Abstract
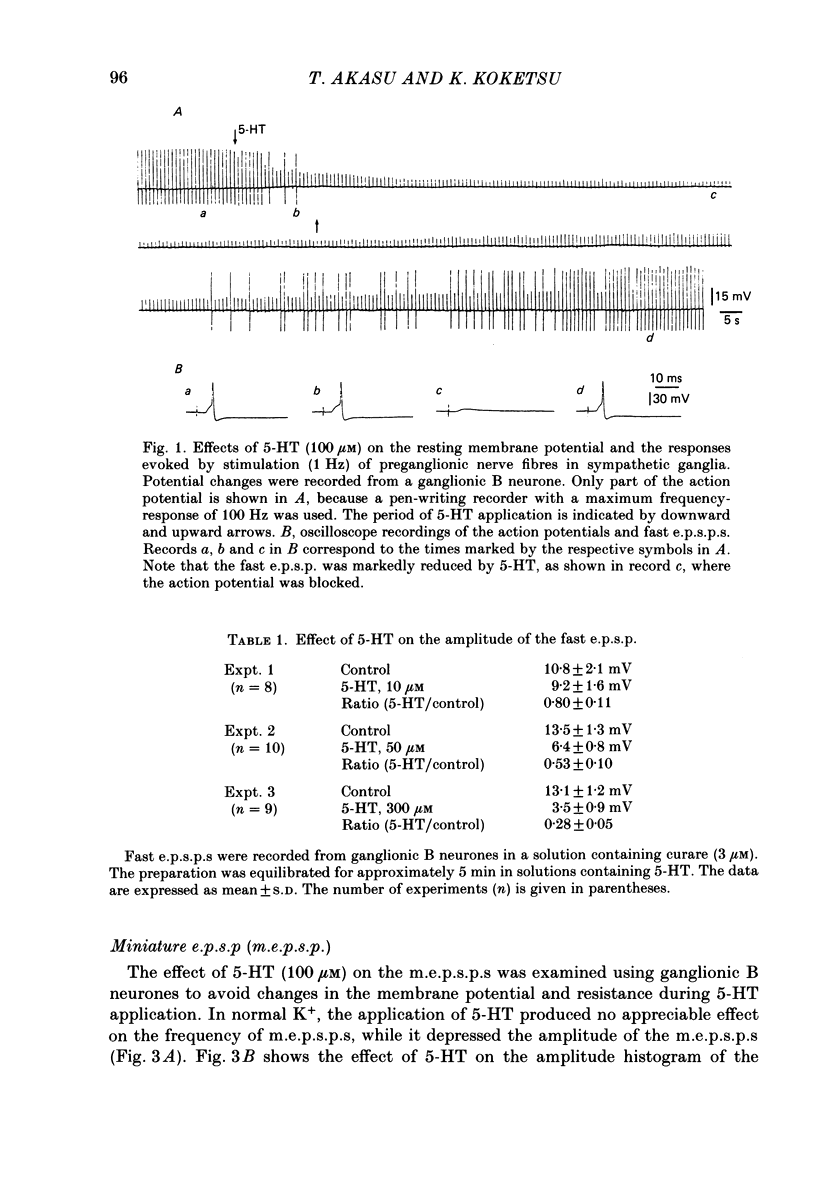
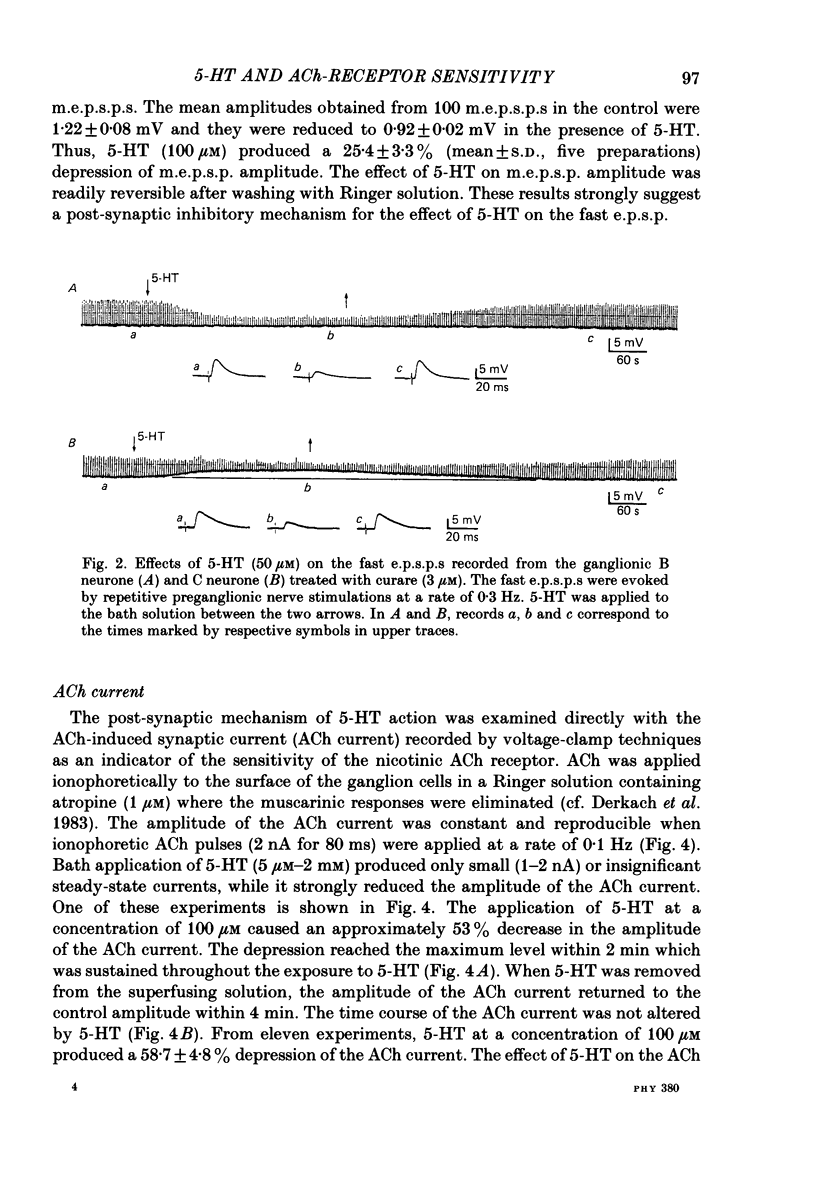
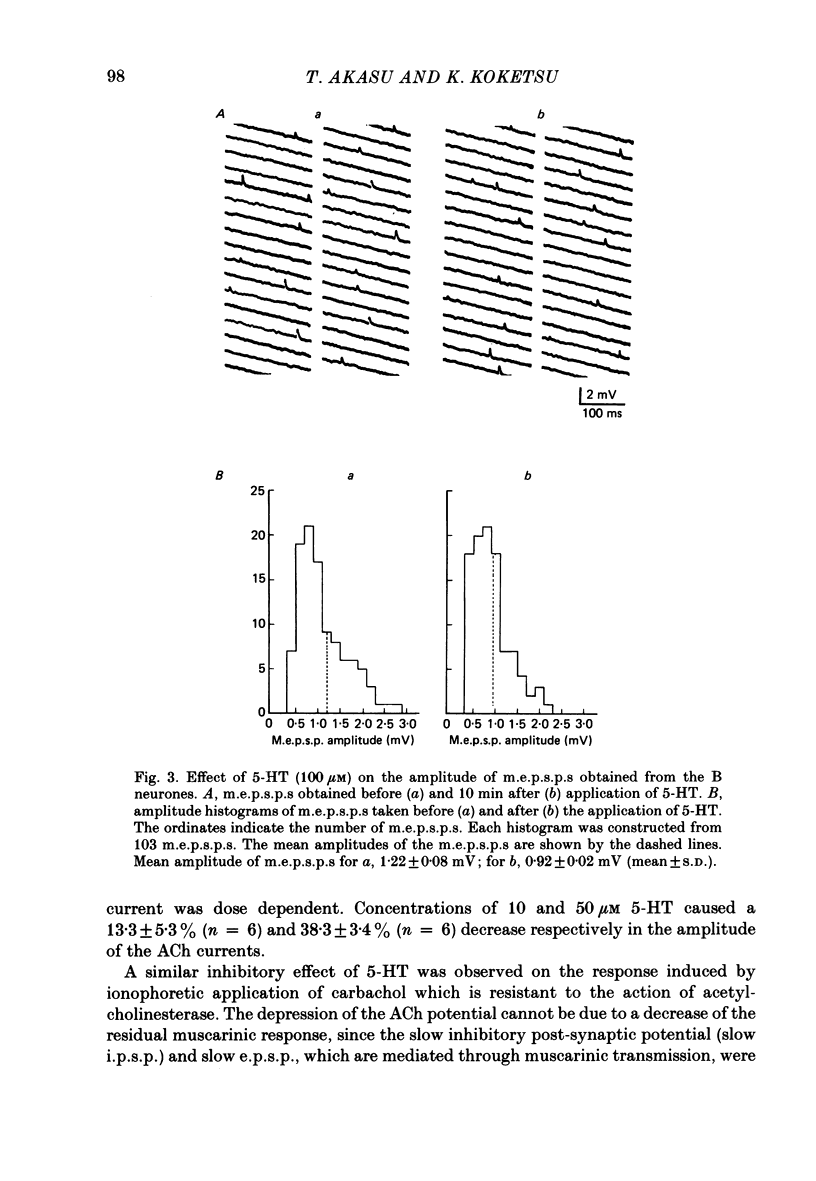
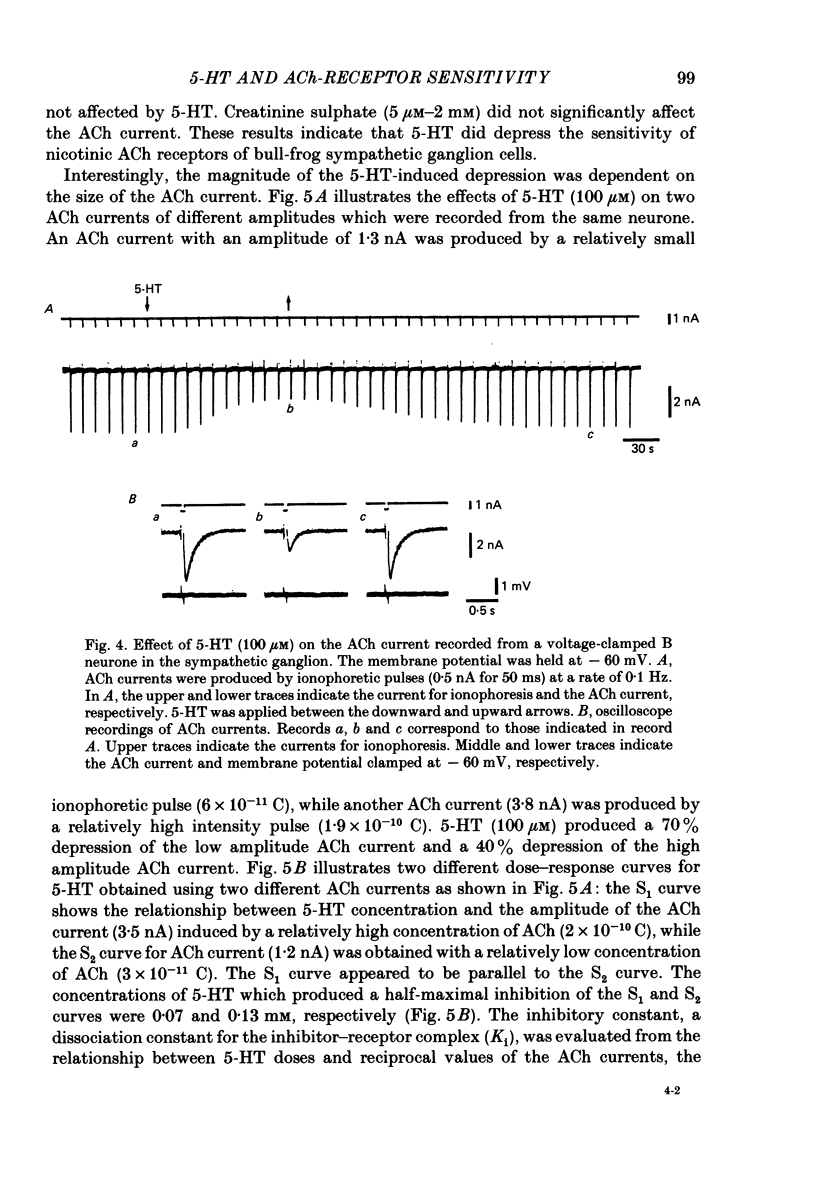
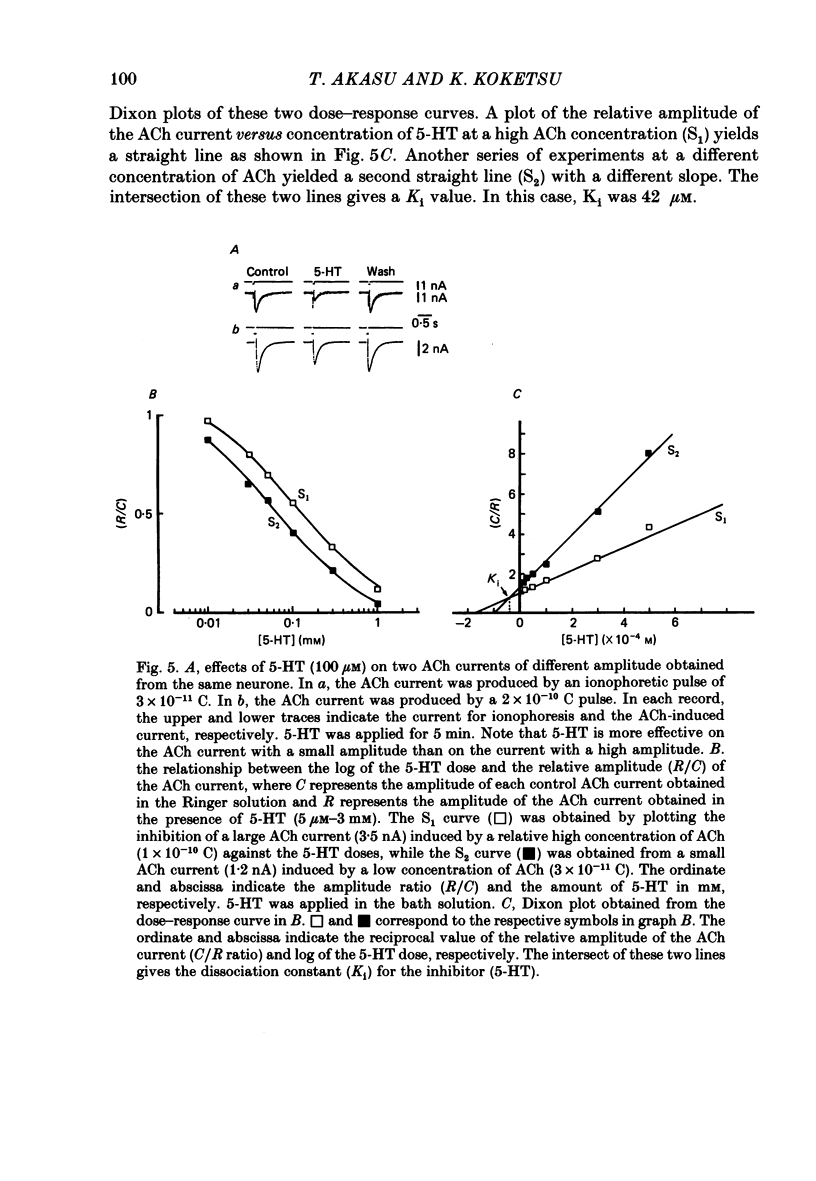
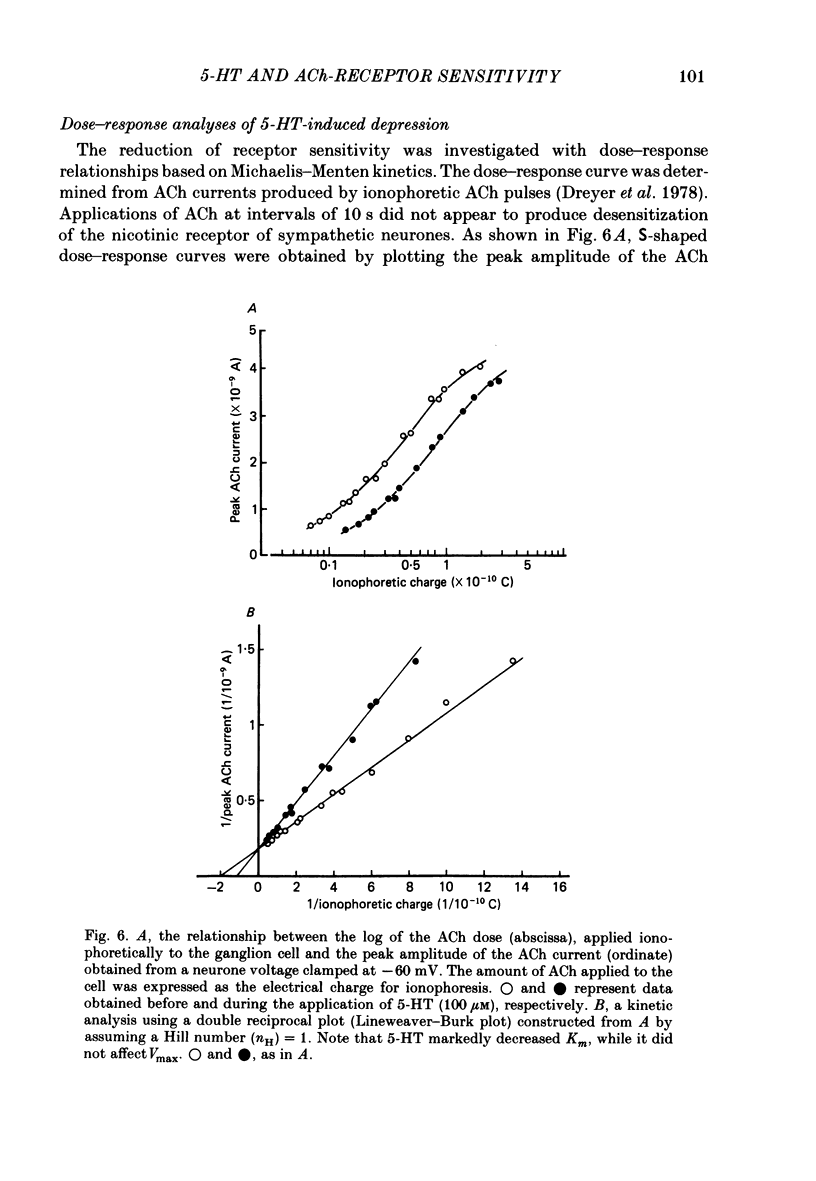
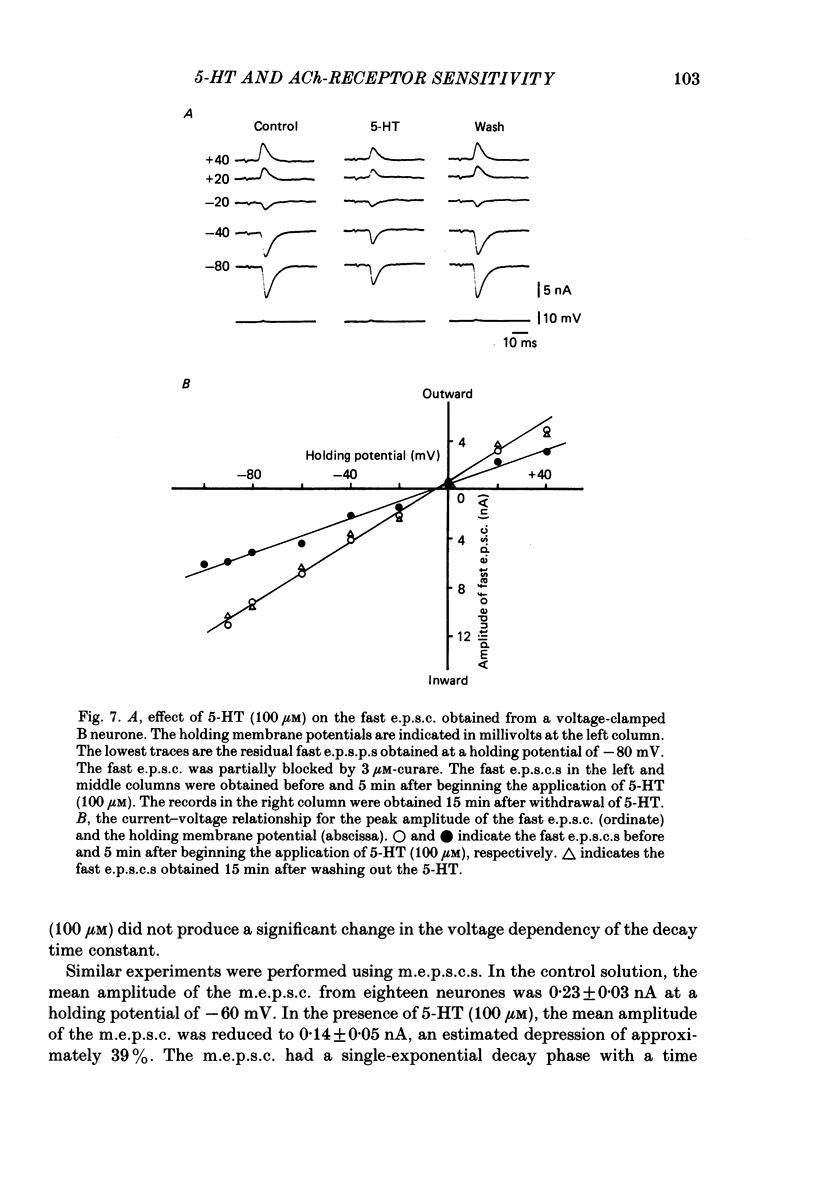
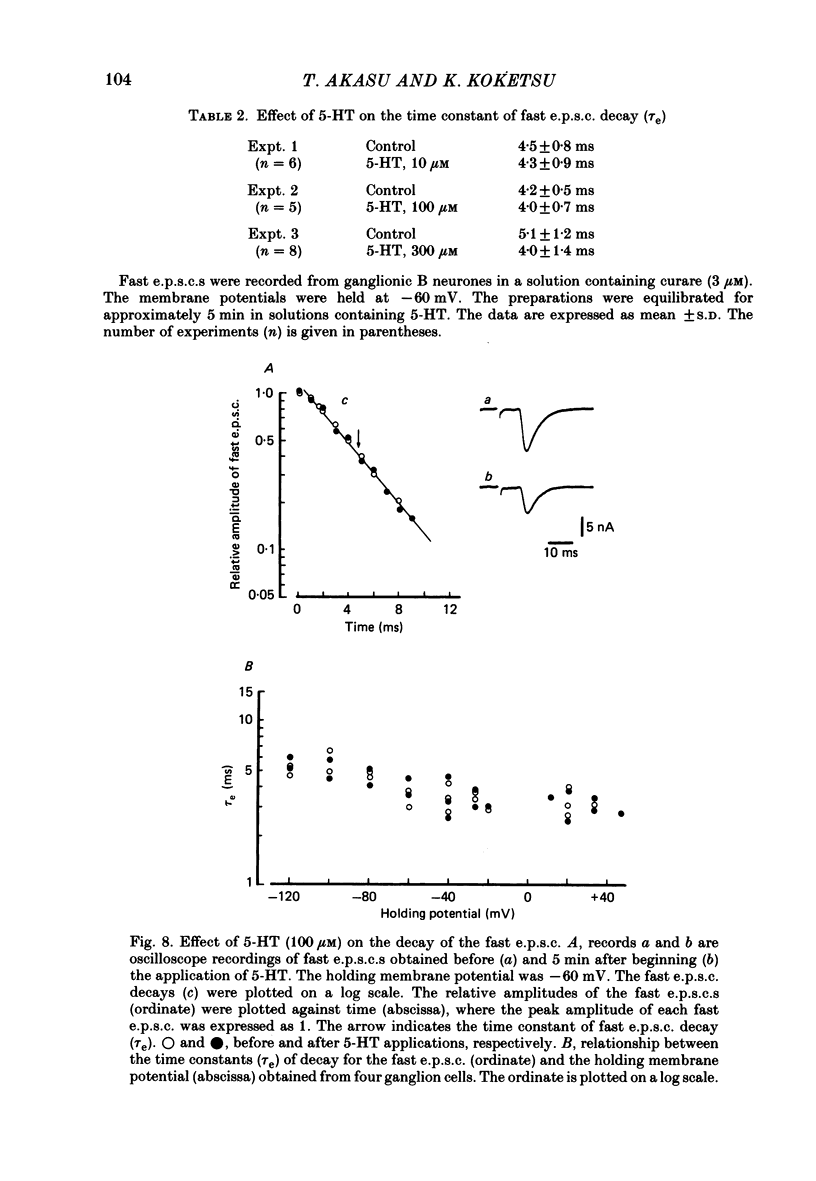
The post-synaptic effects of 5-hydroxytryptamine (5-HT) were examined in neurones of bull-frog sympathetic ganglia with intracellular micro-electrode and voltage-clamp recording techniques. Atropine (1 microM) was used to block the muscarinic cholinoceptors. 5-HT reduced the amplitude of the fast excitatory post-synaptic potential (fast e.p.s.p.). 5-HT also reduced the mean amplitude of the miniature excitatory post-synaptic potentials (m.e.p.s.p.s) without affecting their frequency. Voltage-clamp studies showed that 5-HT decreased in a dose-dependent manner the amplitude of the acetylcholine (ACh) current produced by ionophoretic application of ACh to sympathetic neurones. The relationship between the log of the ACh dose, applied ionophoretically, and the peak ACh current (the dose-response curve) was examined in voltage-clamped neurones. 5-HT caused a parallel shift to the right of the dose-response curve for ACh. Analysis using a double reciprocal plot (Lineweaver-Burk plot) revealed that 5-HT increased the apparent dissociation constant (Km) of ACh for the receptor without changing the maximum ACh current (Vmax), suggesting a competitive antagonism. The