Abstract
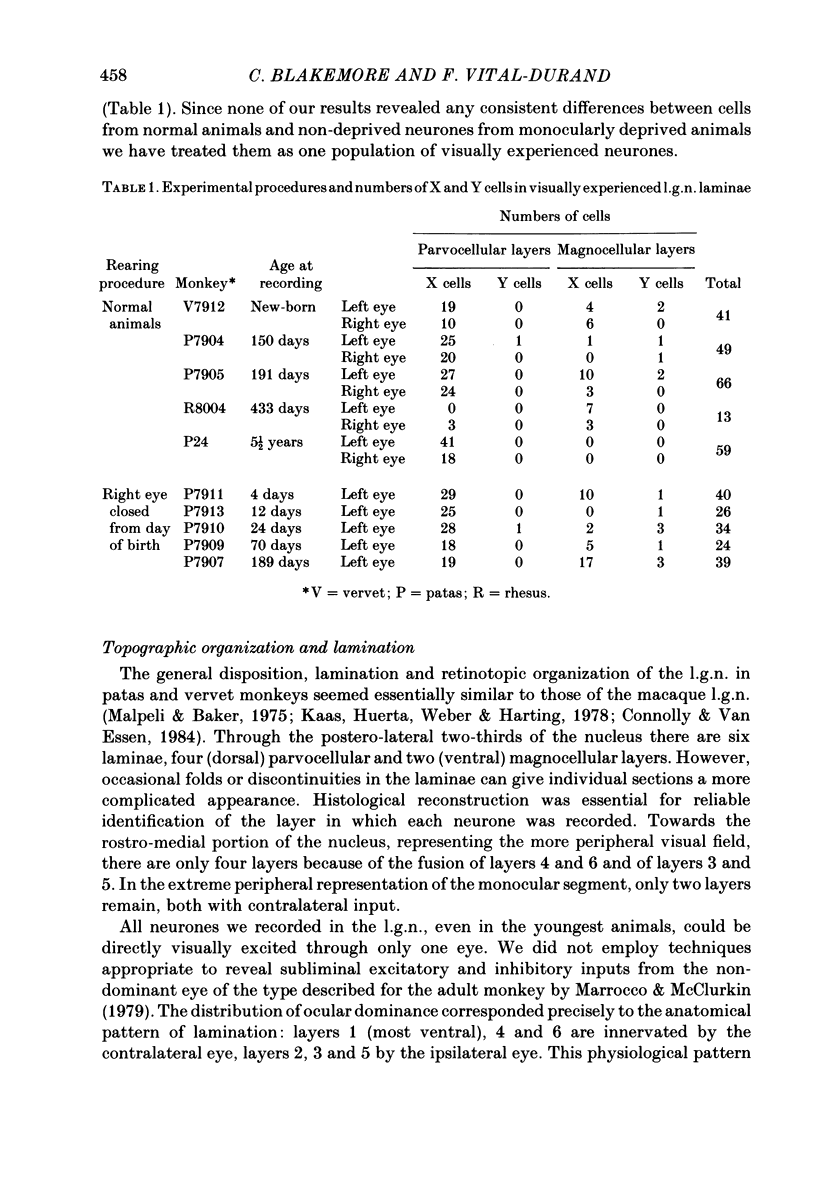
