Abstract
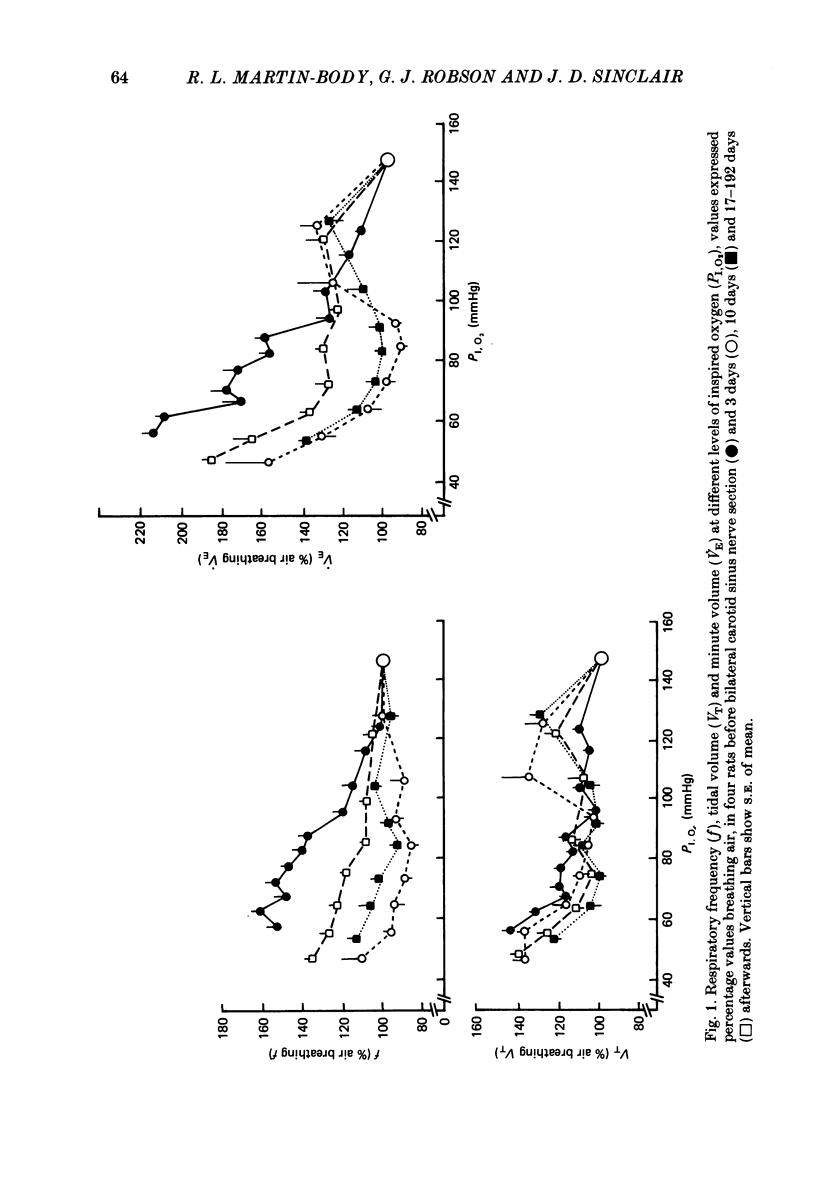
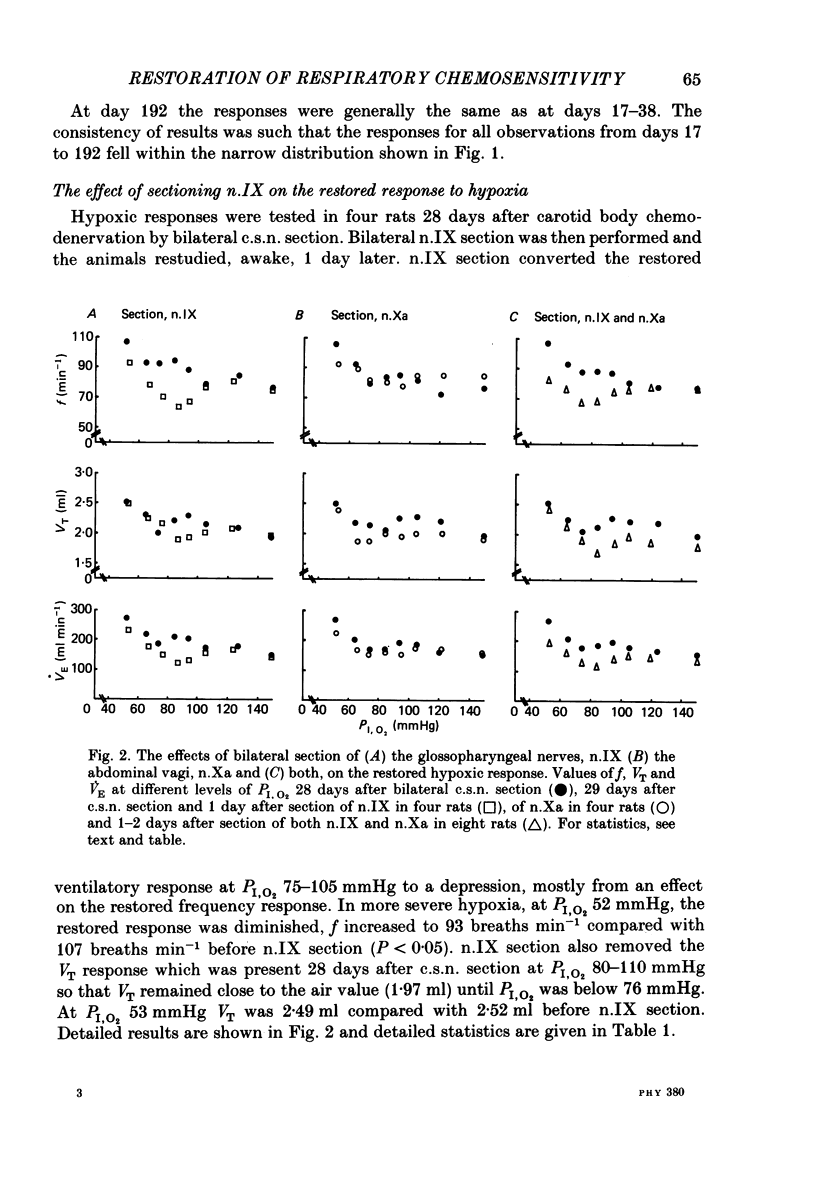
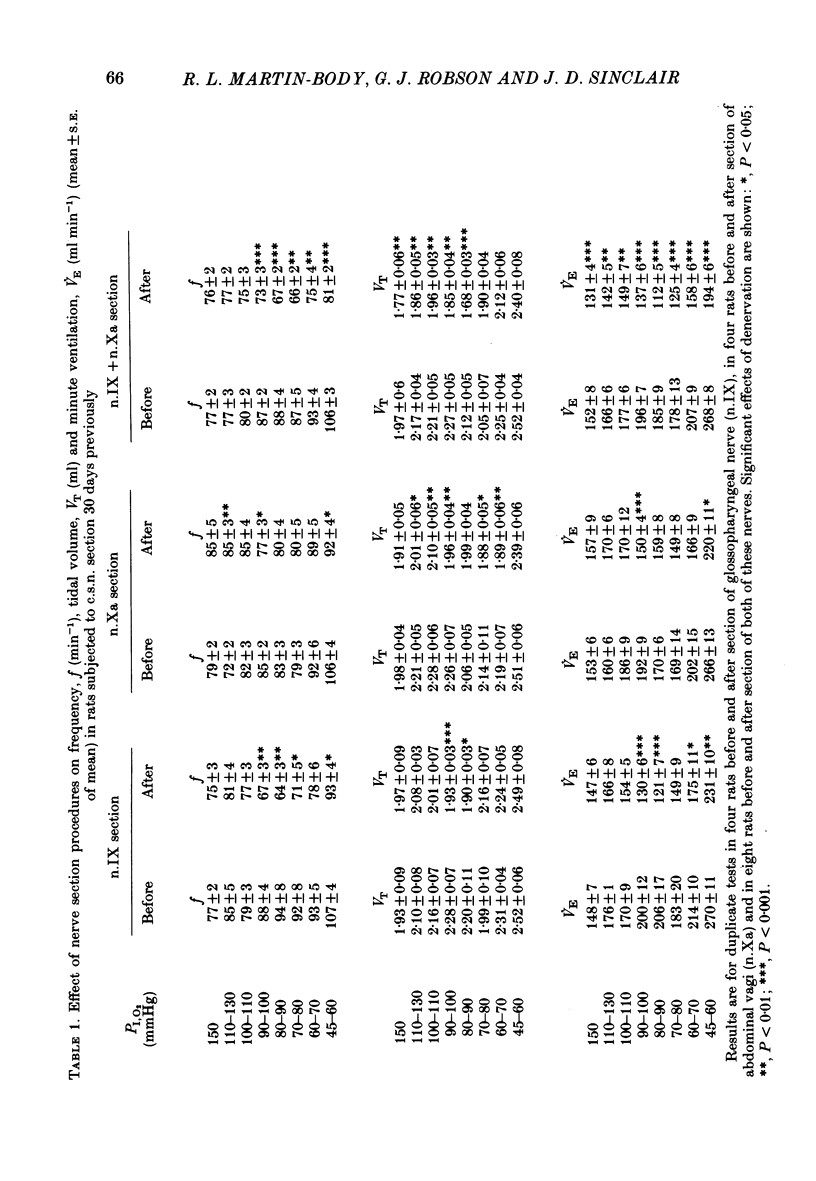
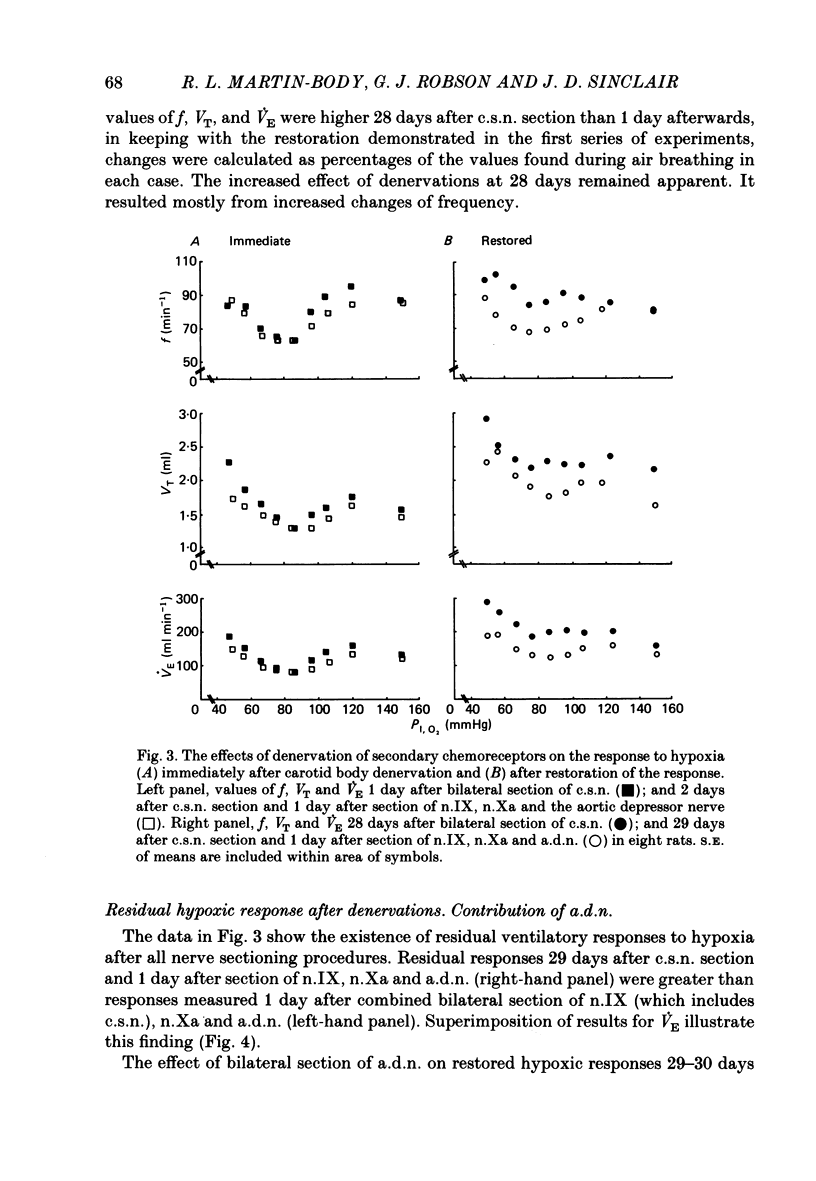
The restoration of ventilatory responses to hypoxia after carotid body denervation was studied in twenty-eight awake rats. The respiratory depression seen in moderate hypoxia (partial pressure of inspired O2, PI,O2, 80-100 mmHg) 3 days after bilateral carotid sinus nerve section disappeared by day 10. By day 17 respiratory stimulation occurred at all levels of PI,O2 below 125 mmHg. The largest restored response, in severe hypoxia (PI,O2 50-60 mmHg), was approximately 55% of the pre-denervation response. The response showed little further change from day 17 to day 192. A comparison of the effect of bilateral section of the glossopharyngeal nerve and of the abdominal vagus 1 and 28 days after carotid sinus nerve section demonstrated that the restoration of hypoxic response resulted in part from an enhanced effect of the inputs from the secondary glomus tissue served by these nerves. A comparison of the effect of bilateral section of glossopharyngeal, abdominal vagal and aortic depressor nerves 1 and 28 days after carotid sinus nerve section demonstrated an increase of a residual hypoxic response which must result either from inputs from unidentified peripheral chemoreceptors or from central mechanisms. Bilateral sectioning of the