relationship between the 5-HT dose and the magnitude of inhibition of the ACh current was obtained using two different amplitudes for the ACh response. The dose-response curve of 5-HT-induced inhibition using a relatively high amplitude ACh current, S1, was parallel with that for a relatively low amplitude ACh current, S2. The Dixon plot of these two curves yielded an apparent inhibition constant (Ki) of 42 microM. Both fast excitatory post-synaptic currents (fast e.p.s.c.s) and miniature excitatory post-synaptic currents (m.e.p.s.c.s) had single-exponential decay time courses. The time constants of fast e.p.s.c. decay (tau e) and m.e.p.s.c. decay (tau m) were not altered by 5-HT, suggesting that 5-HT does not change the kinetics of opening and closing of the ionic channel associated with the nicotinic receptor. 5-HT did not alter the reversal potential of the fast e.p.s.c. These results suggest that 5-HT decreases the sensitivity of the nicotinic receptor of sympathetic neurones, by interfering with ACh binding at the active site on the receptor-ionic-channel complex. 5-HT may physiologically inhibit cholinergic transmission as it is an endogenous substance which antagonizes the nicotinic receptor in post-ganglionic neurones of bull-frog sympathetic ganglia.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akasu T., Hirai K., Koketsu K. 5-hydroxytryptamine controls ACh-receptor sensitivity of bullfrog sympathetic ganglion cells. Brain Res. 1981 Apr 27;211(1):217–220. doi: 10.1016/0006-8993(81)90087-1. [DOI] [PubMed] [Google Scholar]
