Abstract
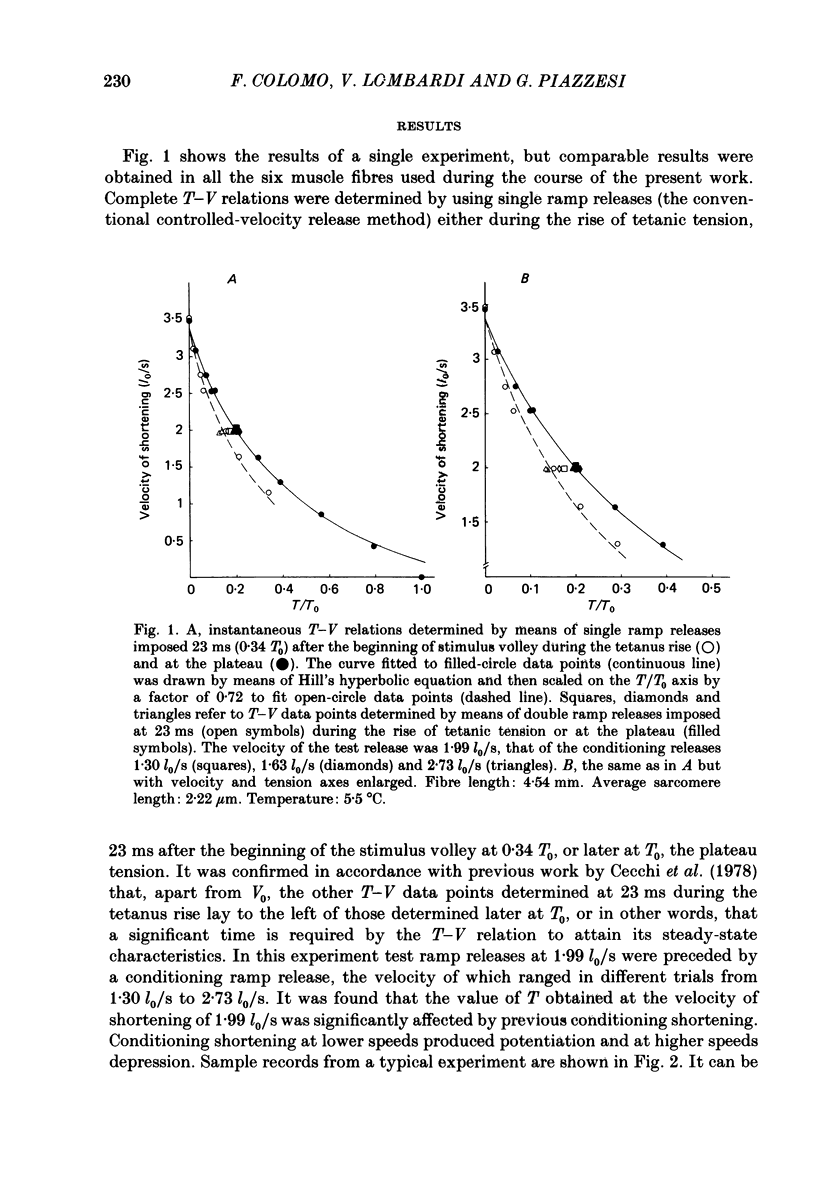
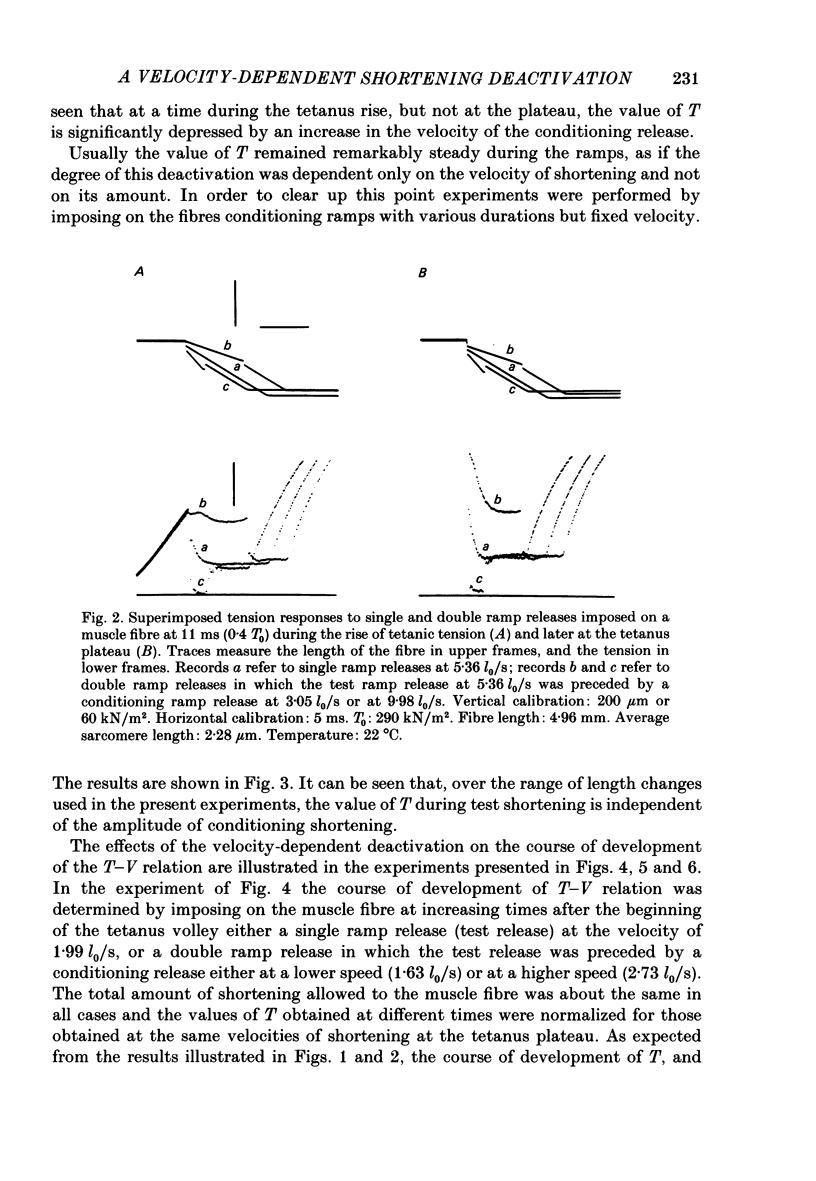
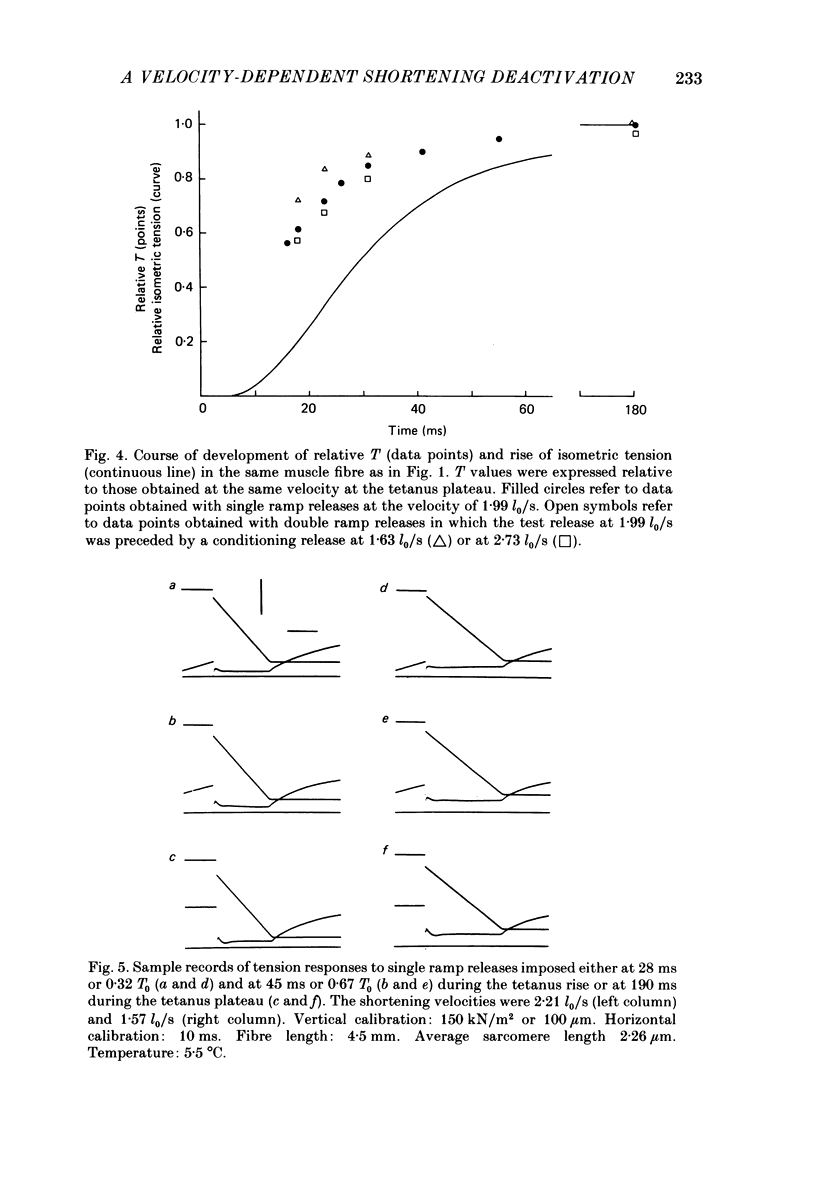
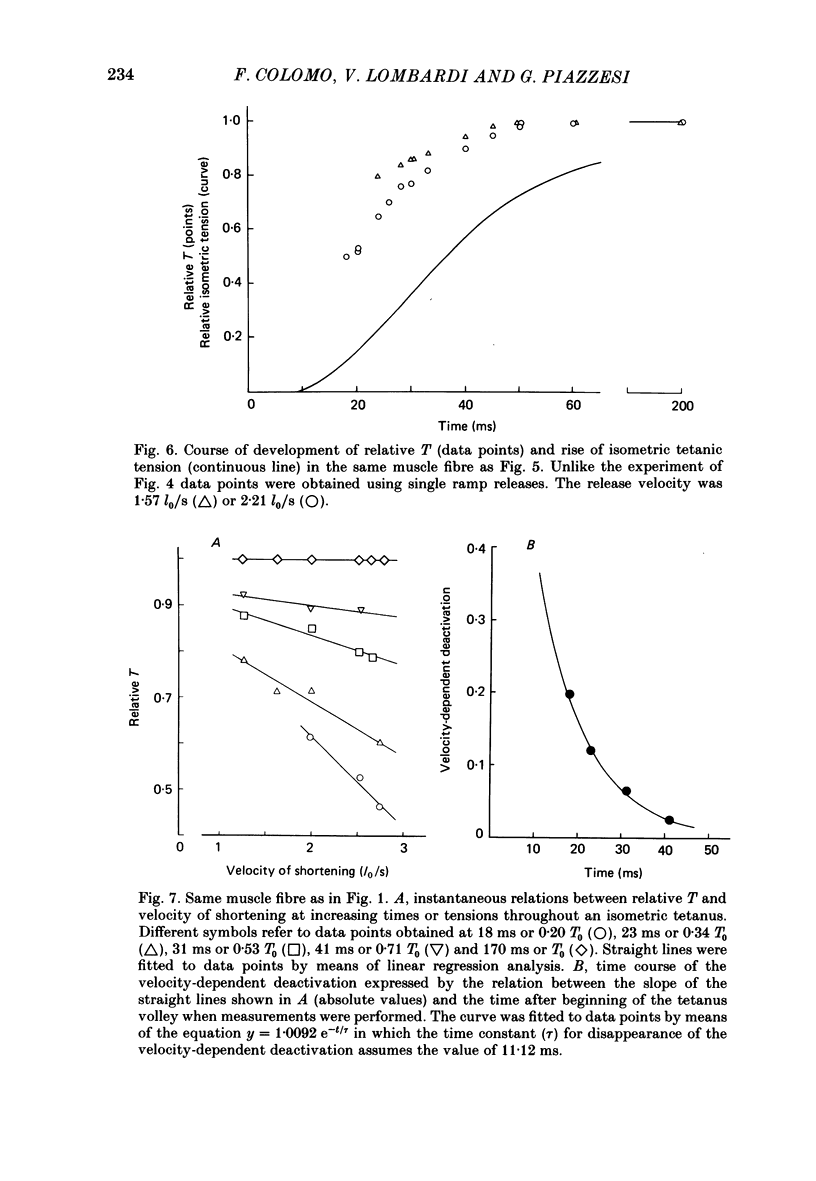
During the onset of activation in isolated frog muscle fibres the development of the force-velocity (T-V) relation was determined by imposing single and double ramp releases. The experiments were performed at 3.5-6 degrees C or 19-22 degrees C and at a starting sarcomere length of about 2.25 micron. A velocity- and time-dependent shortening deactivation was shown to exist during the development of contraction. It was found that, early during the tetanus rise, at submaximal levels of activation, the values of T (the steady force exerted by the muscle fibres at any velocity of shortening V lower than V0) were significantly affected by previous conditioning shortening. Conditioning shortening at lower speeds led to potentiation of T and, at higher speeds, to depression. Both these effects were independent of the amount of shortening and, in addition, were not present at the tetanus plateau. At each given time or isometric tension throughout the tetanus rise the values of T. normalized for those determined at the same velocities at the tetanus plateau, were found to be inversely correlated with the actual velocities of shortening. The slope of this relation (a measure of the velocity-dependent shortening deactivation) decreased exponentially with time, attaining, in six fibres at low temperature, 10% of its initial value within 26-73 