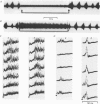
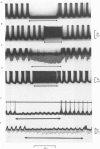
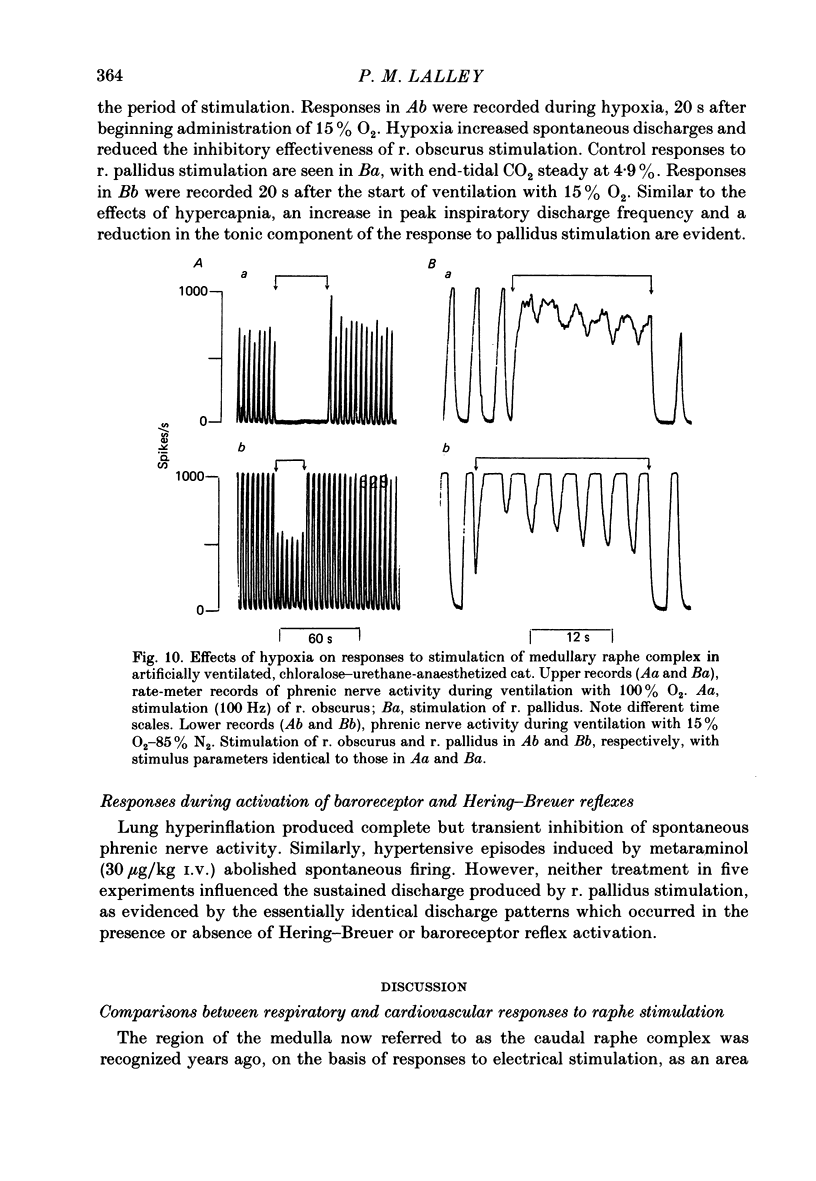
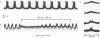Abstract
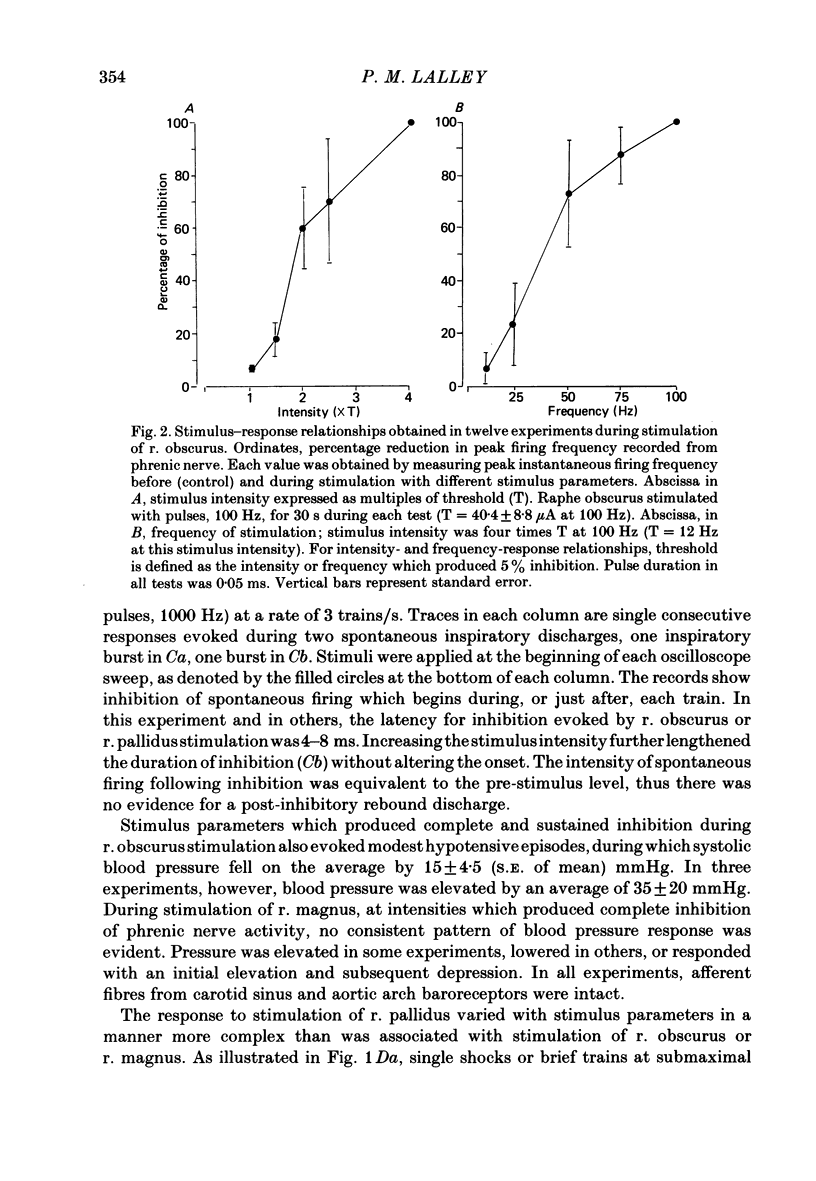
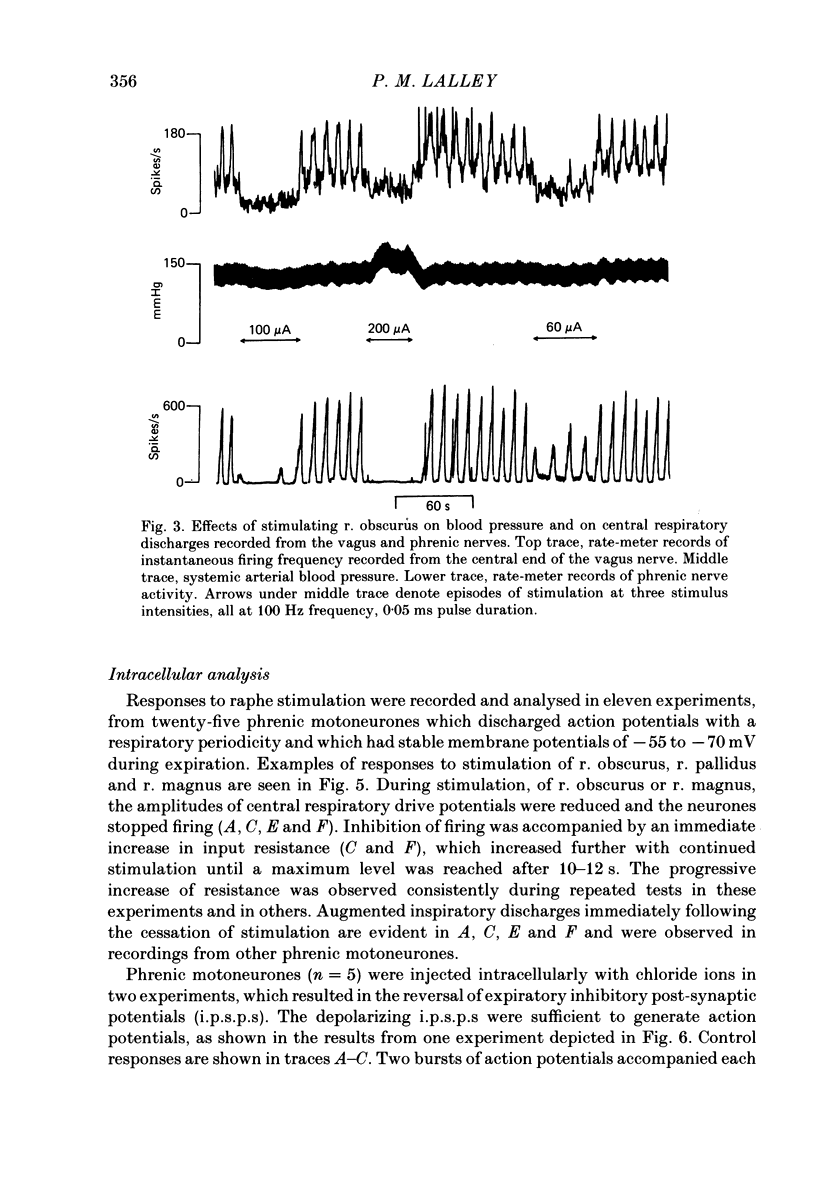
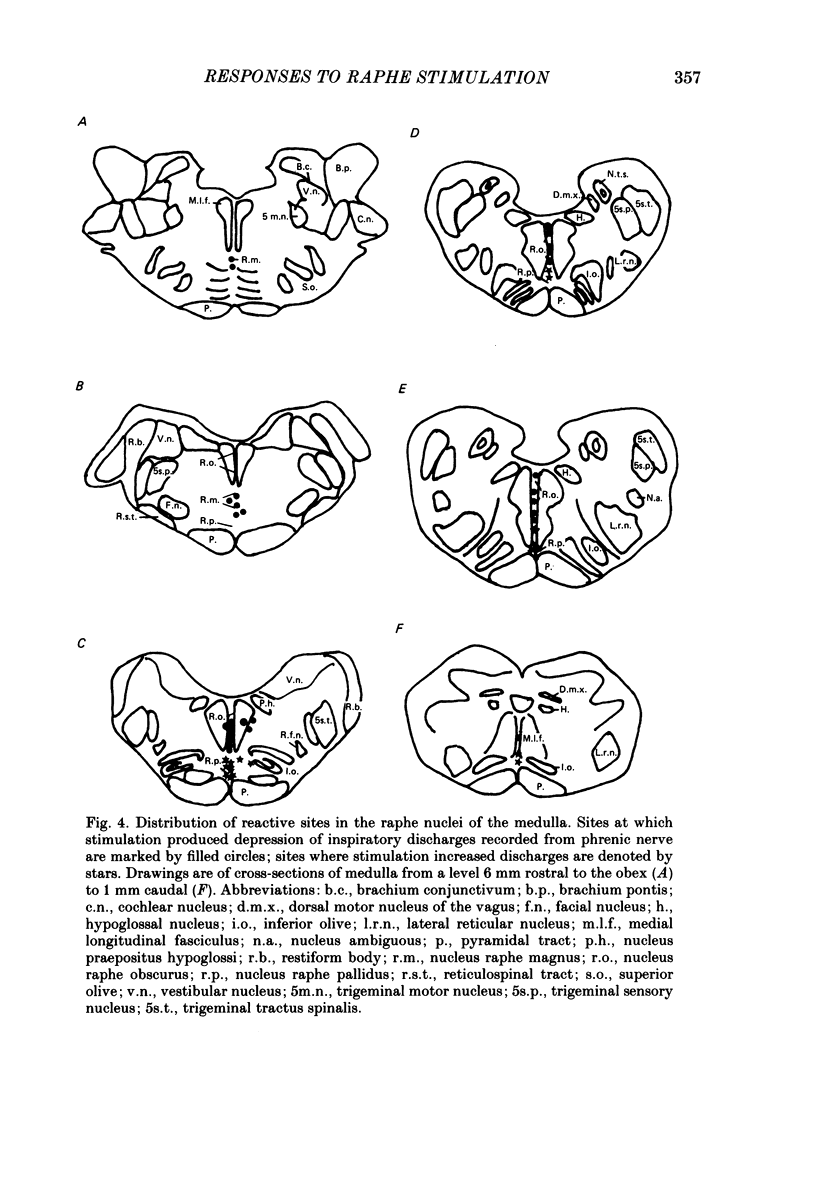
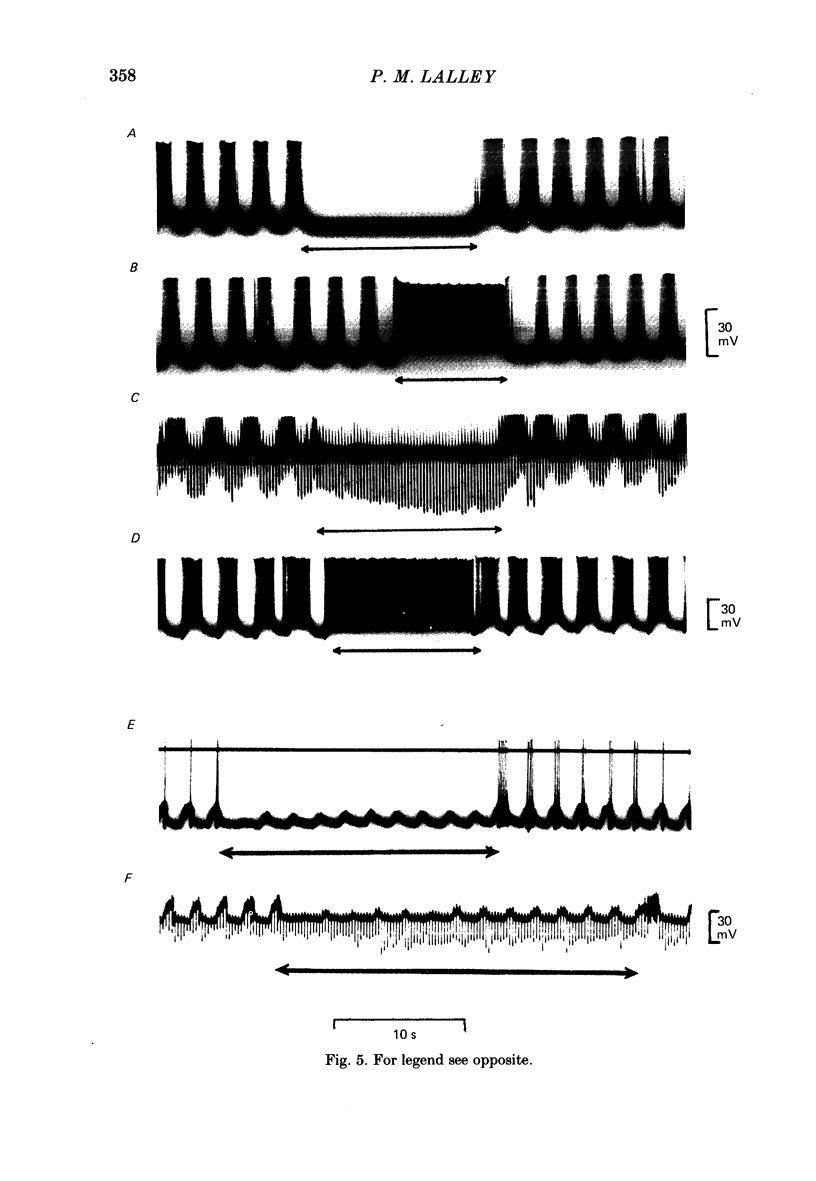
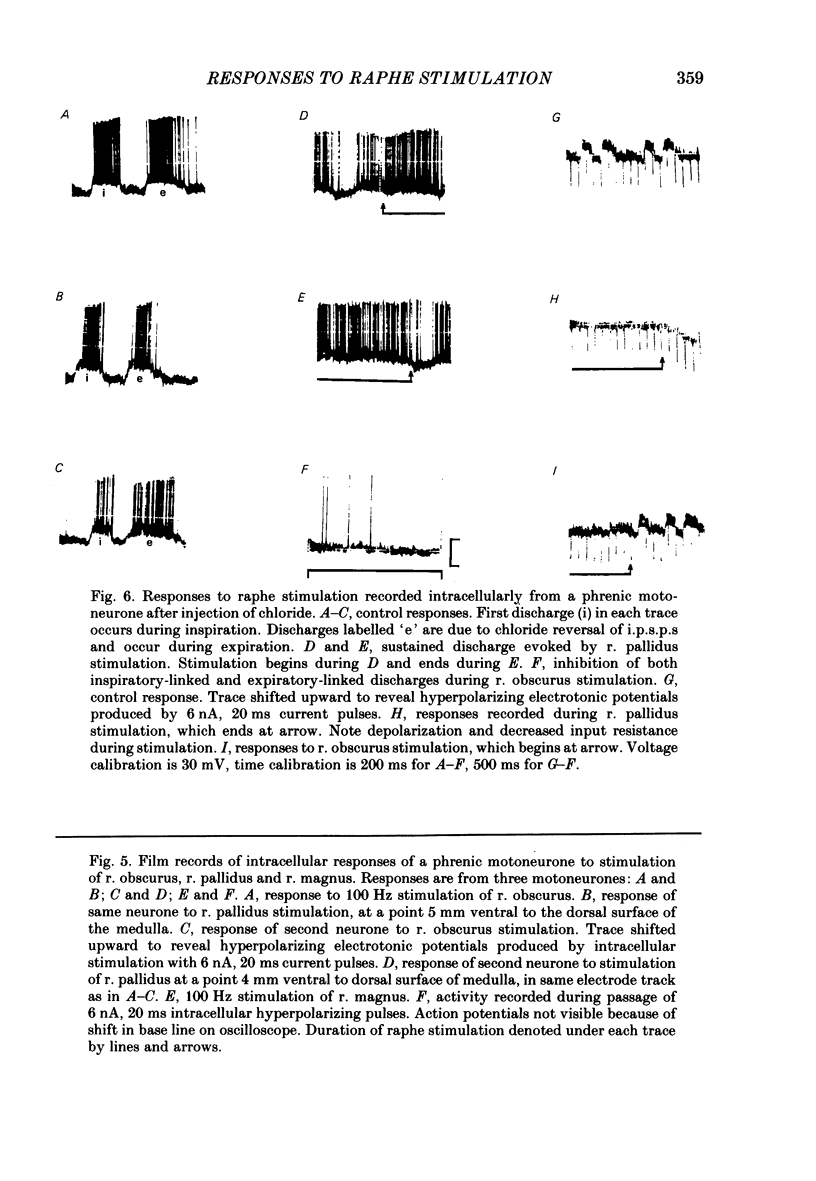
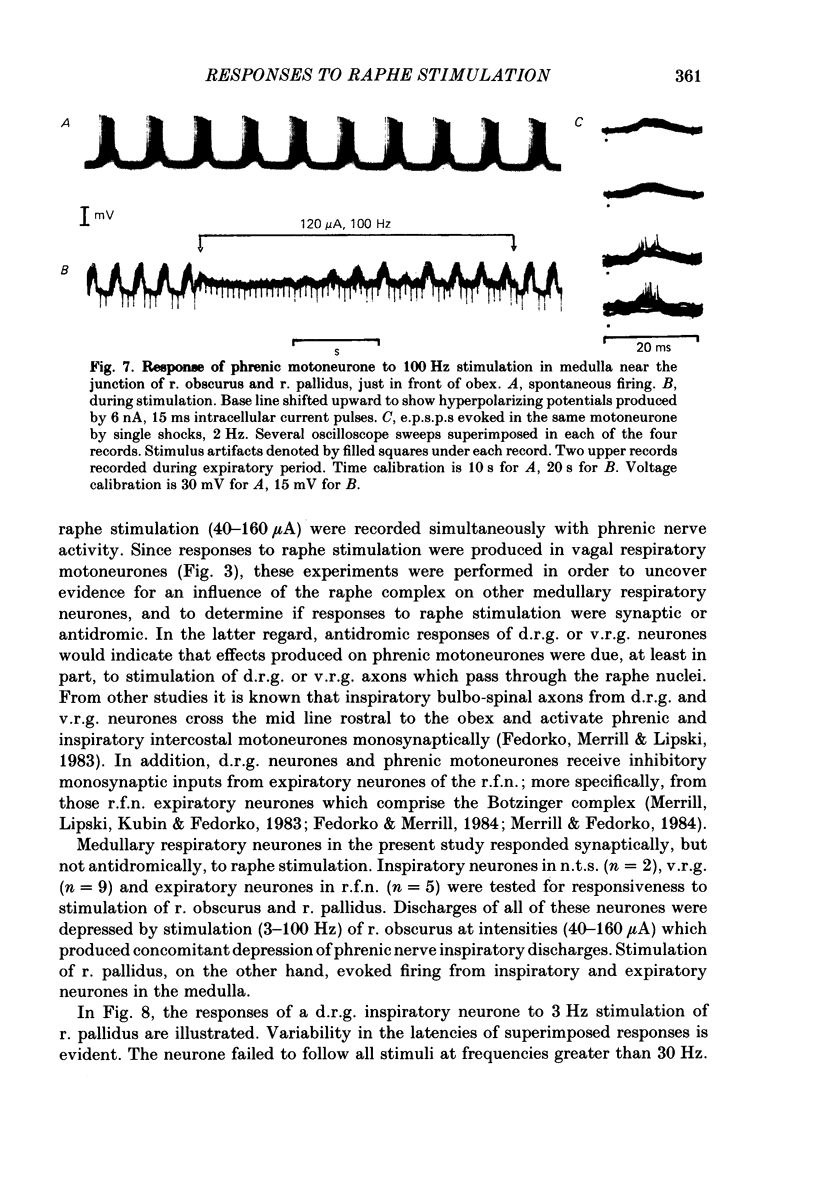
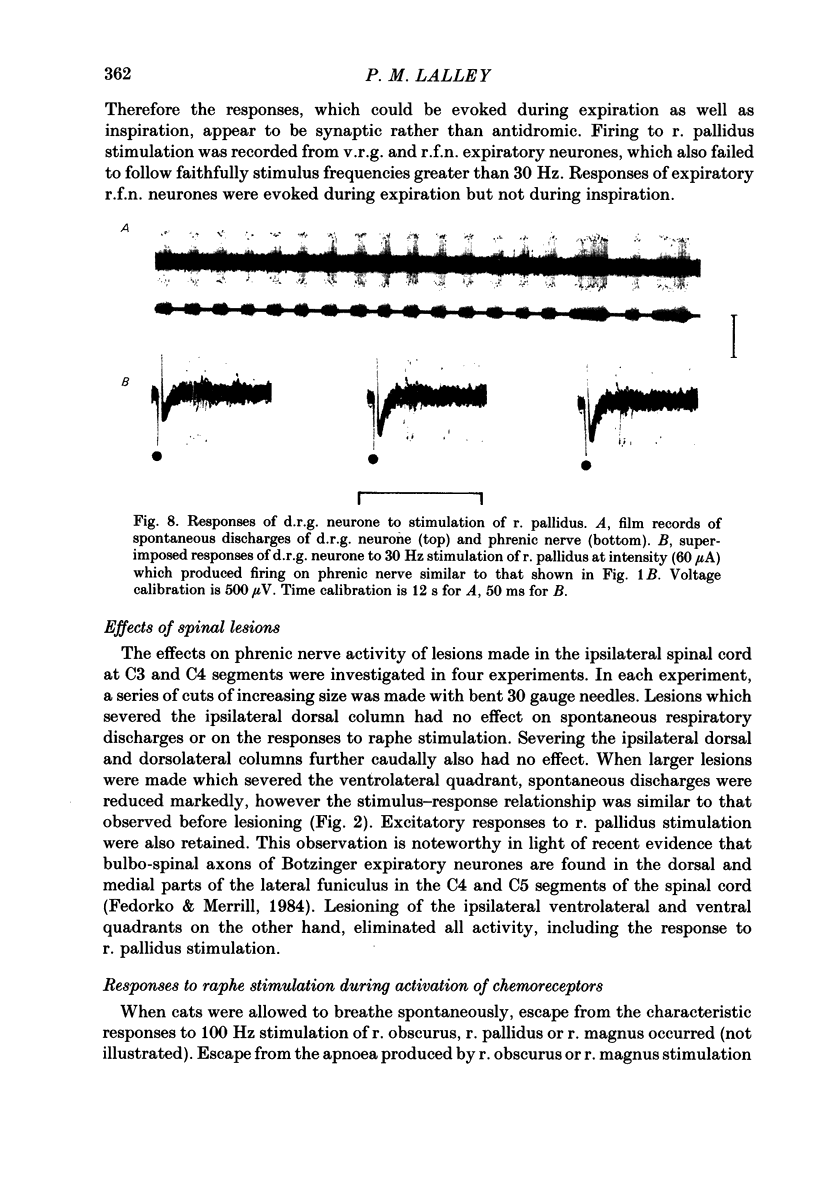
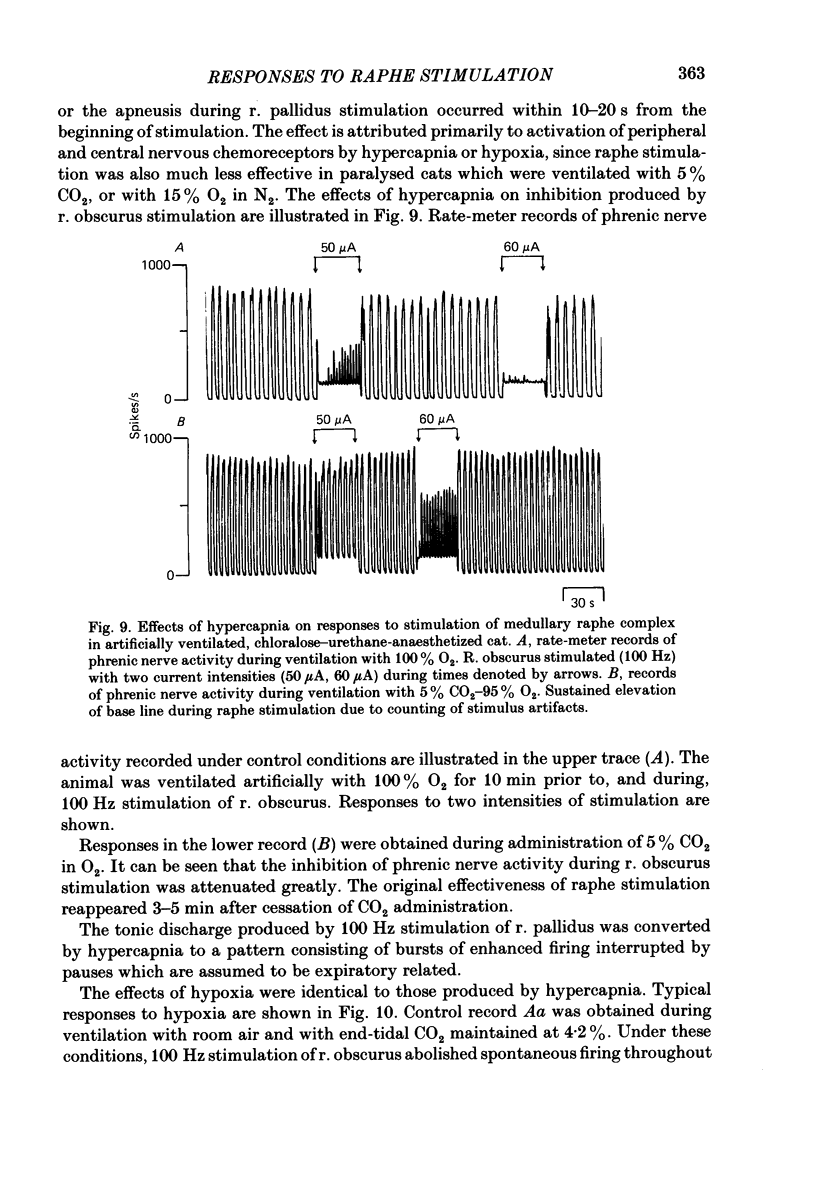
Responses of phrenic motoneurones to stimulation of the three medullary raphe nuclei (raphe magnus (r. magnus), raphe obscurus (r. obscurus) and raphe pallidus (r. pallidus] were recorded in anaesthetized and decerebrated cats. Stimulation of r. magnus or r. obscurus depressed phrenic motoneurones. Stimulation at 100 Hz reduced action potential frequency within each inspiratory burst, without appreciable changes in inspiratory duration, or number of inspiratory bursts per unit time. The depression was proportional to the stimulus intensity (40-160 microA) and frequency (12-100 Hz) and lasted throughout the period of stimulation. Intracellular recording revealed concomitant depression of central respiratory drive potentials (c.r.d.p.s) and increased membrane input resistance during r. obscurus or r. magnus stimulation. In motoneurones which discharged action potentials during expiratory as well as inspiratory phases following intracellular chloride injection, stimulation of r. magnus or r. obscurus depressed cell firing during both