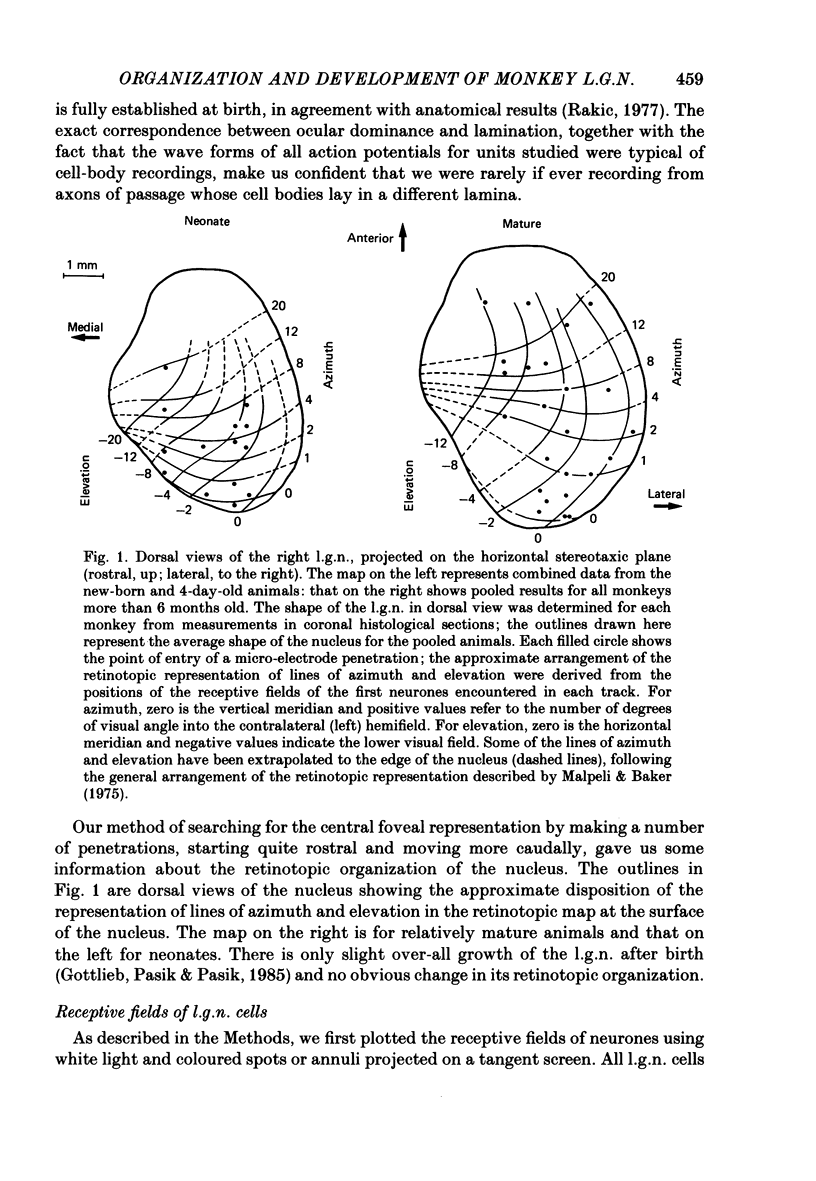
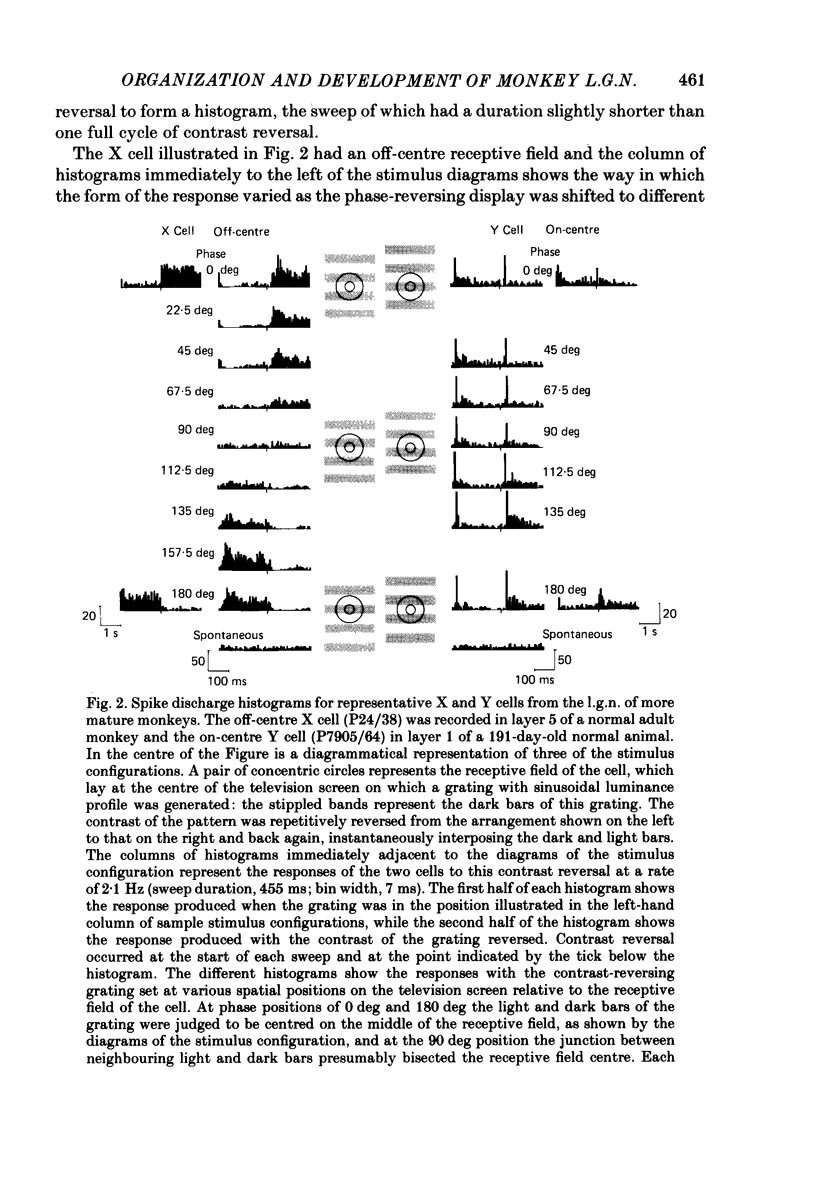
