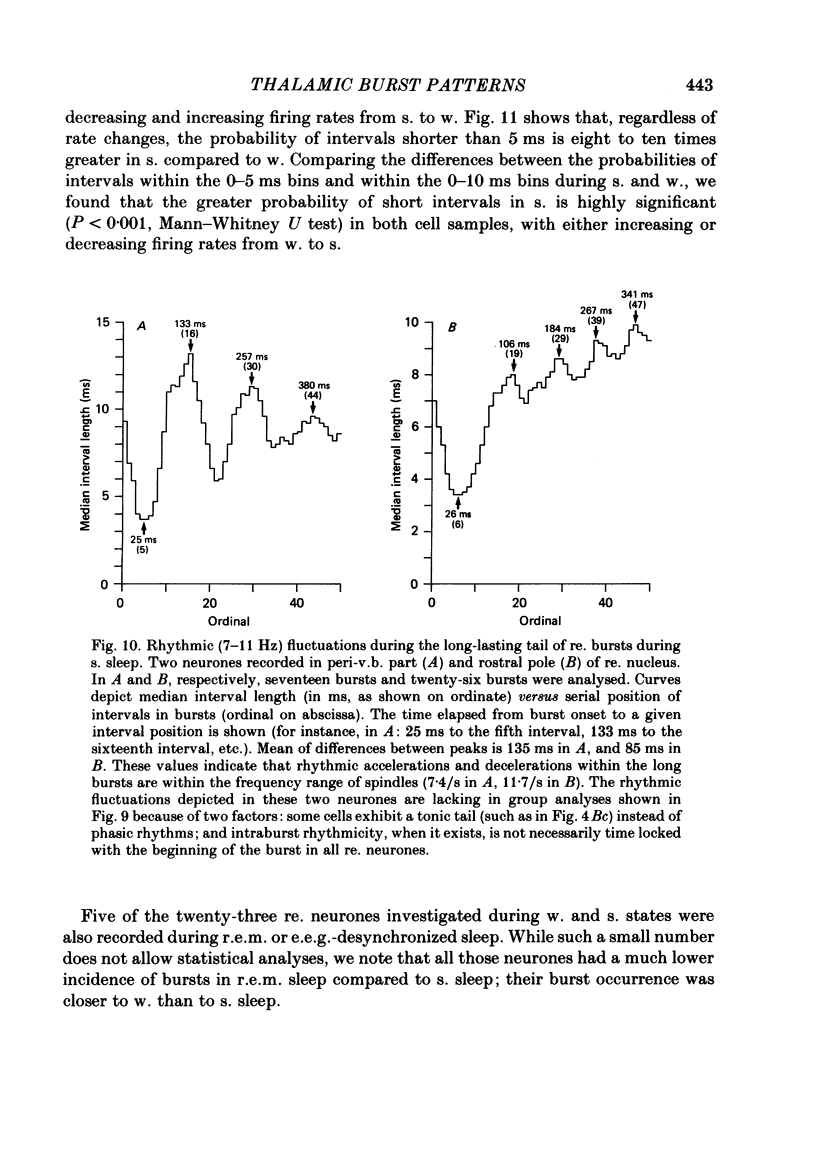
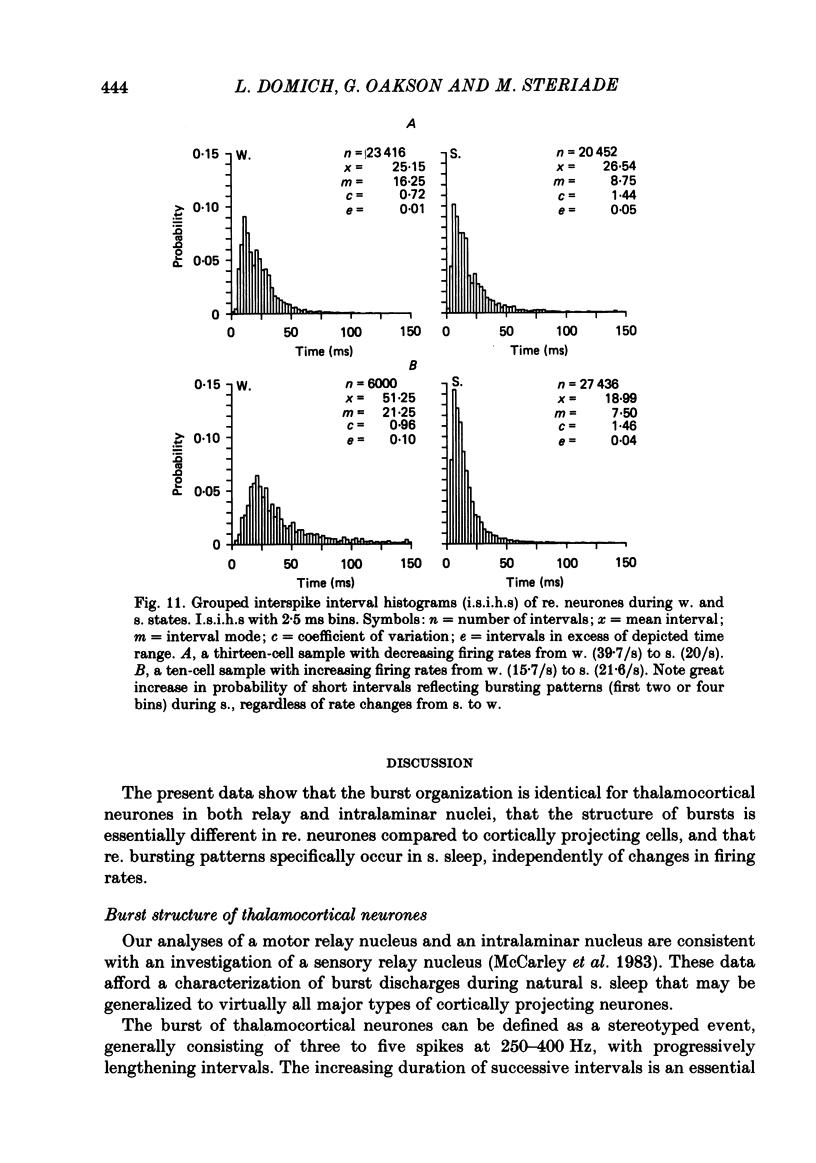
Abstract
Unit discharges were extracellularly recorded from antidromically identified thalamocortical neurones of ventralis lateralis (v.l.) and centralis lateralis (c.l.) nuclei as well as from reticularis thalami (re.) neurones during wakefulness and electroencephalogram-synchronized sleep of the behaving cat. Various parameters of sleep-related discharge bursts were analysed. Statistical analyses revealed striking similarities between motor relay (v.l.) and intralaminar (c.l.) neurones. More than 60% of bursts consist of three to five spikes at 250-400 Hz. The defining feature of bursts in all cortically projecting neurones is a progressive increase in the duration of successive interspike intervals. As in thalamocortical cells, all re. neurones change their tonic discharges in waking to bursting firing in sleep, regardless of the increased or decreased firing rates from wake to sleep in individual neurones. The bursts of re. neurones are essentially different from those of thalamocortical cells. In re. neurones, burst structure consists of an initial progressive decrease in duration of interspike intervals, followed by an increase in duration of successive intervals, eventually leading to a long-lasting tonic spike train at about 100 Hz. In contrast with bursts of thalamocortical neurones, only 6% of re. bursts are shorter than 50 ms; the total duration of the burst extends between 50 ms and 1.5 s. Population periburst histograms show the beginning of a decline in firing probability about 1.5 s prior to burst onset and an increased firing probability persisting for 300-350 ms after burst onset. The different electrophysiological properties underlying the burst structure of cat's thalamocortical and re. neurones are discussed, with emphasis on dissimilar aspects of re. bursts in unanaesthetized and barbituratized preparations. Various factors that may account for the transition from tonic mode in waking to bursting mode in sleep are envisaged.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrionuevo G., Benoit O., Tempier P. Evidence for two types of firing pattern during the sleep-waking cycle in the reticular thalamic nucleus of the cat. Exp Neurol. 1981 May;72(2):486–501. doi: 10.1016/0014-4886(81)90238-7. [DOI] [PubMed] [Google Scholar]