phases. Both c.r.d.p.s and reversed inhibitory post-synaptic potentials (i.p.s.p.s) were depressed. These findings indicate that the depression is not related to post-synaptic inhibition of phrenic motoneurones. Stimulation (100 Hz) of r. pallidus produced discharges of action potentials in phrenic motoneurones. Stimulation lengthened the duration of each inspiratory discharge in proportion to stimulus intensity. Continuous firing occurred throughout the period of stimulation with maximal intensities. Intracellular recordings revealed sustained depolarization and reduction in membrane input resistance during the discharge. Responses were recorded extracellularly from medullary inspiratory neurones of the dorsal respiratory group (d.r.g.) and ventral respiratory group (v.r.g.) and from vagal axons which fired in phase with phrenic nerve activity. Responses to raphe stimulation were similar to those recorded from phrenic motoneurones. Evidence is presented that the responses are not related to stimulation of decussating bulbo-spinal axons from d.r.g. or v.r.g. neurones. It is suggested that medullary respiratory neurones receive inhibitory and excitatory synaptic inputs from medullary raphe neurones. Hypercapnia (5% CO2 in O2) or hypoxia (15% O2 in N2) reduced markedly the inhibition produced during stimulation of r. obscurus or r. magnus, and restored expiratory-linked silent periods during stimulation of r. pallidus. Activation of Hering-Breuer or baroreceptor reflexes did not alter responses to r. pallidus stimulation.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF



