Abstract
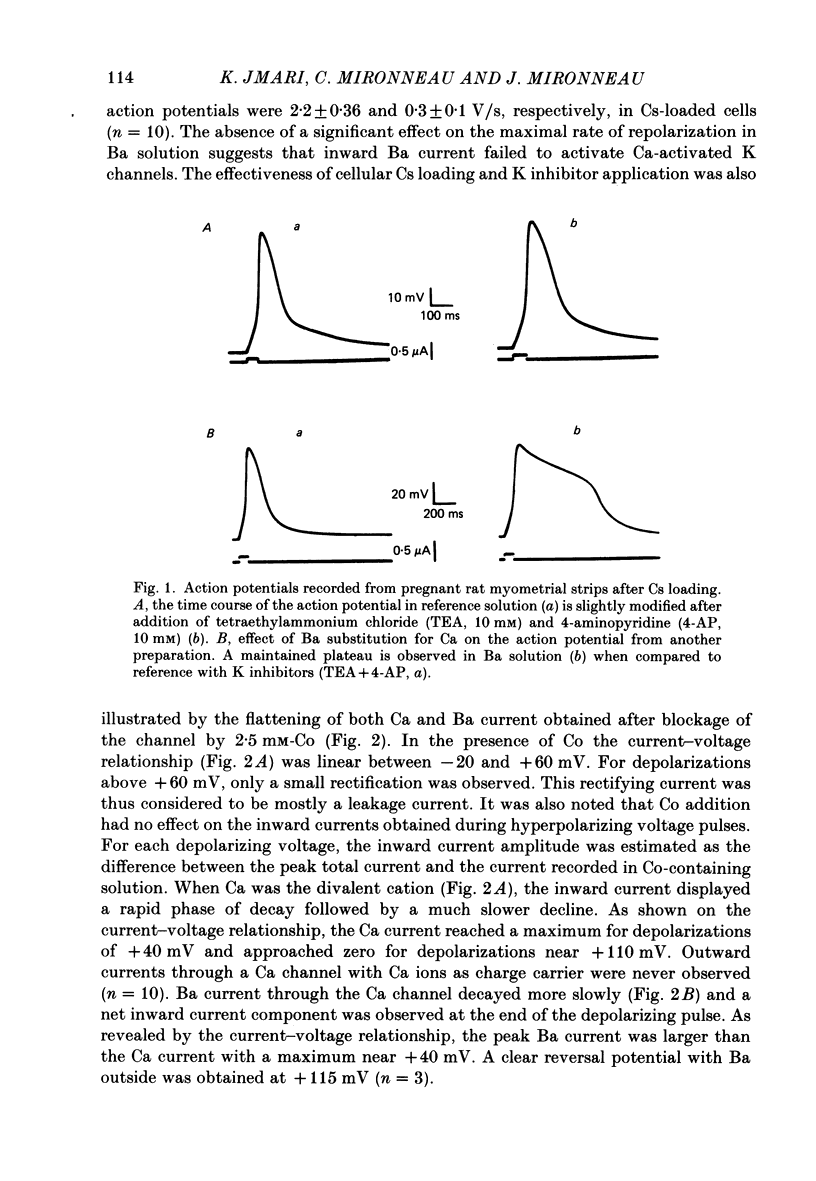
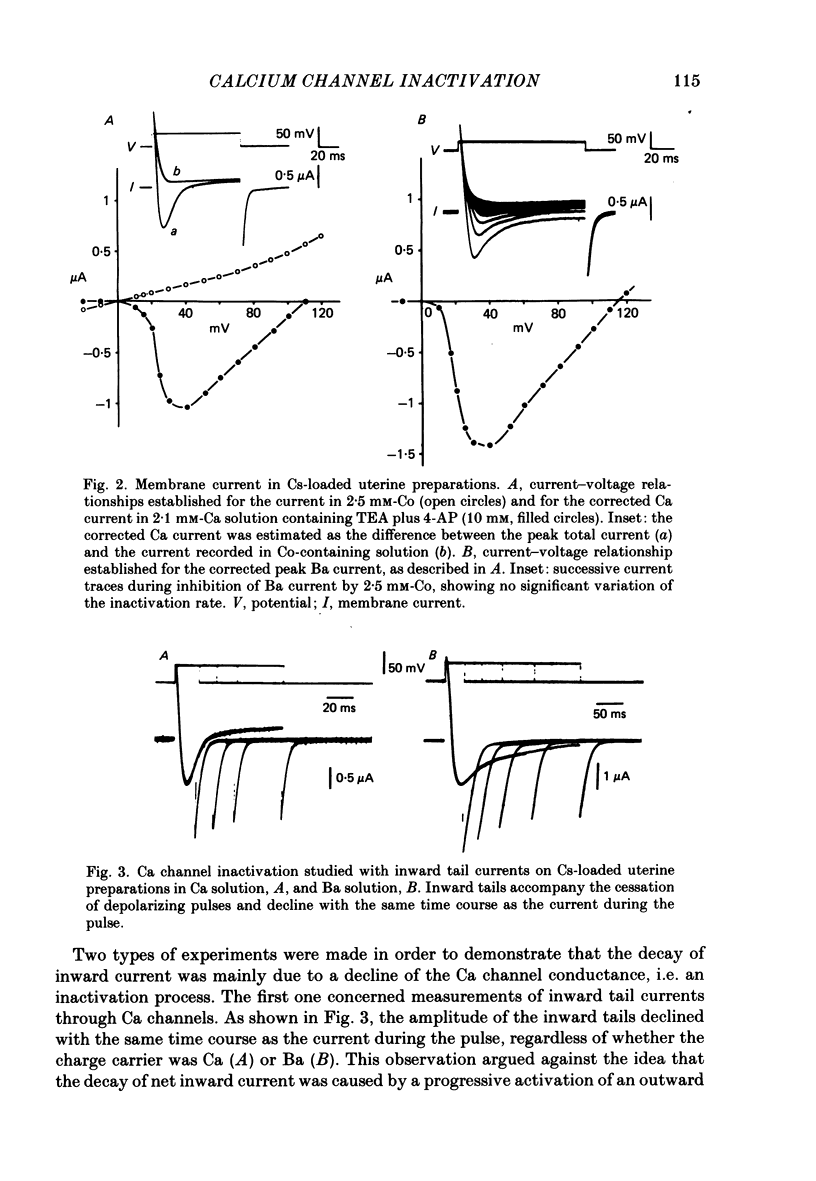
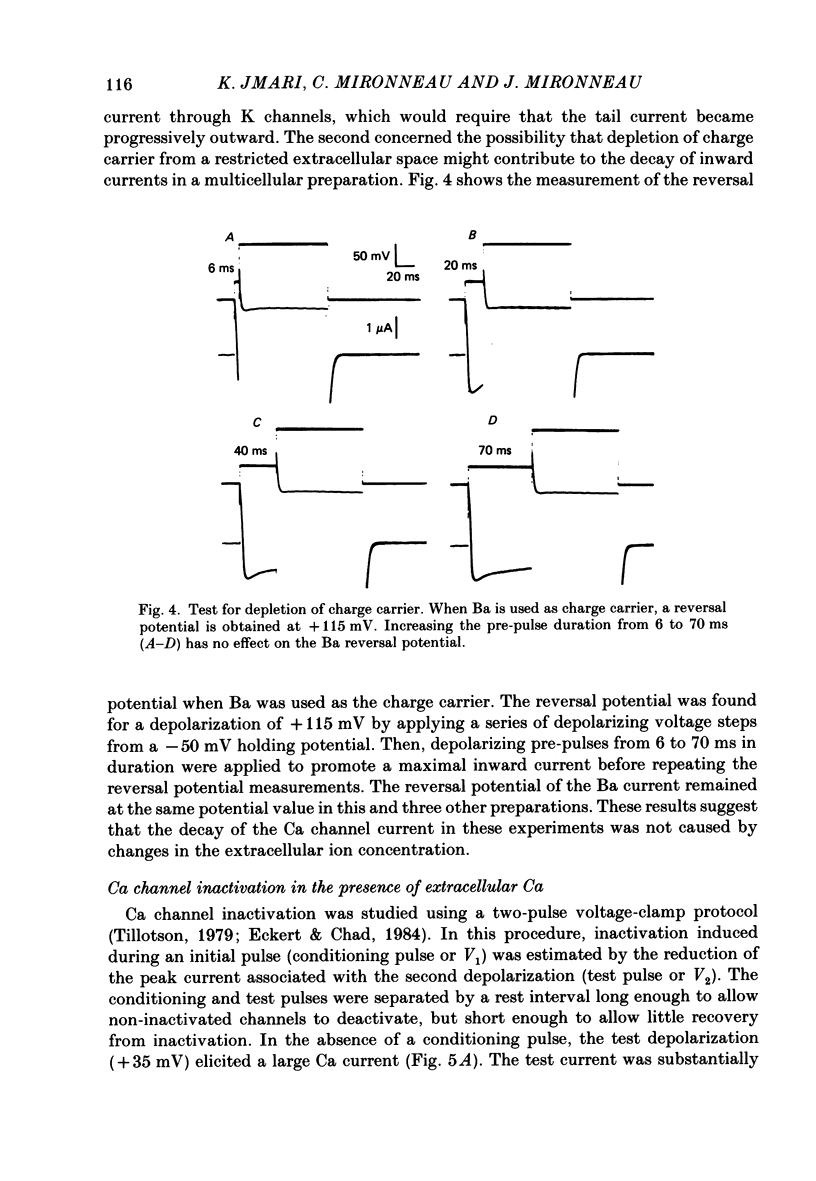
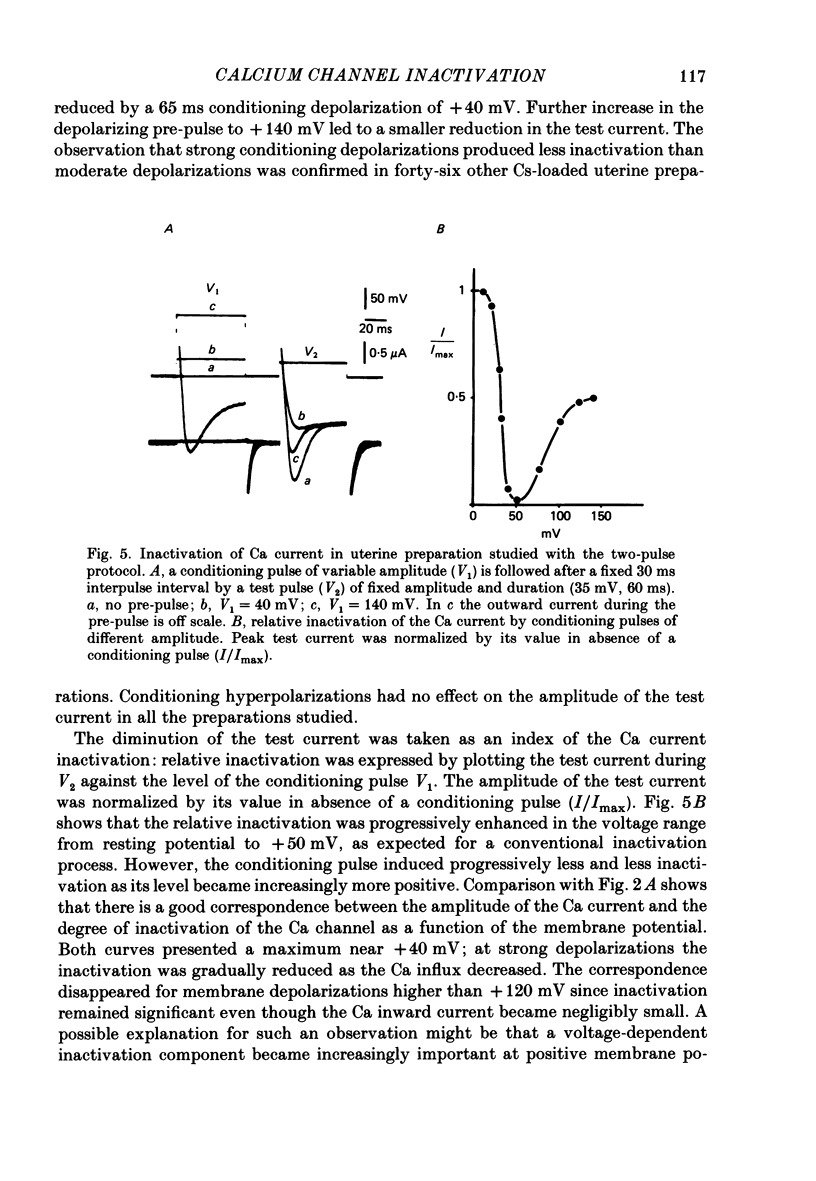
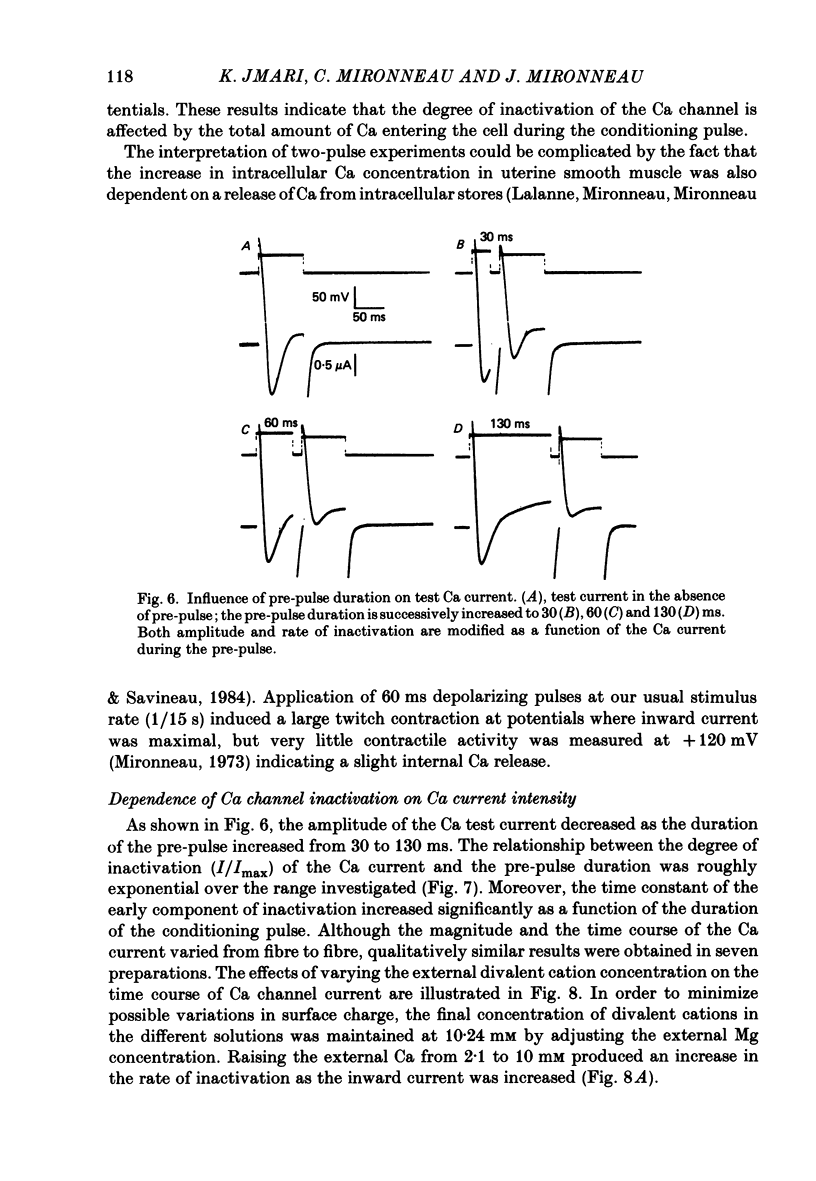
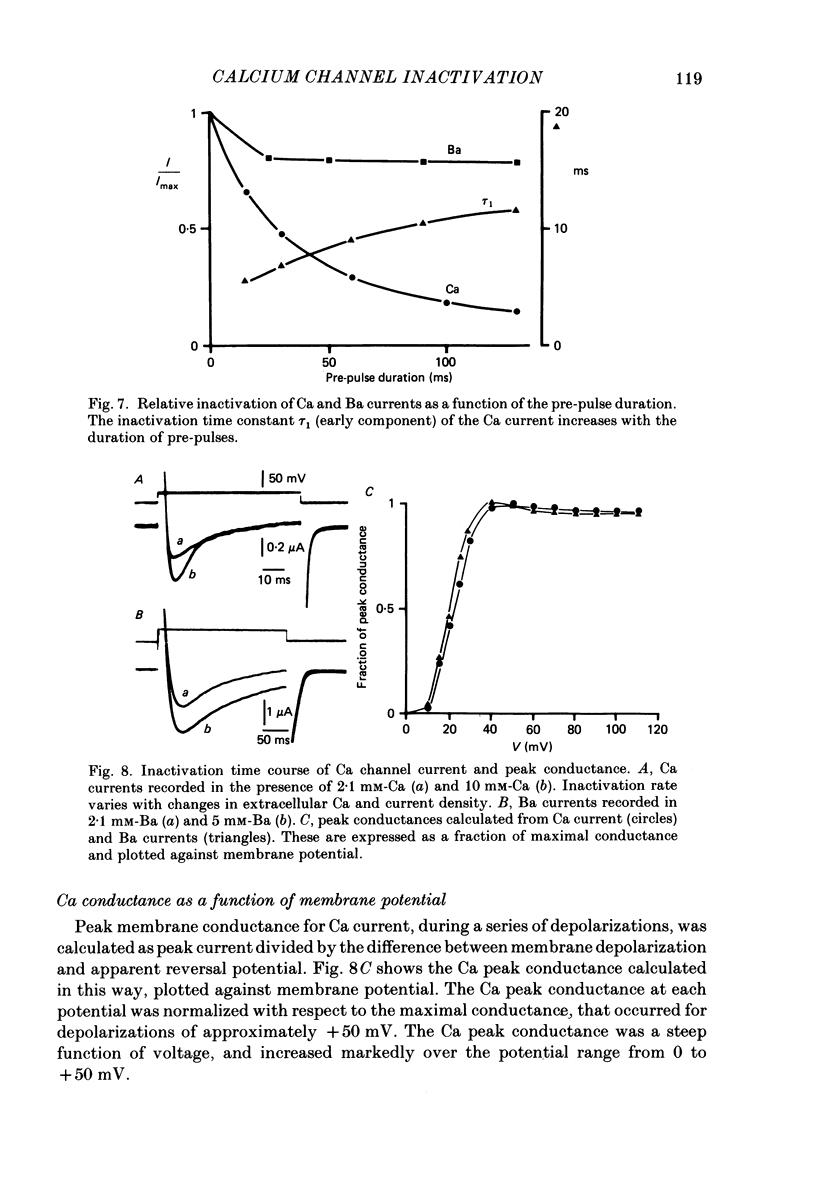
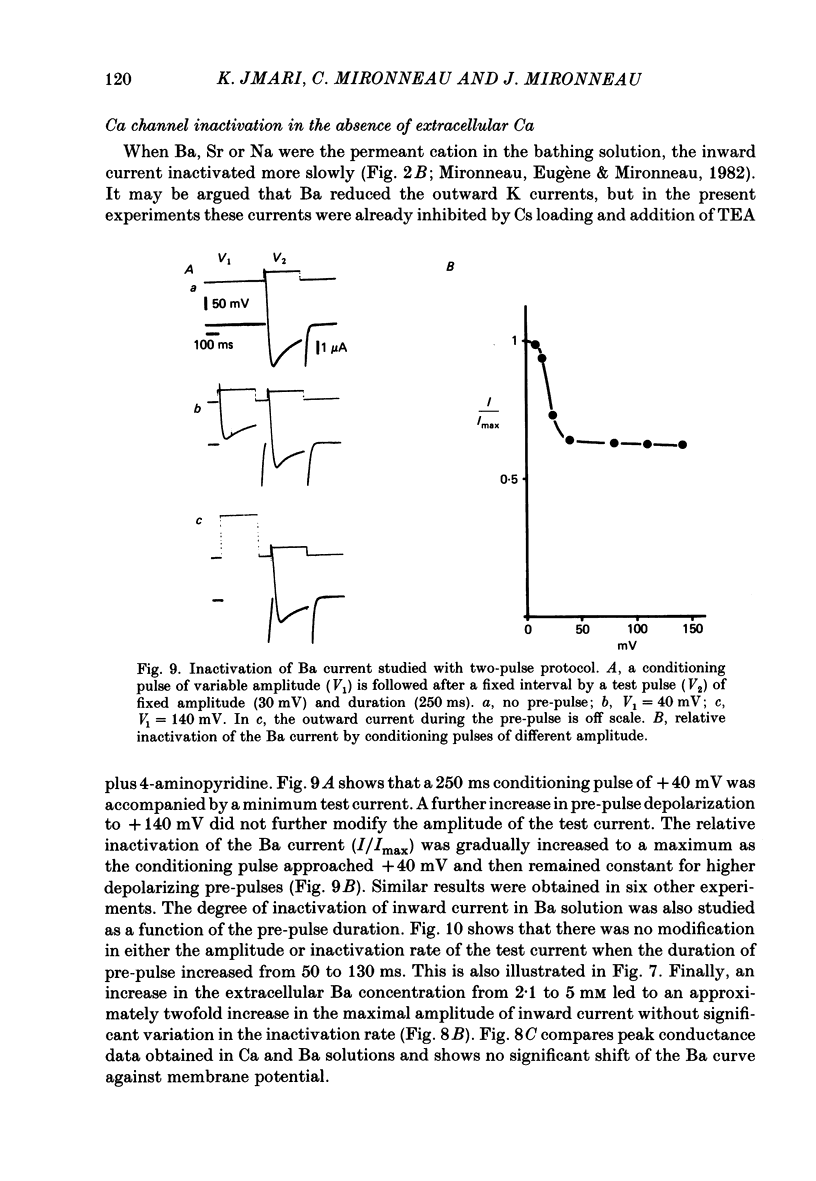
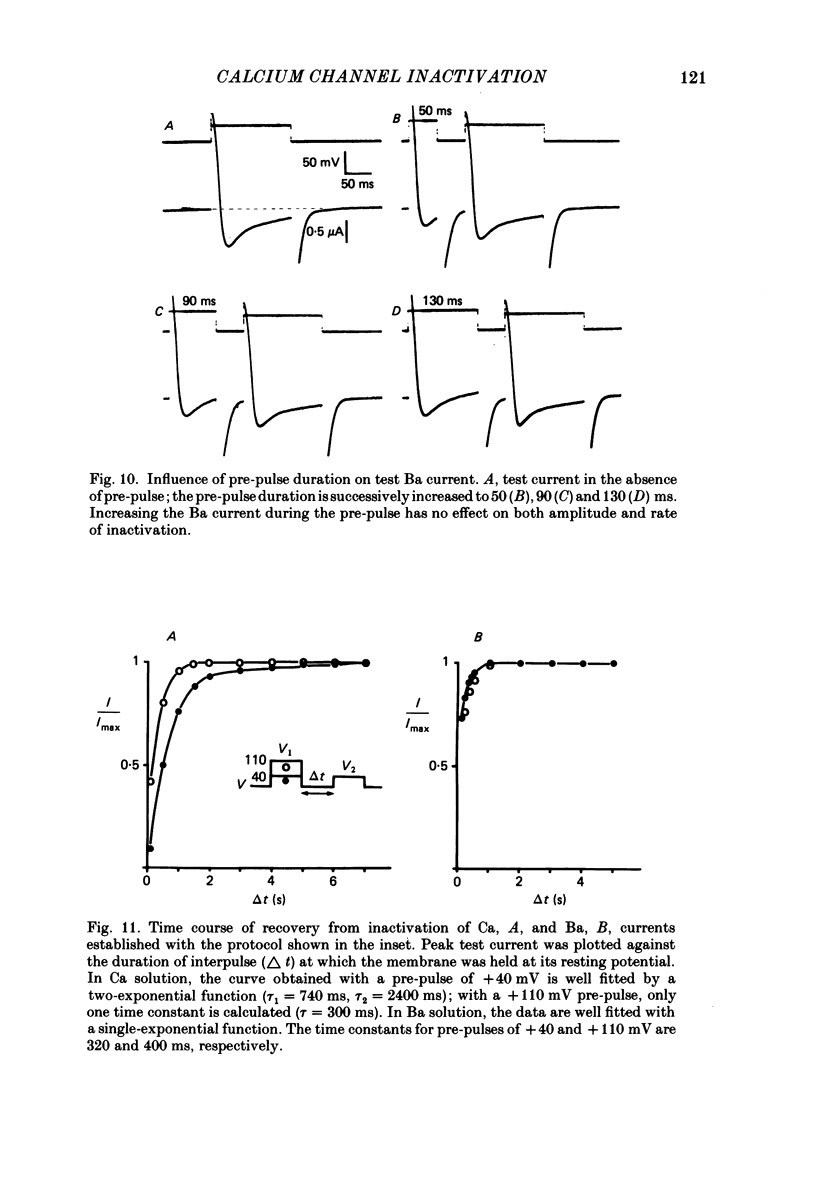
Ca channel currents were recorded in Cs-loaded myometrial strips from pregnant rats after addition of tetraethylammonium chloride and 4-aminopyridine (10 mM each) by means of a double sucrose-gap technique. During a depolarizing pulse, the decay of Ca channel current was slowed down when external Ca was replaced by Ba or Sr. This decay represented an inactivation phenomenon, as assessed by the decreased amplitude of inward tail currents following progressively longer depolarizations, the absence of shift in peak conductance curves against membrane potential, and the stable value of the reversal potential when Ba current was increased during conditioning pulses. Inactivation of Ca and Ba currents through Ca channels was studied using the double-pulse method. Conditioning pulses that produced maximal Ca current induced maximal inactivation; with stronger depolarizations, inactivation decreased but was not completely prevented