- Akasu T., Karczmar A. G., Koketsu K. Effects of serotonin (5-hydroxytryptamine) on amphibian neuromuscular junction. Eur J Pharmacol. 1983 Mar 18;88(1):63–70. doi: 10.1016/0014-2999(83)90392-8. [DOI] [PubMed] [Google Scholar]
- Akasu T., Koketsu K. Voltage-clamp studies of a slow inward current in bullfrog sympathetic ganglion cells. Neurosci Lett. 1981 Nov 4;26(3):259–262. doi: 10.1016/0304-3940(81)90142-7. [DOI] [PubMed] [Google Scholar]
- Colomo F., Rahamimoff R., Stefani E. An action of 5-hydroxytryptamine on the frog motor end-plate. Eur J Pharmacol. 1968 Jun;3(3):272–274. doi: 10.1016/0014-2999(68)90143-x. [DOI] [PubMed] [Google Scholar]
- De Groat W. C., Lalley P. M. Interaction between picrotoxin and 5-hydroxytryptamine in the superior cervical ganglion of the cat. Br J Pharmacol. 1973 Jun;48(2):233–244. doi: 10.1111/j.1476-5381.1973.tb06909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Groat W. C., Volle R. L. Interactions between the catecholamines and ganglionic stimulating agents in sympathetic ganglia. J Pharmacol Exp Ther. 1966 Nov;154(2):200–215. [PubMed] [Google Scholar]
- Derkach V. A., Selyanko A. A., Skok V. I. Acetylcholine-induced current fluctuations and fast excitatory post-synaptic currents in rabbit sympathetic neurones. J Physiol. 1983 Mar;336:511–526. doi: 10.1113/jphysiol.1983.sp014595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer F., Peper K., Sterz R. Determination of dose-response curves by quantitative ionophoresis at the frog neuromuscular junction. J Physiol. 