ms. The results may be explained in terms of a cross-bridge model of contraction by assuming that the rate of development of activation is controlled by the rate of release of the Ca2+ as well as by the velocity at which the muscle fibres are allowed to shorten and in turn by the actual number of attached cross-bridges.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brandt P. W., Cox R. N., Kawai M. Can the binding of Ca2+ to two regulatory sites on troponin C determine the steep pCa/tension relationship of skeletal muscle? Proc Natl Acad Sci U S A. 1980 Aug;77(8):4717–4720. doi: 10.1073/pnas.77.8.4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremel R. D., Weber A. Cooperation within actin filament in vertebrate skeletal muscle. Nat New Biol. 1972 Jul 26;238(82):97–101. doi: 10.1038/newbio238097a0. [DOI] [PubMed] [Google Scholar]
- Cecchi G. A circuit specially suited for use with high-frequency capacitance gauge force transducers. Arch Ital Biol. 1983 Aug;121(3):215–217. [PubMed] [Google Scholar]
- Cecchi G., Colomo F., Lombardi V. A loudspeaker servo system for determination of mechanical characteristics of isolated muscle fibres. Boll Soc Ital Biol Sper. 1976 May 30;52(10):733–736. [PubMed] [Google Scholar]
- Cecchi G., Colomo F., Lombardi V. Force-velocity relation in deuterium oxide-treated frog single muscle fibres during the rise of tension in an isometric tetanus. J Physiol. 