- Deschênes M., Madariaga-Domich A., Steriade M. Dendrodendritic synapses in the cat reticularis thalami nucleus: a structural basis for thalamic spindle synchronization. Brain Res. 1985 May 13;334(1):165–168. doi: 10.1016/0006-8993(85)90580-3. [DOI] [PubMed] [Google Scholar]
- Deschênes M., Paradis M., Roy J. P., Steriade M. Electrophysiology of neurons of lateral thalamic nuclei in cat: resting properties and burst discharges. J Neurophysiol. 1984 Jun;51(6):1196–1219. doi: 10.1152/jn.1984.51.6.1196. [DOI] [PubMed] [Google Scholar]
- Dingledine R., Kelly J. S. Brain stem stimulation and the acetylcholine-evoked inhibition of neurones in the feline nucleus reticularis thalami. J Physiol. 1977 Sep;271(1):135–154. doi: 10.1113/jphysiol.1977.sp011994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filion M., Lamarre Y., Cordeau J. P. Neuronal discharges of the ventrolateral nucleus of the thalamus during sleep and wakefulness in the cat. II. Evoked activity. Exp Brain Res. 1971 Jun 29;12(5):499–508. doi: 10.1007/BF00234245. [DOI] [PubMed] [Google Scholar]
- Glenn L. L., Steriade M. Discharge rate and excitability of cortically projecting intralaminar thalamic neurons during waking and sleep states. J Neurosci. 1982 Oct;2(10):1387–1404. doi: 10.1523/JNEUROSCI.02-10-01387.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBEL D. H. Single unit activity in lateral geniculate body and optic tract of unrestrained cats. J Physiol. 1960 Jan;150:91–104. doi: 10.1113/jphysiol.1960.sp006375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch J. C., Fourment A., Marc M. E. Sleep-related variations of membrane potential in the lateral geniculate body relay neurons of the cat. Brain Res. 1983 Jan 24;259(2):308–312. doi: 10.1016/0006-8993(83)91264-7. [DOI] [PubMed] [Google Scholar]
- Houser C. R., Vaughn J. E., Barber R. P., Roberts E. GABA neurons are the major cell type of the nucleus reticularis thalami. Brain Res. 1980 Nov 3;200(2):341–354. doi: 10.1016/0006-8993(80)90925-7. [DOI] [PubMed] [Google Scholar]
- Jahnsen H., Llinás R. Electrophysiological properties of guinea-pig thalamic neurones: an in vitro study. J Physiol. 1984 Apr;349:205–226. doi: 10.1113/jphysiol.1984.sp015153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahnsen H., Llinás R. Ionic basis for the electro-responsiveness and oscillatory properties of guinea-pig thalamic neurones in vitro. J Physiol. 1984 Apr;349:227–247. doi: 10.1113/jphysiol.1984.sp015154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Greenfield S. A., Jahnsen H. Electrophysiology of pars compacta cells in the in vitro substantia nigra--a possible mechanism for dendritic release. Brain Res. 1984 Feb 27;294(1):127–132. doi: 10.1016/0006-8993(84)91316-7. [DOI] [PubMed] [Google Scholar]