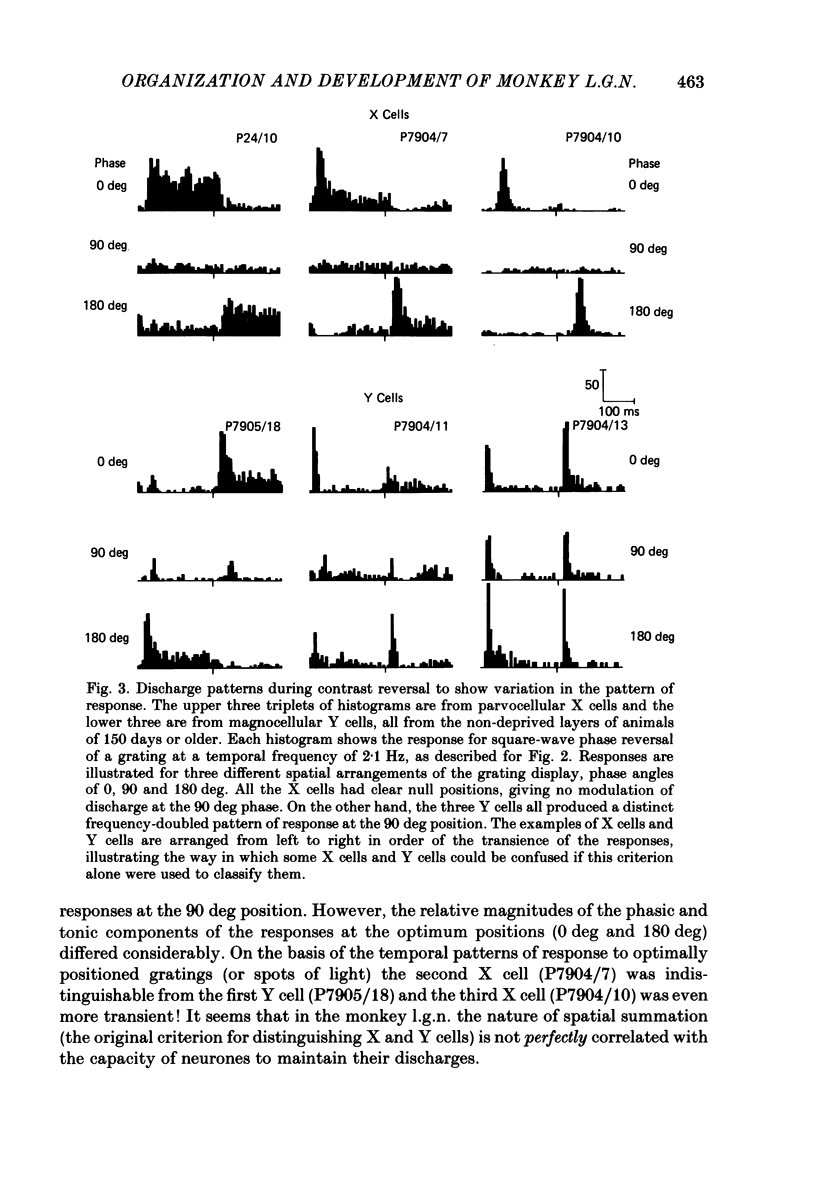