aortic depressor nerves produced no additional effect on restored responses to sectioning glossopharyngeal and abdominal vagal nerves, providing further evidence against significant aortic body function in the rat. The studies support the hypothesis that central neural reorganization provides compensation for loss of carotid body function by enhancement of effects of normally subsidiary inputs.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews W. H., Deane B. M., Howe A., Orbach J. Abdominal chemoreceptors in the rat. J Physiol. 1972 Apr;222(1):84P–85P. [PubMed] [Google Scholar]
- Bartlett D., Jr, Tenney S. M. Control of breathing in experimental anemia. Respir Physiol. 1970 Oct;10(3):384–395. doi: 10.1016/0034-5687(70)90056-3. [DOI] [PubMed] [Google Scholar]
- Bisgard G. E., Forster H. V., Klein J. P. Recovery of peripheral chemoreceptor function after denervation in ponies. J Appl Physiol Respir Environ Exerc Physiol. 1980 Dec;49(6):964–970. doi: 10.1152/jappl.1980.49.6.964. [DOI] [PubMed] [Google Scholar]
- Breslav I. S., Konza E. A. Vosstanovlenie khemoretseptornoi funktsii posle deafferentatsii sinokarotidnykh zon u krys. Fiziol Zh SSSR Im I M Sechenova. 1975 Jan;61(1):84–89. [PubMed] [Google Scholar]