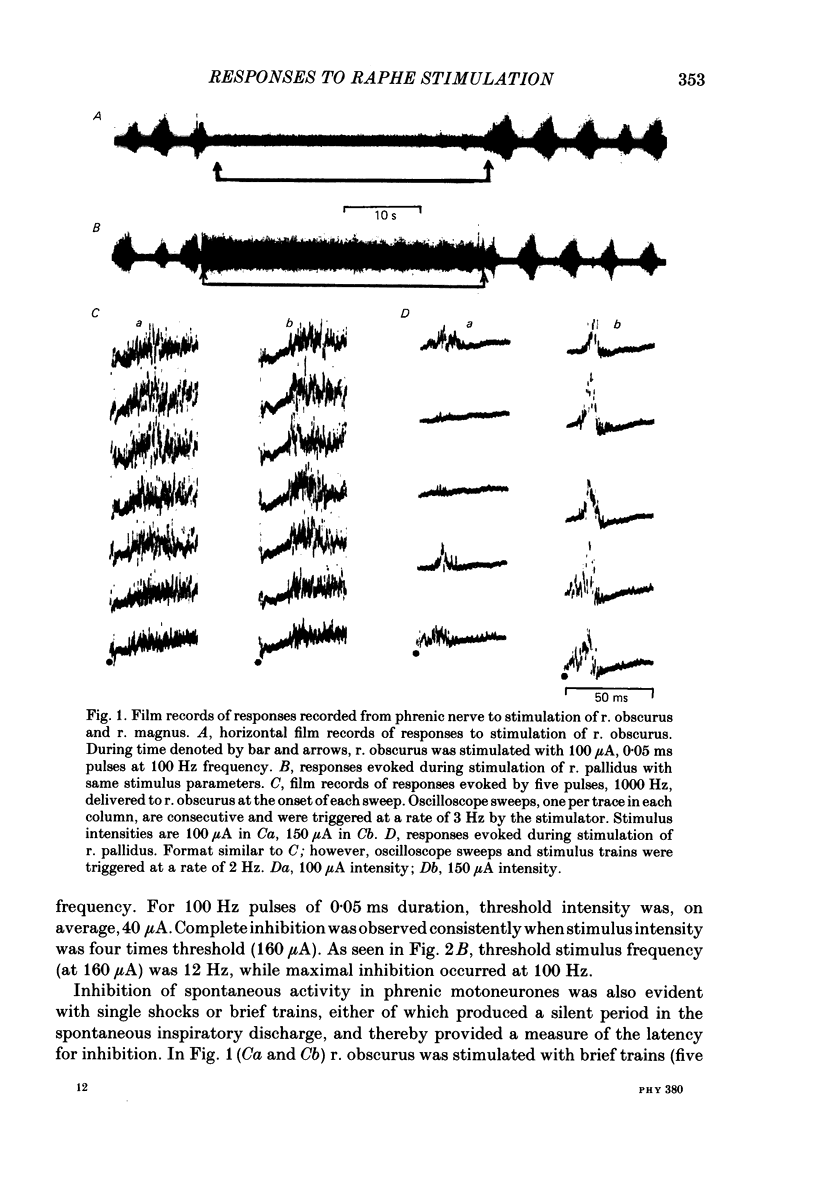









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adair J. R., Hamilton B. L., Scappaticci K. A., Helke C. J., Gillis R. A. Cardiovascular responses to electrical stimulation of the medullary raphe area of the cat. Brain Res. 1977 Jun 3;128(1):141–145. doi: 10.1016/0006-8993(77)90241-4. [DOI] [PubMed] [Google Scholar]
- Andersen P., Sears T. A. Medullary activation of intercostal fusimotor and alpha motoneurones. J Physiol. 1970 Aug;209(3):739–755. doi: 10.1113/jphysiol.1970.sp009189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainton C. R., Kirkwood P. A., Sears T. A. On the transmission of the stimulating effects of carbon dioxide to the muscles of respiration. J Physiol. 1978 Jul;280:249–272. doi: 10.1113/jphysiol.1978.sp012383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainton C. R., Kirkwood P. A. The effect of carbon dioxide on the tonic and the rhythmic discharges of expiratory bulbospinal neurones. J Physiol. 1979 Nov;296:291–314. doi: 10.1113/jphysiol.1979.sp013006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballantyne D., Richter D. W. Post-synaptic inhibition of bulbar inspiratory neurones in the cat. J Physiol. 1984 Mar;348:67–87. doi: 10.1113/jphysiol.1984.sp015100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batsel H. L. Activity of bulbar respiratory neurons during passive hyperventilation. Exp Neurol. 1967 Nov;19(3):357–374. doi: 10.1016/0014-4886(67)90032-5. [DOI] [PubMed] [Google Scholar]