at the expected Ca reversal potential. Increasing the amount of Ca entering the cell during the pre-pulse reduced both amplitude and kinetics of test Ca currents. These results were not observed with Ba as charge carrier suggesting the participation of different mechanisms in inactivation. With Ca as charge carrier, increasing the external Ca speeded the rate of inactivation. This was not observed with Ba outside. Addition of Co (2.5 mM) reduced the amplitude of both Ca and Ba currents but slowed the inactivation of only the Ca current. Recovery from inactivation was described as a two-exponential process only when the conditioning pulse elicited a Ca inward current. In all other cases, recovery from inactivation was represented as a single exponential curve. It is suggested that inactivation of Ca channels in rat uterine smooth muscle is mediated by both internal Ca-dependent and potential-dependent mechanisms.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almers W., Fink R., Palade P. T. Calcium depletion in frog muscle tubules: the decline of calcium current under maintained depolarization. J Physiol. 1981 Mar;312:177–207. doi: 10.1113/jphysiol.1981.sp013623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson N. C., Jr Voltage-clamp studies on uterine smooth muscle. J Gen Physiol. 1969 Aug;54(2):145–165. doi: 10.1085/jgp.54.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft F. M., Stanfield P. R. Calcium inactivation in skeletal muscle fibres of the stick insect, Carausius morosus. J Physiol. 1982 Sep;330:349–372. doi: 10.1113/jphysiol.1982.sp014345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BULBRING E., KURIYAMA H. Effects of changes in the external sodium and calcium concentrations on spontaneous electrical activity in smooth muscle of guinea-pig taenia coli. J Physiol. 1963 Apr;166:29–58. doi: 10.1113/jphysiol.1963.sp007089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton T. B., Lang R. J., Takewaki T., Benham C. D. Patch and whole-cell voltage clamp of single mammalian visceral and vascular smooth muscle cells. Experientia. 1985 Jul 15;41(7):887–894. doi: 10.1007/BF01970006. [DOI] [PubMed] [Google Scholar]
- Brading A., Bülbring E., Tomita T. The effect of sodium and calcium on the action potential of the smooth muscle of the guinea-pig taenia coli. J Physiol. 1969 Feb;200(3):637–654. doi: 10.1113/jphysiol.1969.sp008713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. M., Lux H. D., Wilson D. L. Activation and inactivation of single calcium channels in snail neurons. J Gen Physiol. 