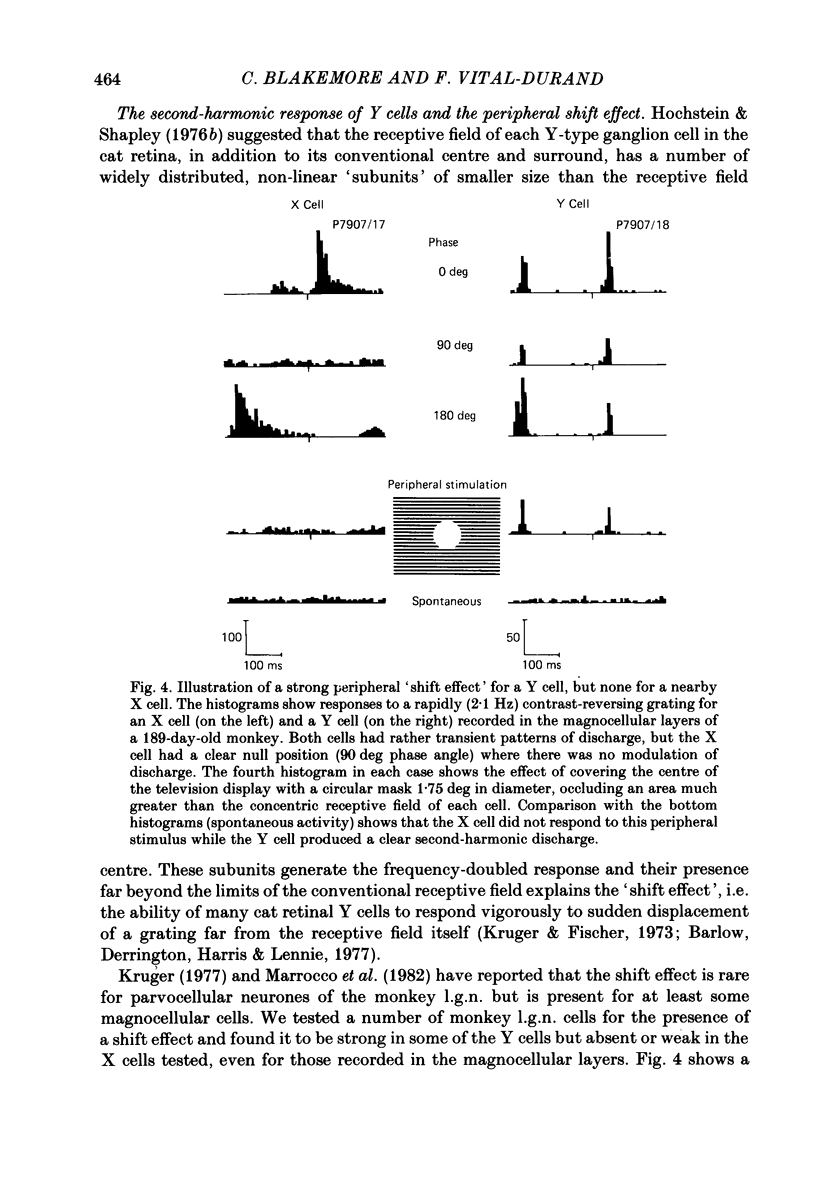