1978 Aug;281:395–419. doi: 10.1113/jphysiol.1978.sp012430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dun N. J., Karczmar A. G. Evidence for a presynaptic inhibitory action of 5-hydroxytryptamine in a mammalian sympathetic ganglion. J Pharmacol Exp Ther. 1981 Jun;217(3):714–718. [PubMed] [Google Scholar]
- Dun N. J., Kiraly M., Ma R. C. Evidence for a serotonin-mediated slow excitatory potential in the guinea-pig coeliac ganglia. J Physiol. 1984 Jun;351:61–76. doi: 10.1113/jphysiol.1984.sp015232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES R. M., LIBET B. Origin and blockade of the synaptic responses of curarized sympathetic ganglia. J Physiol. 1961 Aug;157:484–503. doi: 10.1113/jphysiol.1961.sp006738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller R. W. Pharmacology of central serotonin neurons. Annu Rev Pharmacol Toxicol. 1980;20:111–127. doi: 10.1146/annurev.pa.20.040180.000551. [DOI] [PubMed] [Google Scholar]
- GERTNER S. B., PASSONEN M. K., GIARMAN N. J. Studies concerning the presence of 5-hydroxytryptamine (serotonin) in the perfusate from the superior cervical ganglion. J Pharmacol Exp Ther. 1959 Dec;127:268–275. [PubMed] [Google Scholar]
- GYERMEK L., BINDLER E. Blockade of the ganglionic stimulant action of 5-hydroxytryptamin. J Pharmacol Exp Ther. 1962 Mar;135:344–348. [PubMed] [Google Scholar]