1984 May;83(5):751–769. doi: 10.1085/jgp.83.5.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. M., Morimoto K., Tsuda Y., wilson D. L. Calcium current-dependent and voltage-dependent inactivation of calcium channels in Helix aspersa. J Physiol. 1981 Nov;320:193–218. doi: 10.1113/jphysiol.1981.sp013944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byerly L., Chase P. B., Stimers J. R. Permeation and interaction of divalent cations in calcium channels of snail neurons. J Gen Physiol. 1985 Apr;85(4):491–518. doi: 10.1085/jgp.85.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert R., Chad J. E. Inactivation of Ca channels. Prog Biophys Mol Biol. 1984;44(3):215–267. doi: 10.1016/0079-6107(84)90009-9. [DOI] [PubMed] [Google Scholar]
- Eckert R., Tillotson D. L. Calcium-mediated inactivation of the calcium conductance in caesium-loaded giant neurones of Aplysia californica. J Physiol. 1981 May;314:265–280. doi: 10.1113/jphysiol.1981.sp013706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The action of calcium on the electrical properties of squid axons. J Physiol. 1957 Jul 11;137(2):218–244. doi: 10.1113/jphysiol.1957.sp005808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox A. P. Voltage-dependent inactivation of a calcium channel. Proc Natl Acad Sci U S A. 1981 Feb;78(2):953–956. doi: 10.1073/pnas.78.2.