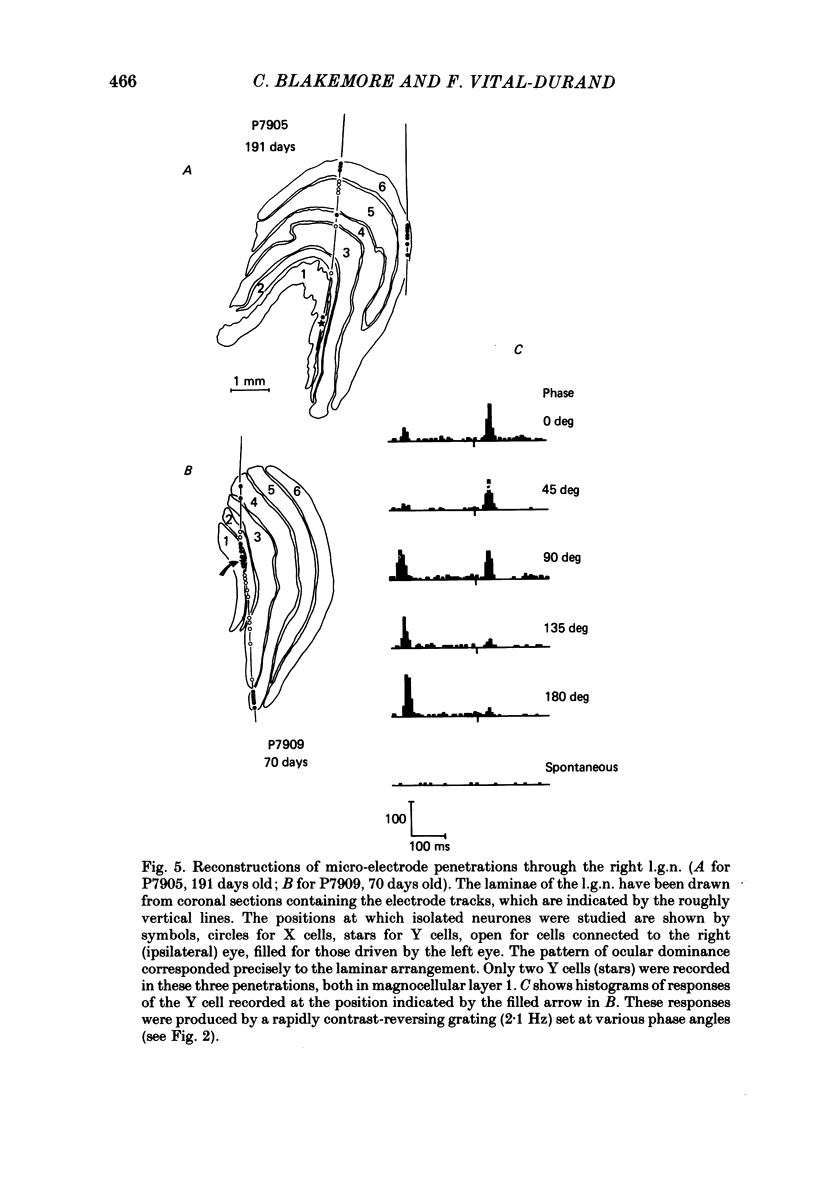
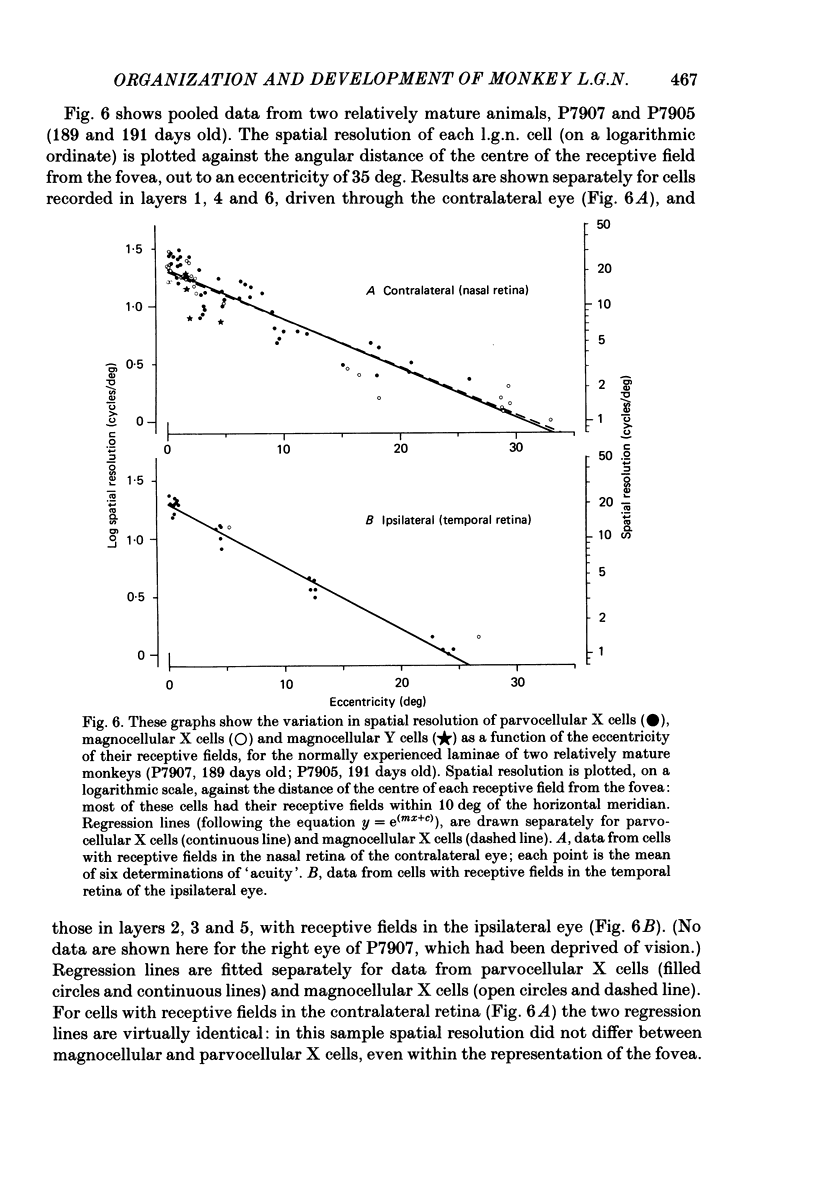