- Llinás R., Jahnsen H. Electrophysiology of mammalian thalamic neurones in vitro. Nature. 1982 Jun 3;297(5865):406–408. doi: 10.1038/297406a0. [DOI] [PubMed] [Google Scholar]
- MacLeod N. K., James T. A. Regulation of cerebello-cortical transmission in the rat ventromedial thalamic nucleus. Exp Brain Res. 1984;55(3):535–552. doi: 10.1007/BF00235285. [DOI] [PubMed] [Google Scholar]
- McCarley R. W., Benoit O., Barrionuevo G. Lateral geniculate nucleus unitary discharge in sleep and waking: state- and rate-specific aspects. J Neurophysiol. 1983 Oct;50(4):798–818. doi: 10.1152/jn.1983.50.4.798. [DOI] [PubMed] [Google Scholar]
- Montero V. M., Scott G. L. Synaptic terminals in the dorsal lateral geniculate nucleus from neurons of the thalamic reticular nucleus: a light and electron microscope autoradiographic study. Neuroscience. 1981;6(12):2561–2577. doi: 10.1016/0306-4522(81)90102-0. [DOI] [PubMed] [Google Scholar]
- Mukhametov L. M., Rizzolatti G., Tradardi V. Spontaneous activity of neurones of nucleus reticularis thalami in freely moving cats. J Physiol. 1970 Oct;210(3):651–667. doi: 10.1113/jphysiol.1970.sp009233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohara P. T., Lieberman A. R. The thalamic reticular nucleus of the adult rat: experimental anatomical studies. J Neurocytol. 1985 Jun;14(3):365–411. doi: 10.1007/BF01217752. [DOI] [PubMed] [Google Scholar]
- Ohara P. T., Sefton A. J., Lieberman A. R. Mode of termination of afferents from the thalamic reticular nucleus in the dorsal lateral geniculate nucleus of the rat. Brain Res. 1980 Sep 22;197(2):503–506. doi: 10.1016/0006-8993(80)91136-1. [DOI] [PubMed] [Google Scholar]
- Roy J. P., Clercq M., Steriade M., Deschênes M. Electrophysiology of neurons of lateral thalamic nuclei in cat: mechanisms of long-lasting hyperpolarizations. J Neurophysiol. 1984 Jun;51(6):1220–1235. doi: 10.1152/jn.1984.51.6.1220. [DOI] [PubMed] [Google Scholar]
- Schlag J., Waszak M. Electrophysiological properties of units of the thalamic reticular complex. Exp Neurol. 1971 Jul;32(1):79–97. doi: 10.1016/0014-4886(71)90167-1. [DOI] [PubMed] [Google Scholar]