- Berger A. J. Phrenic motoneurons in the cat: subpopulations and nature of respiratory drive potentials. J Neurophysiol. 1979 Jan;42(1 Pt 1):76–90. doi: 10.1152/jn.1979.42.1.76. [DOI] [PubMed] [Google Scholar]
- Biscoe T. J., Sampson S. R. An analysis of the inhibition of phrenic motoneurones which occurs on stimulation of some cranial nerve afferents. J Physiol. 1970 Aug;209(2):375–393. doi: 10.1113/jphysiol.1970.sp009170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark F. J., von Euler C. On the regulation of depth and rate of breathing. J Physiol. 1972 Apr;222(2):267–295. doi: 10.1113/jphysiol.1972.sp009797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M. I. Discharge patterns of brain-stem respiratory neurons during Hering-Breuer reflex evoked by lung inflation. J Neurophysiol. 1969 May;32(3):356–374. doi: 10.1152/jn.1969.32.3.356. [DOI] [PubMed] [Google Scholar]
- Cohen M. I. Discharge patterns of brain-stem respiratory neurons in relation to carbon dioxide tension. J Neurophysiol. 1968 Mar;31(2):142–165. doi: 10.1152/jn.1968.31.2.142. [DOI] [PubMed] [Google Scholar]
- Cohen M. I. Neurogenesis of respiratory rhythm in the mammal. Physiol Rev. 1979 Oct;59(4):1105–1173. doi: 10.1152/physrev.1979.59.4.1105. [DOI] [PubMed] [Google Scholar]
- Coote J. H., Macleod V. H. Evidence for the involvement in the baroreceptor reflex of a descending inhibitory pathway. J Physiol. 1974 Sep;241(2):477–496. doi: 10.1113/jphysiol.1974.sp010667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coote J. H., Macleod V. H. The spinal route of sympatho-inhibitory pathways descending from the medulla oblongata. Pflugers Arch. 1975 Sep 29;359(4):335–347. doi: 10.1007/BF00581444. [DOI] [PubMed] [Google Scholar]