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima Y., Hagiwara S. Currents carried by monovalent cations through calcium channels in mouse neoplastic B lymphocytes. J Physiol. 1985 Jan;358:255–284. doi: 10.1113/jphysiol.1985.sp015550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerin M., Wallon G. The reversible replacement of internal potassium by caesium in isolated turtle heart. J Physiol. 1979 Aug;293:525–537. doi: 10.1113/jphysiol.1979.sp012905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Byerly L. Calcium channel. Annu Rev Neurosci. 1981;4:69–125. doi: 10.1146/annurev.ne.04.030181.000441. [DOI] [PubMed] [Google Scholar]
- Inomata H., Kao C. Y. Actions of Ba++ on ionic currents of the guinea-pig taenia coli. J Pharmacol Exp Ther. 1985 Apr;233(1):112–124. [PubMed] [Google Scholar]
- Inomata H., Kao C. Y. Ionic currents in the guinea-pig taenia coli. J Physiol. 1976 Feb;255(2):347–378. doi: 10.1113/jphysiol.1976.sp011284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao C. Y., McCullough J. R. Ionic currents in the uterine smooth muscle. J Physiol. 1975 Mar;246(1):1–36. doi: 10.1113/jphysiol.1975.sp010878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass R. S., Sanguinetti M. C. Inactivation of calcium channel current in the calf cardiac Purkinje fiber. Evidence for voltage- and calcium-mediated mechanisms. J Gen Physiol. 1984 Nov;84(5):705–726. doi: 10.1085/jgp.84.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama H., Suzuki H. Changes in electrical properties of rat myometrium during gestation and following hormonal treatments. J Physiol. 1976 Sep;260(2):315–333. doi: 10.1113/jphysiol.1976.sp011517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalanne C., Mironneau C., Mironneau J., Savineau J. P. Contractions of rat uterine smooth muscle induced by acetylcholine and angiotensin II in Ca2+-free medium. Br J Pharmacol. 1984 Feb;81(2):317–326. doi: 10.1111/j.1476-5381.1984.tb10081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. S., Marban E., Tsien R. W. Inactivation of calcium channels in mammalian heart cells: joint dependence on membrane potential and intracellular calcium. J Physiol. 1985 Jul;364:395–411. doi: 10.1113/jphysiol.1985.sp015752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. S., Tsien R. W. High selectivity of calcium channels in single dialysed heart cells of the guinea-pig. J Physiol. 