- Gerschenfeld H. M., Paupardin-Tritsch D. Ionic mechanisms and receptor properties underlying the responses of molluscan neurones to 5-hydroxytryptamine. J Physiol. 1974 Dec;243(2):427–456. doi: 10.1113/jphysiol.1974.sp010761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERTZLER E. C. 5-Hydroxytryptamine and transmission in sympathetic ganglia. Br J Pharmacol Chemother. 1961 Dec;17:406–413. doi: 10.1111/j.1476-5381.1961.tb01126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigler H. J., Aghajanian G. K. Serotonin receptors in the brain. Fed Proc. 1977 Jul;36(8):2159–2164. [PubMed] [Google Scholar]
- Higashi H., Nishi S. 5-Hydroxytryptamine receptors of visceral primary afferent neurones on rabbit nodose ganglia. J Physiol. 1982 Feb;323:543–567. doi: 10.1113/jphysiol.1982.sp014091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai K., Koketsu K. Presynaptic regulation of the release of acetylcholine by 5-hydroxytryptamine. Br J Pharmacol. 1980 Nov;70(3):499–500. doi: 10.1111/j.1476-5381.1980.tb08729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst G. D., Silinsky E. M. Some effects of 5-hydroxytryptamine, dopamine and noradrenaline on neurones in the submucous plexus of guinea-pig small intestine. J Physiol. 1975 Oct;251(3):817–832. doi: 10.1113/jphysiol.1975.sp011124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaramillo J., Volle R. L. A comparison of the ganglionic stimulating and blocking properties of some nicotinic drugs. Arch Int Pharmacodyn Ther. 1968 Jul;174(1):88–97. [PubMed] [Google Scholar]