- Dostrovsky J. O., Hu J. W., Sessle B. J., Sumino R. Stimulation sites in periaqueductal gray, nucleus raphe magnus and adjacent regions effective in suppressing oral-facial reflexes. Brain Res. 1982 Dec 9;252(2):287–297. doi: 10.1016/0006-8993(82)90396-1. [DOI] [PubMed] [Google Scholar]
- Eldridge F. L., Kiley J. P., Millhorn D. E. Respiratory effects of carbon dioxide-induced changes of medullary extracellular fluid pH in cats. J Physiol. 1984 Oct;355:177–189. doi: 10.1113/jphysiol.1984.sp015413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorko L., Merrill E. G. Axonal projections from the rostral expiratory neurones of the Bötzinger complex to medulla and spinal cord in the cat. J Physiol. 1984 May;350:487–496. doi: 10.1113/jphysiol.1984.sp015214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorko L., Merrill E. G., Lipski J. Two descending medullary inspiratory pathways to phrenic motoneurones. Neurosci Lett. 1983 Dec 30;43(2-3):285–291. doi: 10.1016/0304-3940(83)90202-1. [DOI] [PubMed] [Google Scholar]
- Futuro-Neto H. A., Coote J. H. Desynchronized sleep-like pattern of sympathetic activity elicited by electrical stimulation of sites in the brainstem. Brain Res. 1982 Dec 9;252(2):269–276. doi: 10.1016/0006-8993(82)90394-8. [DOI] [PubMed] [Google Scholar]