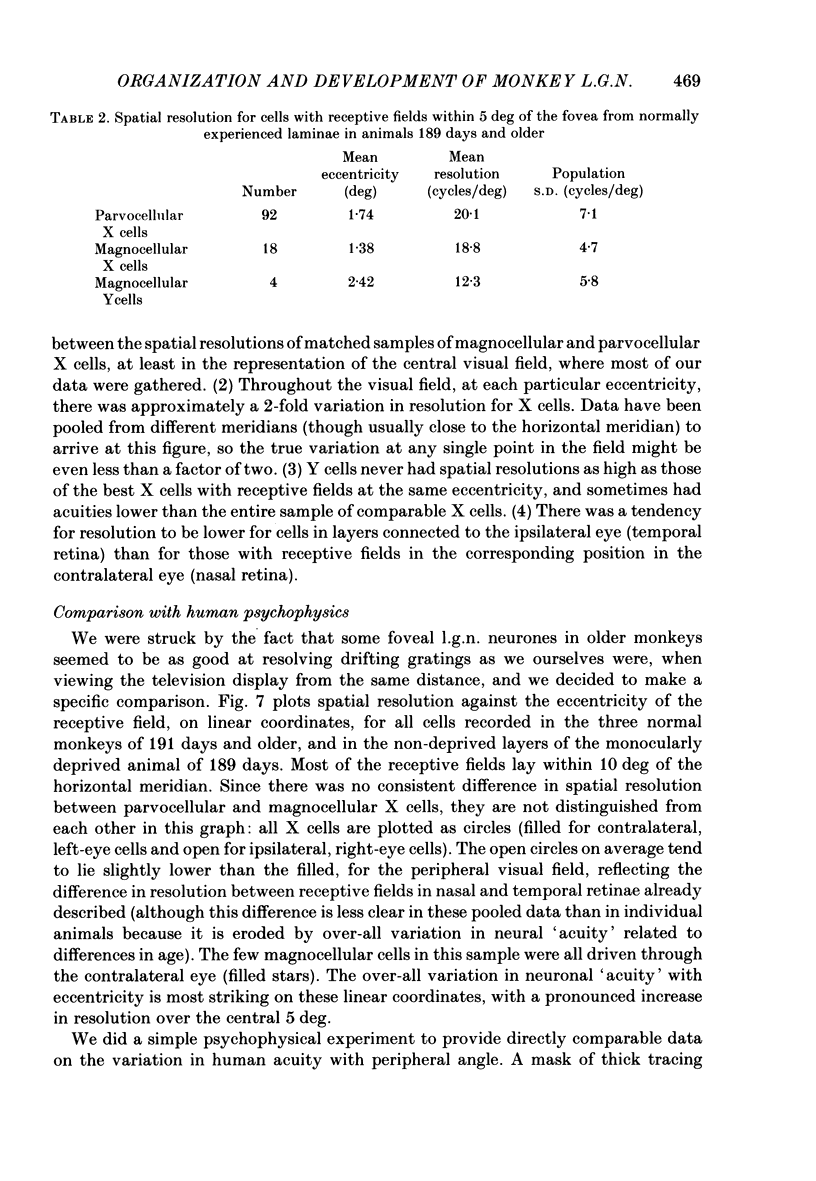