- Kato E., Kuba K., Koketsu K. Effects of elabutoxins on neuromuscular transmission in frog skeletal muscles. J Pharmacol Exp Ther. 1978 Feb;204(2):446–453. [PubMed] [Google Scholar]
- Kato E., Kuba K., Koketsu K. Effects of erabutoxins on the cholinergic receptors of bullfrog sympathetic ganglion cells. Brain Res. 1980 Jun 2;191(1):294–298. doi: 10.1016/0006-8993(80)90336-4. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. The binding of acetylcholine to receptors and its removal from the synaptic cleft. J Physiol. 1973 Jun;231(3):549–574. doi: 10.1113/jphysiol.1973.sp010248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koketsu K., Akasu T., Miyagawa M., Hirai K. Biogenic antagonists of the nicotinic receptor: their interactions with erabutoxin. Brain Res. 1982 Nov 4;250(2):391–393. doi: 10.1016/0006-8993(82)90438-3. [DOI] [PubMed] [Google Scholar]
- Koketsu K. Cholinergic synaptic potentials and the underlying ionic mechasims. Fed Proc. 1969 Jan-Feb;28(1):101–112. [PubMed] [Google Scholar]
- Koketsu K. Modulation of receptor sensitivity and action potentials by transmitters in vertebrate neurones. Jpn J Physiol. 1984;34(6):945–960. doi: 10.2170/jjphysiol.34.945. [DOI] [PubMed] [Google Scholar]
- Kuba K., Koketsu K. Synaptic events in sympathetic ganglia. Prog Neurobiol. 1978;11(2):77–169. doi: 10.1016/0301-0082(78)90010-2. [DOI] [PubMed] [Google Scholar]
- Kuba K., Nishi S. Characteristics of fast excitatory postsynaptic current in bullfrog sympathetic ganglion cells. Effects of membrane potential, temperature and Ca ions. Pflugers Arch. 1979 Jan 31;378(3):205–212. doi: 10.1007/BF00592737. [DOI] [PubMed] [Google Scholar]
