Abstract
A single glass micropipette voltage-clamp technique was used to study a potential-dependent calcium inward current in isolated smooth muscle cells of the guinea-pig taenia caeci. Experiments were performed at 22-24 degrees C. With potassium as the main cation in the pipette solution, a transient inward current appeared in response to a depolarizing pulse, followed by an outward current. The replacement of potassium ions by caesium ions and TEA (tetraethyl-ammonium) in the pipette solution resulted in an effective suppression of potassium outward current permitting a study of the calcium current solely. The calcium inward current was blocked by 5 mM-cobalt and 5 X 10(-6) M-verapamil. Activation of the calcium current occurred at a membrane potential of between -35 and -25 mV. The calcium current was maximal in the potential range +10 to +20 mV and did not reverse even at +60 mV. Inactivation of the calcium current had a complex nature. It did not inactivate completely even during depolarizations lasting many seconds. During the first 400 ms the decay of the calcium current followed a time course described by two exponentials. The fast time constant of decay was in the range of 40 to 53 ms (n = 3) and the slow time constant was approximately 10-fold greater (at 0 mV). The fast time constant did not depend on the membrane potential while the slow time constant decreased with depolarization. Availability of the calcium current was estimated in double-pulse experiments. It had a U-shaped dependence on the conditioning potential; maximal inactivation was observed at potentials corresponding to the maximal calcium current. It was suggested that a component of inactivation was dependent on the calcium current which flowed. Calculations of calcium entry at various depolarizations showed that large amounts of calcium ions enter the cell. Also, it was suggested that calcium ions are effectively bound within the smooth muscle cell.
Full text
PDF

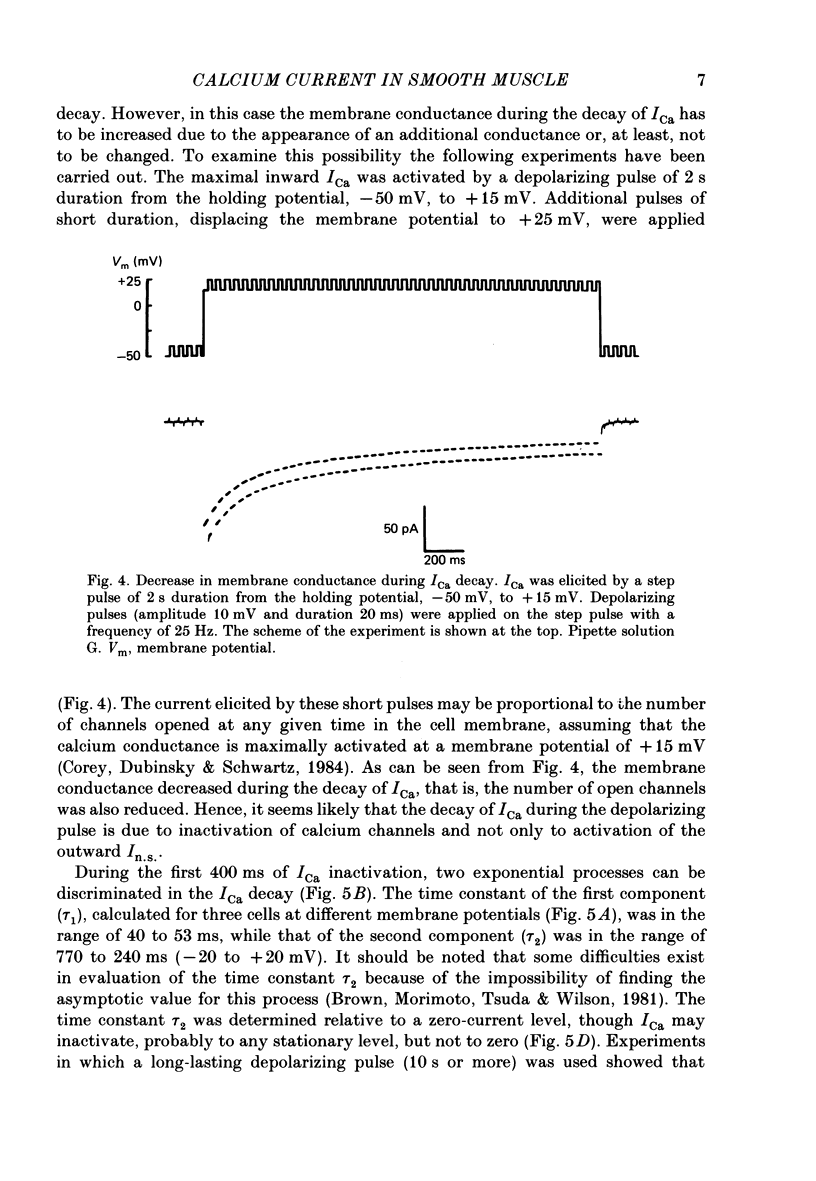
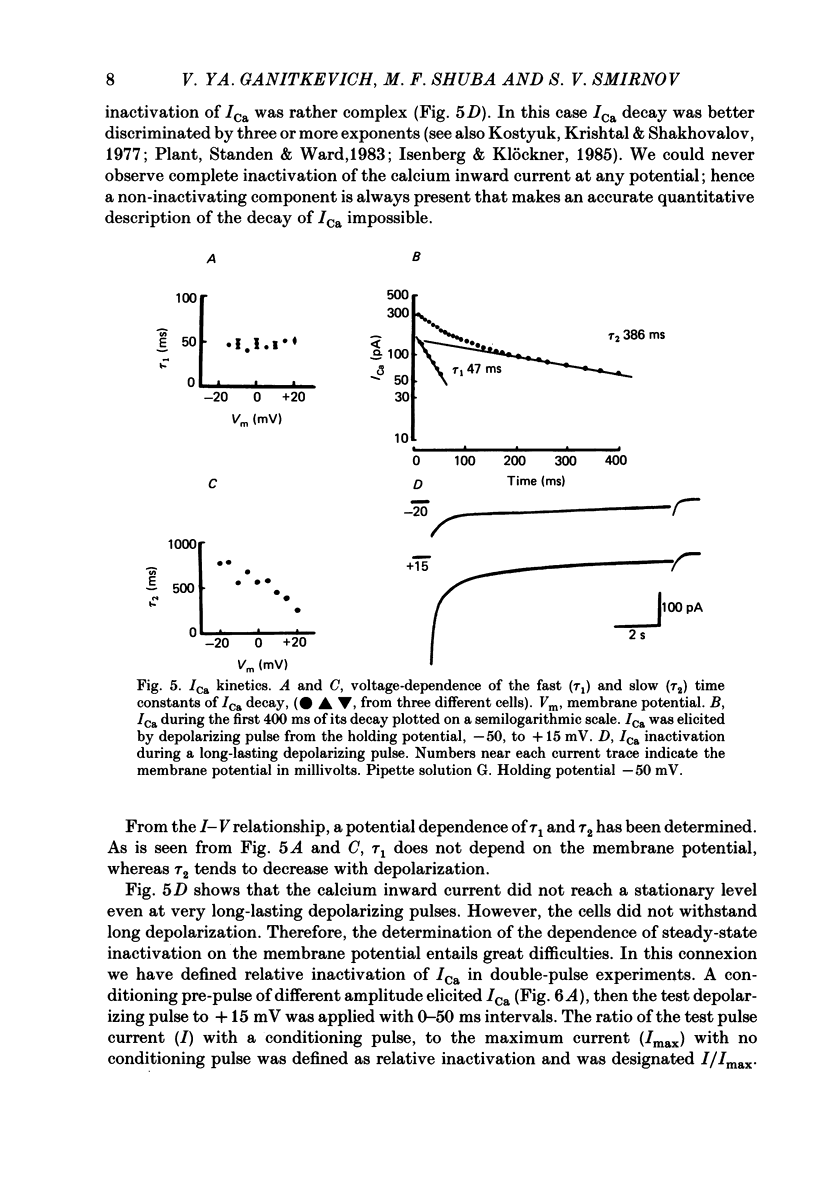
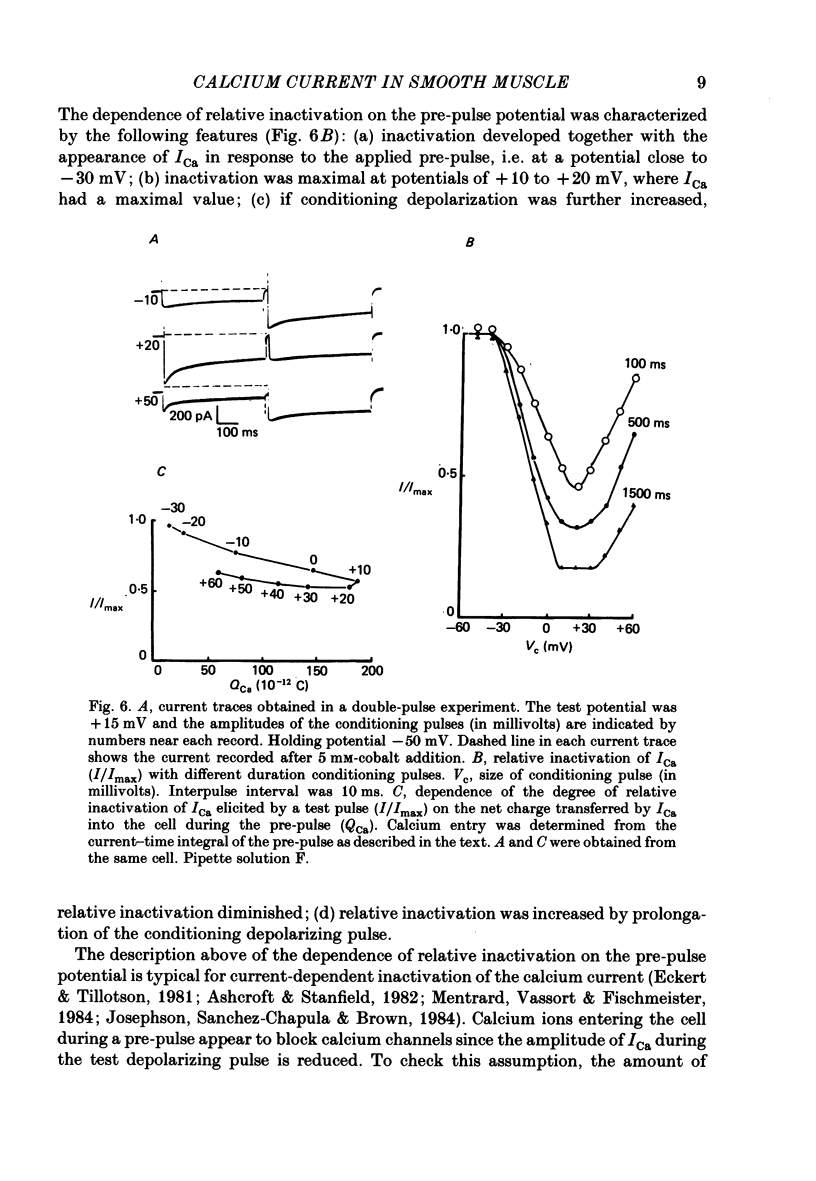
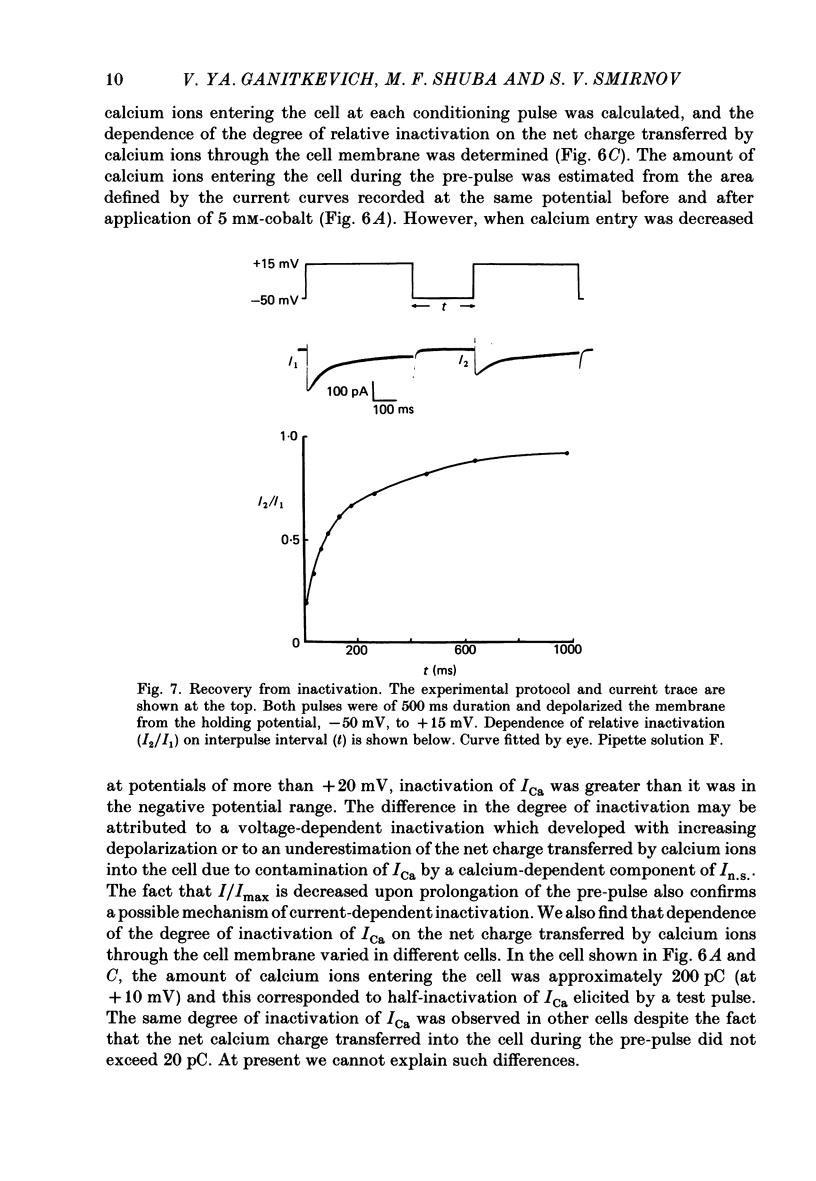
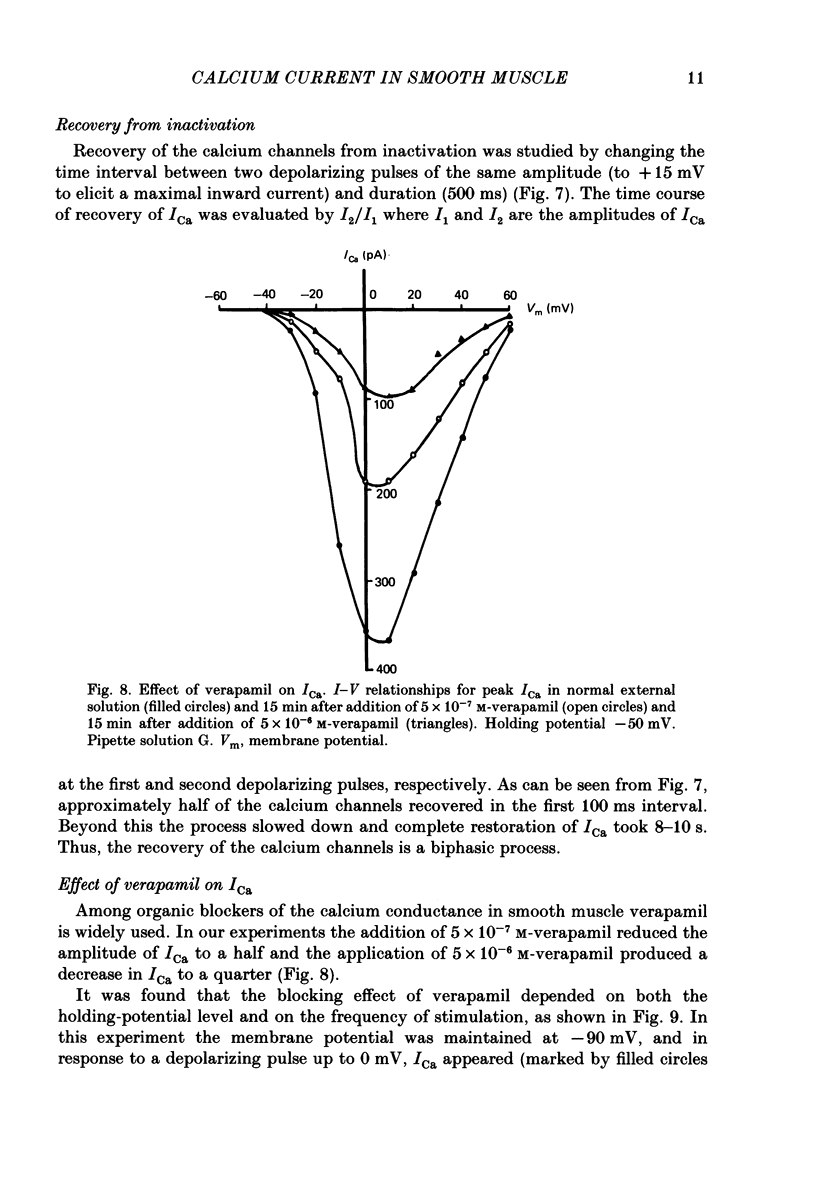
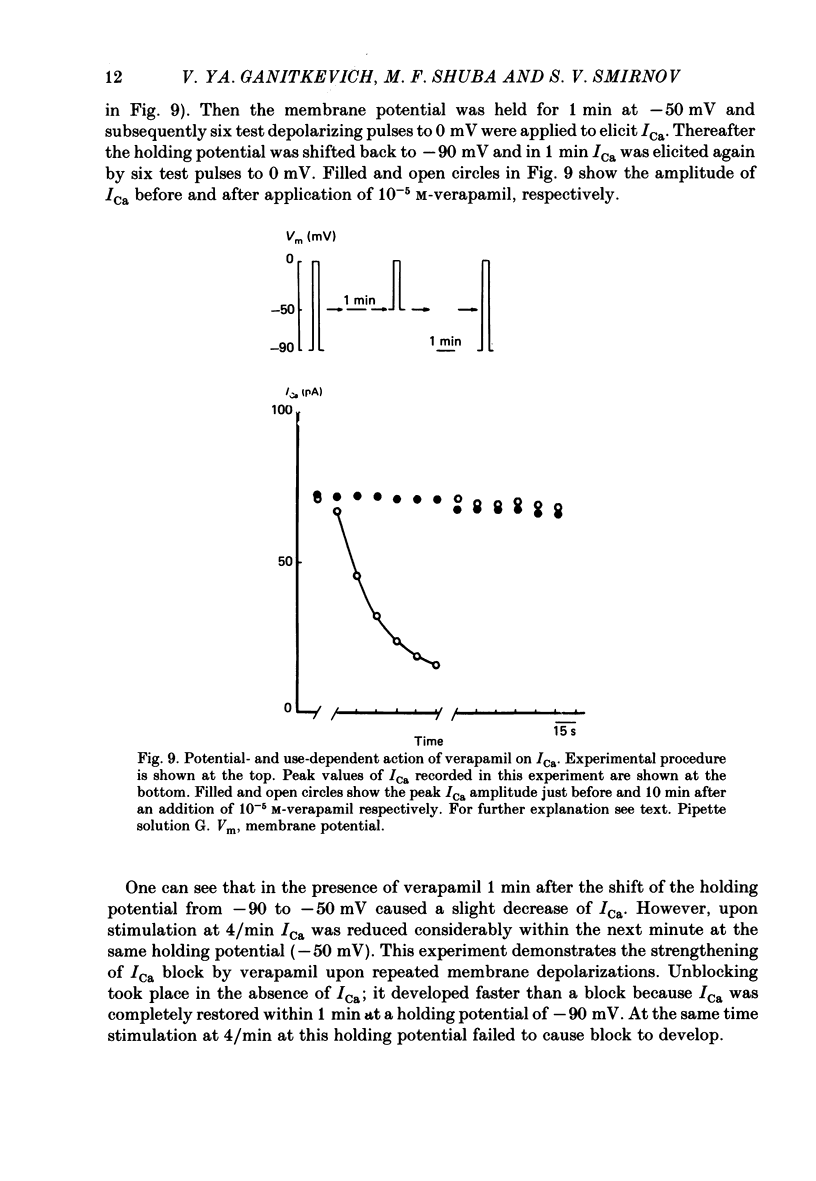