1981 Aug;317:207–221. doi: 10.1113/jphysiol.1981.sp013821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecchi G., Colomo F., Lombardi V. Force-velocity relation in normal and nitrate-treated frog single muscle fibres during rise of tension in an isometric tetanus. J Physiol. 1978 Dec;285:257–273. doi: 10.1113/jphysiol.1978.sp012570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecchi G., Colomo F., Lombardi V., Piazzesi G. Development of activation and rise of tension in an isometric tetanus. Pflugers Arch. 1979 Jul;381(1):71–74. doi: 10.1007/BF00582334. [DOI] [PubMed] [Google Scholar]
- Cecchi G., Colomo F., Piazzesi G. The development of the force-velocity relation in normal and dantrolene-treated frog single muscle fibres. J Muscle Res Cell Motil. 1983 Aug;4(4):395–404. doi: 10.1007/BF00711946. [DOI] [PubMed] [Google Scholar]
- Cecchi G., Lombardi V., Menchetti G. Development of force-velocity relation and rise of isometric tetanic tension measure the time course of different processes. Pflugers Arch. 1984 Aug;401(4):396–401. doi: 10.1007/BF00584342. [DOI] [PubMed] [Google Scholar]
- Edman K. A. Depression of mechanical activity induced by active shortening in frog skeletal muscle fibres. Acta Physiol Scand. 1976 Nov;98(3):384–386. doi: 10.1111/j.1748-1716.1976.tb10325.x. [DOI] [PubMed] [Google Scholar]
- Edman K. A. Mechanical deactivation induced by active shortening in isolated muscle fibres of the frog. J Physiol. 1975 Mar;246(1):255–275. doi: 10.1113/jphysiol.1975.sp010889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman K. A., Mulieri L. A., Scubon-Mulieri B. Non-hyperbolic force-velocity relationship in single muscle fibres. Acta Physiol Scand. 1976 Oct;98(2):143–156. doi: 10.1111/j.1748-1716.1976.tb00234.x. [DOI] [PubMed] [Google Scholar]