- GILL P. K., KUNO M. PROPERTIES OF PHRENIC MOTONEURONES. J Physiol. 1963 Sep;168:258–273. doi: 10.1113/jphysiol.1963.sp007191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel M., Seller H. Excitation of expiratory neurones adjacent to the nucleus ambiguus by carotid sinus baroreceptor and trigeminal afferents. Pflugers Arch. 1969;313(1):1–10. doi: 10.1007/BF00586323. [DOI] [PubMed] [Google Scholar]
- Holstege G., Kuypers H. G. The anatomy of brain stem pathways to the spinal cord in cat. A labeled amino acid tracing study. Prog Brain Res. 1982;57:145–175. doi: 10.1016/S0079-6123(08)64128-X. [DOI] [PubMed] [Google Scholar]
- Lalley P. M. Biphasic effects of baclofen on phrenic motoneurons: possible involvement of two types of gamma-aminobutyric acid (GABA) receptors. J Pharmacol Exp Ther. 1983 Aug;226(2):616–624. [PubMed] [Google Scholar]
- Lalley P. M. Serotoninergic and non-serotoninergic responses of phrenic motoneurones to raphe stimulation in the cat. J Physiol. 1986 Nov;380:373–385. doi: 10.1113/jphysiol.1986.sp016291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipski J., McAllen R. M., Spyer K. M. The carotid chemoreceptor input to the respiratory neurones of the nucleus of tractus solitarus. J Physiol. 1977 Aug;269(3):797–810. doi: 10.1113/jphysiol.1977.sp011930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall R. B. Evidence for a serotonergically mediated sympathoexcitatory response to stimulation of medullary raphe nuclei. Brain Res. 1984 Oct 8;311(1):131–139. doi: 10.1016/0006-8993(84)91405-7. [DOI] [PubMed] [Google Scholar]