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashcroft F. M., Stanfield P. R. Calcium inactivation in skeletal muscle fibres of the stick insect, Carausius morosus. J Physiol. 1982 Sep;330:349–372. doi: 10.1113/jphysiol.1982.sp014345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. M., Morimoto K., Tsuda Y., wilson D. L. Calcium current-dependent and voltage-dependent inactivation of calcium channels in Helix aspersa. J Physiol. 1981 Nov;320:193–218. doi: 10.1113/jphysiol.1981.sp013944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buryi V. A., Gurkovskaia A. V., Shuba M. F. Vydelenie transmembrannogo kal'tsievogo toka gladkomyshechnykh kletok v beskalievoi srede. Dokl Akad Nauk SSSR. 1983;268(2):481–485. [PubMed] [Google Scholar]
- Byerly L., Hagiwara S. Calcium currents in internally perfused nerve cell bodies of Limnea stagnalis. J Physiol. 1982 Jan;322:503–528. doi: 10.1113/jphysiol.1982.sp014052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byerly L., Moody W. J. Intracellular calcium ions and calcium currents in perfused neurones of the snail, Lymnaea stagnalis. J Physiol. 1984 Jul;352:637–652. doi: 10.1113/jphysiol.1984.sp015314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey D. P., Dubinsky J. M., Schwartz E. A. The calcium current in inner segments of rods from the salamander (Ambystoma tigrinum) retina. J Physiol. 1984 Sep;354:557–575. doi: 10.1113/jphysiol.1984.sp015393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert R., Tillotson D. L. Calcium-mediated inactivation of the calcium conductance in caesium-loaded giant neurones of Aplysia californica. J Physiol. 1981 May;314:265–280. doi: 10.1113/jphysiol.1981.sp013706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fay F. S., Hoffmann R., Leclair S., Merriam P. Preparation of individual smooth muscle cells from the stomach of Bufo marinus. Methods Enzymol. 1982;85(Pt B):284–292. doi: 10.1016/0076-6879(82)85027-1. [DOI] [PubMed] [Google Scholar]
- Ganitkevich V. Ia, Smirnov S. V., Shuba M. F. Vydelenie kal'tsievogo toka v izolirovannykh gladkomyshechnykh kletkakh. Dokl Akad Nauk SSSR. 1985;282(3):717–720. [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Inomata H., Kao C. Y. Ionic currents in the guinea-pig taenia coli. J Physiol. 