- Libet B. Generation of slow inhibitory and excitatory postsynaptic potentials. Fed Proc. 1970 Nov-Dec;29(6):1945–1956. [PubMed] [Google Scholar]
- MacDermott A. B., Connor E. A., Dionne V. E., Parsons R. L. Voltage clamp study of fast excitatory synaptic currents in bullfrog sympathetic ganglion cells. J Gen Physiol. 1980 Jan;75(1):39–60. doi: 10.1085/jgp.75.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NISHI S., KOKETSU K. Electrical properties and activities of single sympathetic neurons in frogs. J Cell Comp Physiol. 1960 Feb;55:15–30. doi: 10.1002/jcp.1030550104. [DOI] [PubMed] [Google Scholar]
- Nishi S., Soeda H., Koketsu K. Studies on sympathetic B and C neurons and patterns of pregnaglionic innervation. J Cell Physiol. 1965 Aug;66(1):19–32. doi: 10.1002/jcp.1030660103. [DOI] [PubMed] [Google Scholar]
- Peroutka S. J., Snyder S. H. Multiple serotonin receptors and their physiological significance. Fed Proc. 1983 Feb;42(2):213–217. [PubMed] [Google Scholar]
- TAKEUCHI A., TAKEUCHI N. Active phase of frog's end-plate potential. J Neurophysiol. 1959 Jul;22(4):395–411. doi: 10.1152/jn.1959.22.4.395. [DOI] [PubMed] [Google Scholar]
- TRENDELENBURG U. The action of histamine, pilocarpine and 5-hydroxytryptamine on transmission through the superior cervical ganglion. J Physiol. 