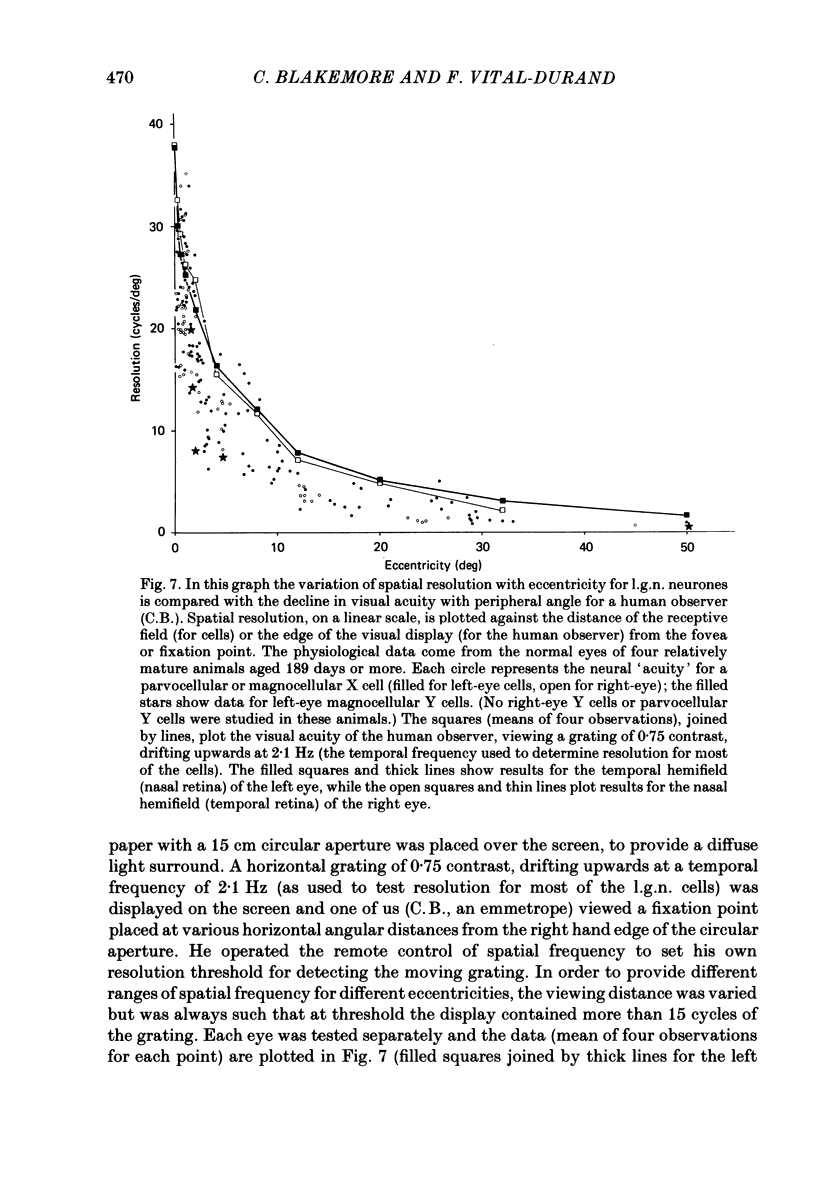
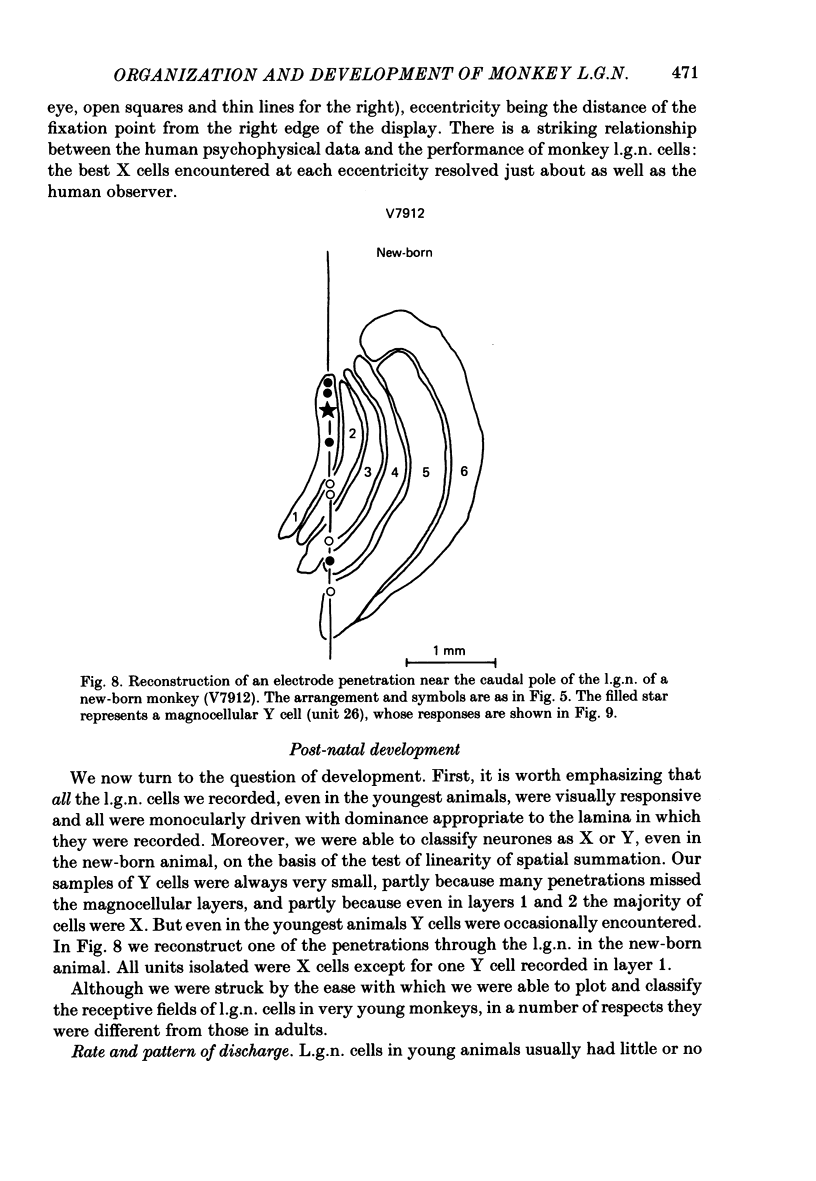