1976 Feb;255(2):347–378. doi: 10.1113/jphysiol.1976.sp011284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephson I. R., Sanchez-Chapula J., Brown A. M. A comparison of calcium currents in rat and guinea pig single ventricular cells. Circ Res. 1984 Feb;54(2):144–156. doi: 10.1161/01.res.54.2.144. [DOI] [PubMed] [Google Scholar]
- Kostyuk P. G., Krishtal O. A., Shakhovalov Y. A. Separation of sodium and calcium currents in the somatic membrane of mollusc neurones. J Physiol. 1977 Sep;270(3):545–568. doi: 10.1113/jphysiol.1977.sp011968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law R. O. Characteristics of ionic binding by rat renal tissue in vitro. J Physiol. 1984 Aug;353:67–80. doi: 10.1113/jphysiol.1984.sp015322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magura I. S. Long-lasting inward current in snail neurons in barium solutions in voltage-clamp conditions. J Membr Biol. 1977 Jul 14;35(3):239–256. doi: 10.1007/BF01869952. [DOI] [PubMed] [Google Scholar]
- Mentrard D., Vassort G., Fischmeister R. Calcium-mediated inactivation of the calcium conductance in cesium-loaded frog heart cells. J Gen Physiol. 1984 Jan;83(1):105–131. doi: 10.1085/jgp.83.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obara K. Isolation and contractile properties of single smooth muscle cells from guinea pig taenia caeci. Jpn J Physiol. 1984;34(1):41–54. doi: 10.2170/jjphysiol.34.41. [DOI] [PubMed] [Google Scholar]
- Plant T. D., Standen N. B., Ward T. A. The effects of injection of calcium ions and calcium chelators on calcium channel inactivation in Helix neurones. J Physiol. 1983 Jan;334:189–212. doi: 10.1113/jphysiol.1983.sp014489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer J. J., Walsh J. V. Large conductance ca-activated k channels in smooth muscle cell membrane: reduction in unitary currents due to internal na ions. Biophys J. 1984 Jan;45(1):68–70. doi: 10.1016/s0006-3495(84)84112-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh J. V., Jr, Singer J. J. Voltage clamp of single freshly dissociated smooth muscle cells: current-voltage relationships for three currents. Pflugers Arch. 1981 May;390(2):207–210. doi: 10.1007/BF00590209. [DOI] [PubMed] [Google Scholar]