- Eldridge F. L., Gill-Kumar P., Millhorn D. E. Input-output relationships of central neural circuits involved in respiration in cats. J Physiol. 1981 Feb;311:81–95. doi: 10.1113/jphysiol.1981.sp013574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming P. J., Levine M. R., Goncalves A. L., Woollard S. Barometric plethysmograph: advantages and limitations in recording infant respiration. J Appl Physiol Respir Environ Exerc Physiol. 1983 Dec;55(6):1924–1931. doi: 10.1152/jappl.1983.55.6.1924. [DOI] [PubMed] [Google Scholar]
- Forster H. V., Pan L. G., Bisgard G. E., Kaminski R. P., Dorsey S. M., Busch M. A. Hyperpnea of exercise at various PIO2 in normal and carotid body-denervated ponies. J Appl Physiol Respir Environ Exerc Physiol. 1983 May;54(5):1387–1393. doi: 10.1152/jappl.1983.54.5.1387. [DOI] [PubMed] [Google Scholar]
- Gallego R., Kuno M., Núez R., Snider W. D. Disuse enhances synaptic efficacy in spinal mononeurones. J Physiol. 1979 Jun;291:191–205. doi: 10.1113/jphysiol.1979.sp012807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda Y., Watanabe S., Hashizume I., Satomura Y., Hata N., Sakakibara Y., Severinghaus J. W. Hypoxic chemosensitivity in asthmatic patients two decades after carotid body resection. J Appl Physiol Respir Environ Exerc Physiol. 1979 Apr;46(4):632–638. doi: 10.1152/jappl.1979.46.4.632. [DOI] [PubMed] [Google Scholar]
- Howe A., Pack R. J., Wise J. C. Arterial chemoreceptor-like activity in the abdominal vagus of the rat. J Physiol. 1981 Nov;320:309–318. doi: 10.1113/jphysiol.1981.sp013951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kienecker E. W., Knoche H., Bingmann D. Functional properties of regenerating sinus nerve fibres in the rabbit. Neuroscience. 1978;3(10):977–988. doi: 10.1016/0306-4522(78)90118-5. [DOI] [PubMed] [Google Scholar]