1957 Jan 23;135(1):66–72. doi: 10.1113/jphysiol.1957.sp005695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhofstad A. A., Steinbusch H. W., Penke B., Varga J., Joosten H. W. Serotonin-immunoreactive cells in the superior cervical ganglion of the rat. Evidence for the existence of separate serotonin- and catecholamine-containing small ganglionic cells. Brain Res. 1981 May 11;212(1):39–49. doi: 10.1016/0006-8993(81)90030-5. [DOI] [PubMed] [Google Scholar]
- Wallis D. I., North R. A. The action of 5-hydroxytryptamine on single neurones of the rabbit superior cervical ganglion. Neuropharmacology. 1978 Dec;17(12):1023–1028. doi: 10.1016/0028-3908(78)90028-x. [DOI] [PubMed] [Google Scholar]
- Wallis D. I., Woodward B. The facilitatory actions of 5-hydroxytryptamine and bradykinin in the superior cervical ganglion of the rabbit. Br J Pharmacol. 1974 Aug;51(4):521–531. doi: 10.1111/j.1476-5381.1974.tb09670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S., Koketsu K. 5-HT hyperpolarization of bullfrog sympathetic ganglion cell membrane. Experientia. 1973 Nov 15;29(11):1370–1372. doi: 10.1007/BF01922825. [DOI] [PubMed] [Google Scholar]
- Weber M., Changeux J. P. Binding of Naja nigricollis (3H)alpha-toxin to membrane fragments from Electrophorus and Torpedo electric organs. 3. Effects of local anaesthetics on the binding of the tritiated alpha-neurotoxin. Mol Pharmacol. 1974 Jan;10(1):35–40. [PubMed] [Google Scholar]
- Wood J. D., Mayer C. J. Serotonergic activation of tonic-type enteric neurons in guinea pig small bowel. J Neurophysiol. 1979 Mar;42(2):582–593. doi: 10.1152/jn.1979.42.2.582. [DOI] [PubMed] [Google Scholar]