- Fenn W. O. A quantitative comparison between the energy liberated and the work performed by the isolated sartorius muscle of the frog. J Physiol. 1923 Dec 28;58(2-3):175–203. doi: 10.1113/jphysiol.1923.sp002115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenn W. O., Marsh B. S. Muscular force at different speeds of shortening. J Physiol. 1935 Nov 22;85(3):277–297. doi: 10.1113/jphysiol.1935.sp003318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford L. E., Huxley A. F., Simmons R. M. Tension responses to sudden length change in stimulated frog muscle fibres near slack length. J Physiol. 1977 Jul;269(2):441–515. doi: 10.1113/jphysiol.1977.sp011911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford L. E., Huxley A. F., Simmons R. M. Tension transients during steady shortening of frog muscle fibres. J Physiol. 1985 Apr;361:131–150. doi: 10.1113/jphysiol.1985.sp015637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUXLEY A. F. Muscle structure and theories of contraction. Prog Biophys Biophys Chem. 1957;7:255–318. [PubMed] [Google Scholar]
- Homsher E., Yamada T., Wallner A., Tsai J. Energy balance studies in frog skeletal muscles shortening at one-half maximal velocity. J Gen Physiol. 1984 Sep;84(3):347–359. doi: 10.1085/jgp.84.