- Steriade M., Apostol V., Oakson G. Control of unitary activities in cerebellothalamic pathway during wakefulness and synchronized sleep. J Neurophysiol. 1971 May;34(3):389–413. doi: 10.1152/jn.1971.34.3.389. [DOI] [PubMed] [Google Scholar]
- Steriade M., Deschenes M. The thalamus as a neuronal oscillator. Brain Res. 1984 Nov;320(1):1–63. doi: 10.1016/0165-0173(84)90017-1. [DOI] [PubMed] [Google Scholar]
- Steriade M., Deschênes M., Domich L., Mulle C. Abolition of spindle oscillations in thalamic neurons disconnected from nucleus reticularis thalami. J Neurophysiol. 1985 Dec;54(6):1473–1497. doi: 10.1152/jn.1985.54.6.1473. [DOI] [PubMed] [Google Scholar]
- Steriade M., Domich L., Oakson G. Reticularis thalami neurons revisited: activity changes during shifts in states of vigilance. J Neurosci. 1986 Jan;6(1):68–81. doi: 10.1523/JNEUROSCI.06-01-00068.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M., Glenn L. L. Neocortical and caudate projections of intralaminar thalamic neurons and their synaptic excitation from midbrain reticular core. J Neurophysiol. 1982 Aug;48(2):352–371. doi: 10.1152/jn.1982.48.2.352. [DOI] [PubMed] [Google Scholar]
- Steriade M., Oakson G., Ropert N. Firing rates and patterns of midbrain reticular neurons during steady and transitional states of the sleep-waking cycle. Exp Brain Res. 1982;46(1):37–51. doi: 10.1007/BF00238096. [DOI] [PubMed] [Google Scholar]
- Steriade M., Parent A., Hada J. Thalamic projections of nucleus reticularis thalami of cat: a study using retrograde transport of horseradish peroxidase and fluorescent tracers. J Comp Neurol. 1984 Nov 10;229(4):531–547. doi: 10.1002/cne.902290407. [DOI] [PubMed] [Google Scholar]
- Steriade M., Wyzinski P. Cortically elicited activities in thalamic reticularis neurons. Brain Res. 1972 Jul 20;42(2):514–520. doi: 10.1016/0006-8993(72)90552-5. [DOI] [PubMed] [Google Scholar]
- Waszak M. Effect of barbiturate anesthesia on discharge pattern in nucleus reticularis thalami. Pharmacol Biochem Behav. 1974 May;2(3):339–345. doi: 10.1016/0091-3057(74)90078-1. [DOI] [PubMed] [Google Scholar]
- Yen C. T., Conley M., Hendry S. H., Jones E. G. The morphology of physiologically identified GABAergic neurons in the somatic sensory part of the thalamic reticular nucleus in the cat. J Neurosci. 1985 Aug;5(8):2254–2268. doi: 10.1523/JNEUROSCI.05-08-02254.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]