- Merrill E. G., Fedorko L. Monosynaptic inhibition of phrenic motoneurons: a long descending projection from Bötzinger neurons. J Neurosci. 1984 Sep;4(9):2350–2353. doi: 10.1523/JNEUROSCI.04-09-02350.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill E. G., Lipski J., Kubin L., Fedorko L. Origin of the expiratory inhibition of nucleus tractus solitarius inspiratory neurones. Brain Res. 1983 Mar 14;263(1):43–50. doi: 10.1016/0006-8993(83)91198-8. [DOI] [PubMed] [Google Scholar]
- REDGATE E. S., GELLHORN E. Respiratory activity and the hypothalamus. Am J Physiol. 1958 Apr;193(1):189–194. doi: 10.1152/ajplegacy.1958.193.1.189. [DOI] [PubMed] [Google Scholar]
- Ranck J. B., Jr Which elements are excited in electrical stimulation of mammalian central nervous system: a review. Brain Res. 1975 Nov 21;98(3):417–440. doi: 10.1016/0006-8993(75)90364-9. [DOI] [PubMed] [Google Scholar]
- Richter D. W. Generation and maintenance of the respiratory rhythm. J Exp Biol. 1982 Oct;100:93–107. doi: 10.1242/jeb.100.1.93. [DOI] [PubMed] [Google Scholar]
- Richter D. W., Seller H. Baroreceptor effects on medullary respiratory neurones of the cat. Brain Res. 1975 Mar 14;86(1):168–171. doi: 10.1016/0006-8993(75)90651-4. [DOI] [PubMed] [Google Scholar]
- SEARS T. A. THE SLOW POTENTIALS OF THORACIC RESPIRATORY MOTONEURONES AND THEIR RELATION TO BREATHING. J Physiol. 1964 Dec;175:404–424. doi: 10.1113/jphysiol.1964.sp007524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUMI T. NEURONAL MECHANISMS IN SWALLOWING. Pflugers Arch Gesamte Physiol Menschen Tiere. 1964;278:467–477. doi: 10.1007/BF00363579. [DOI] [PubMed] [Google Scholar]