1984 Sep;354:253–272. doi: 10.1113/jphysiol.1984.sp015374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mentrard D., Vassort G., Fischmeister R. Calcium-mediated inactivation of the calcium conductance in cesium-loaded frog heart cells. J Gen Physiol. 1984 Jan;83(1):105–131. doi: 10.1085/jgp.83.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mironneau J., Eugene D., Mironneau C. Sodium action potentials induced by calcium chelation in rat uterine smooth muscle. Pflugers Arch. 1982 Nov 11;395(3):232–238. doi: 10.1007/BF00584815. [DOI] [PubMed] [Google Scholar]
- Mironneau J. Excitation-contraction coupling in voltage clamped uterine smooth muscle. J Physiol. 1973 Aug;233(1):127–141. doi: 10.1113/jphysiol.1973.sp010301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mironneau J., Savineau J. P. Effects of calcium ions on outward membrane currents in rat uterine smooth muscle. J Physiol. 1980 May;302:411–425. doi: 10.1113/jphysiol.1980.sp013253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mironneau J., Savineau J. P., Mironneau C. Fast outward current controlling electrical activity in rat uterine smooth muscle during gestation. J Physiol (Paris) 1981 Mar;77(8):851–859. [PubMed] [Google Scholar]
- Mironneau J. Voltage clamp analysis of the ionic currents in uterine smooth muscle using the double sucrose gap method. Pflugers Arch. 1974;352(3):197–120. doi: 10.1007/BF00590485. [DOI] [PubMed] [Google Scholar]
- Muramatsu I., Kumamoto M., Fujiwara M. Effects of conditioning polarization on the membrane ionic currents in rat myometrium. J Membr Biol. 1978 Dec 29;44(3-4):331–352. doi: 10.1007/BF01944228. [DOI] [PubMed] [Google Scholar]
- Tillotson D. Inactivation of Ca conductance dependent on entry of Ca ions in molluscan neurons. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1497–1500. doi: 10.1073/pnas.76.3.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. W. Calcium channels in excitable cell membranes. Annu Rev Physiol. 1983;45:341–358. doi: 10.1146/annurev.ph.45.030183.002013. [DOI] [PubMed] [Google Scholar]
- Vassort G. Voltage-clamp analysis of transmembrane ionic currents in guinea-pig myometrium: evidence for an initial potassium activation triggered by calcium influx. J Physiol. 1975 Nov;252(3):713–734. doi: 10.1113/jphysiol.1975.sp011167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh J. V., Jr, Singer J. J. Calcium action potentials in single freshly isolated smooth muscle cells. Am J Physiol. 1980 Nov;239(5):C162–C174. doi: 10.1152/ajpcell.1980.239.5.C162. [DOI] [PubMed] [Google Scholar]