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JEWELL B. R., WILKIE D. R. An analysis of the mechanical components in frog's striated muscle. J Physiol. 1958 Oct 31;143(3):515–540. doi: 10.1113/jphysiol.1958.sp006075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian F. J. Activation in a skeletal muscle contraction model with a modification for insect fibrillar muscle. Biophys J. 1969 Apr;9(4):547–570. doi: 10.1016/S0006-3495(69)86403-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian F. J., Morgan D. L. Variation of muscle stiffness with tension during tension transients and constant velocity shortening in the frog. J Physiol. 1981;319:193–203. doi: 10.1113/jphysiol.1981.sp013901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian F. J., Sollins M. R. Variation of muscle stiffness with force at increasing speeds of shortening. J Gen Physiol. 1975 Sep;66(3):287–302. doi: 10.1085/jgp.66.3.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B. The relation between force and speed in muscular contraction. J Physiol. 1939 Jun 14;96(1):45–64. doi: 10.1113/jphysiol.1939.sp003756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardi V., Menchetti G. The maximum velocity of shortening during the early phases of the contraction in frog single muscle fibres. J Muscle Res Cell Motil. 1984 Oct;5(5):503–513. doi: 10.1007/BF00713257. [DOI] [PubMed] [Google Scholar]
- Ridgway E. B., Gordon A. M., Martyn D. A. Hysteresis in the force-calcium relation in muscle. Science. 1983 Mar 4;219(4588):1075–1077. doi: 10.1126/science.6823567. [DOI] [PubMed] [Google Scholar]