- Lugliani R., Whipp B. J., Seard C., Wasserman K. Effect of bilateral carotid-body resection on ventilatory control at rest and during exercise in man. N Engl J Med. 1971 Nov;285(20):1105–1111. doi: 10.1056/NEJM197111112852002. [DOI] [PubMed] [Google Scholar]
- Majumdar S., Smith P. G., Mills E. Evidence for central reorganization of ventilatory chemoreflex pathways in the cat during regeneration of visceral afferents in the carotid sinus nerve. Neuroscience. 1982 May;7(5):1309–1316. doi: 10.1016/0306-4522(82)91136-8. [DOI] [PubMed] [Google Scholar]
- Martin-Body R. L., Robson G. J., Sinclair J. D. Respiratory effects of sectioning the carotid sinus glossopharyngeal and abdominal vagal nerves in the awake rat. J Physiol. 1985 Apr;361:35–45. doi: 10.1113/jphysiol.1985.sp015631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Body R. L., Sinclair J. D. Analysis of respiratory patterns in the awake and in the halothane anaesthetised rat. Respir Physiol. 1985 Jul;61(1):105–113. doi: 10.1016/0034-5687(85)90032-5. [DOI] [PubMed] [Google Scholar]
- Matsuura S. Chemoreceptor properties of glomus tissue found in the carotid region of the cat. J Physiol. 1973 Nov;235(1):57–73. doi: 10.1113/jphysiol.1973.sp010378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell R. A., Sinha A. K., Mcdonald D. M. Chemoreceptive properties of regenerated endings of the carotid sinus nerve. Brain Res. 1972 Aug 25;43(2):681–685. doi: 10.1016/0006-8993(72)90430-1. [DOI] [PubMed] [Google Scholar]
- Palkama A., Hopsu V. K. The presence of carotid body like structure between the right subclavian and common carotid arteries. Ann Med Exp Biol Fenn. 1965;43(3):169–173. [PubMed] [Google Scholar]
- Sapru H. N., Krieger A. J. Carotid and aortic chemoreceptor function in the rat. J Appl Physiol Respir Environ Exerc Physiol. 1977 Mar;42(3):344–348. doi: 10.1152/jappl.1977.42.3.344. [DOI] [PubMed] [Google Scholar]
- Sinclair J. D., St John W., Bartlett D. Enhancement of respiratory response to carbon dioxide produced by lesioning caudal regions of the nucleus of the tractus solitarius. Brain Res. 1985 Jun 17;336(2):318–320. doi: 10.1016/0006-8993(85)90659-6. [DOI] [PubMed] [Google Scholar]
- Smith P. G., Mills E. Restoration of reflex ventilatory response to hypoxia after removal of carotid bodies in the cat. Neuroscience. 1980;5(3):573–580. doi: 10.1016/0306-4522(80)90054-8. [DOI] [PubMed] [Google Scholar]
- Zapata P., Hess A., Eyzaguirre C. Reinnervation of carotid body and sinus with superior laryngeal nerve fibers. J Neurophysiol. 1969 Mar;32(2):215–228. doi: 10.1152/jn.1969.32.2.215. [DOI] [PubMed] [Google Scholar]