- Sessle B. J., Ball G. J., Lucier G. E. Suppressive influences from periaqueductal gray and nucleus raphe magnus on respiration and related reflex activities and on solitary tract neurons, and effect of naloxone. Brain Res. 1981 Jul 6;216(1):145–161. doi: 10.1016/0006-8993(81)91283-x. [DOI] [PubMed] [Google Scholar]
- TABER E., BRODAL A., WALBERG F. The raphe nuclei of the brain stem in the cat. I. Normal topography and cytoarchitecture and general discussion. J Comp Neurol. 1960 Apr;114:161–187. doi: 10.1002/cne.901140205. [DOI] [PubMed] [Google Scholar]
- West D. C., Wolstencroft J. H. Strength-duration characteristics of myelinated and non-myelinated bulbospinal axons in the cat spinal cord. J Physiol. 1983 Apr;337:37–50. doi: 10.1113/jphysiol.1983.sp014610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson V. J., Yoshida M. Monosynaptic inhibition of neck motoneurons by the medial vestibular nucleus. Exp Brain Res. 1969;9(4):365–380. doi: 10.1007/BF00235245. [DOI] [PubMed] [Google Scholar]
- Yen C. T., Blum P. S. Response properties and functional organization of neurons in midline region of medullary reticular formation of cats. J Neurophysiol. 1984 Nov;52(5):961–979. doi: 10.1152/jn.1984.52.5.961. [DOI] [PubMed] [Google Scholar]
- Yen C. T., Blum P. S., Spath J. A., Jr Control of cardiovascular function by electrical stimulation within the medullary raphe region of the cat. Exp Neurol. 1983 Mar;79(3):666–679. doi: 10.1016/0014-4886(83)90031-6. [DOI] [PubMed] [Google Scholar]