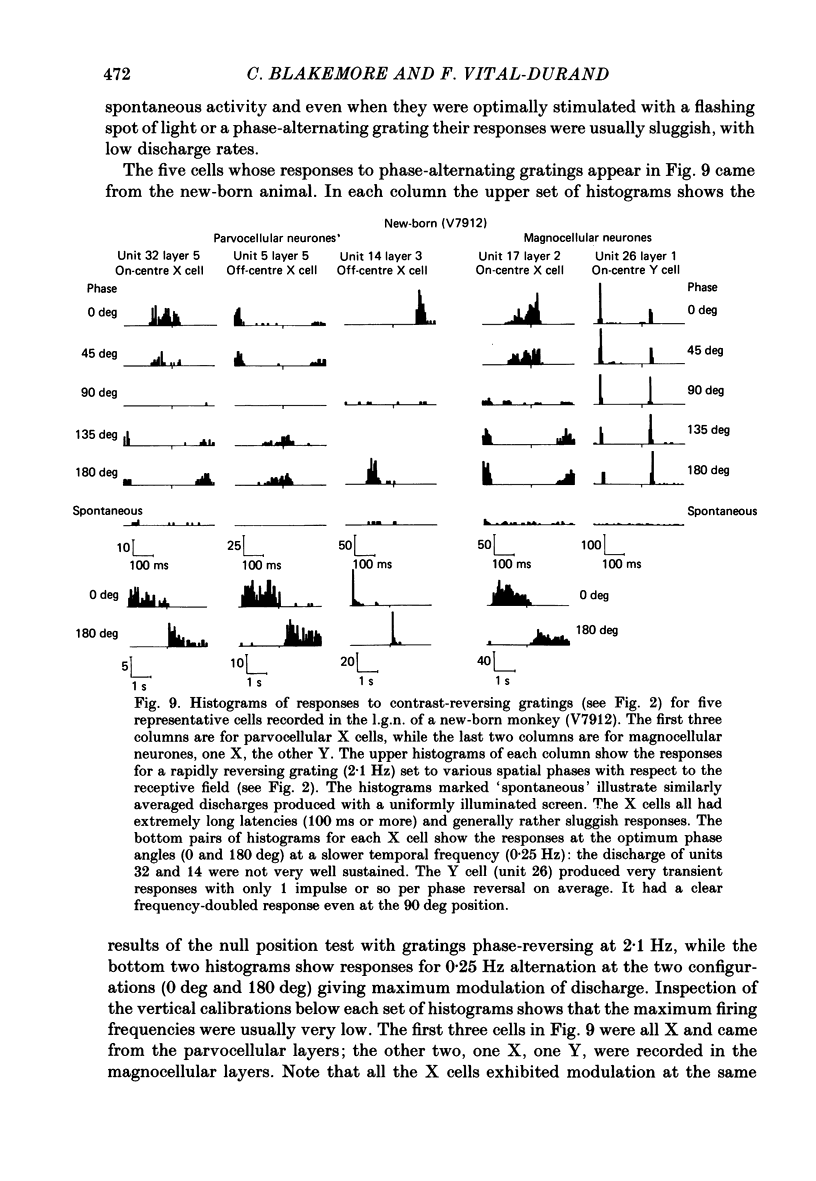
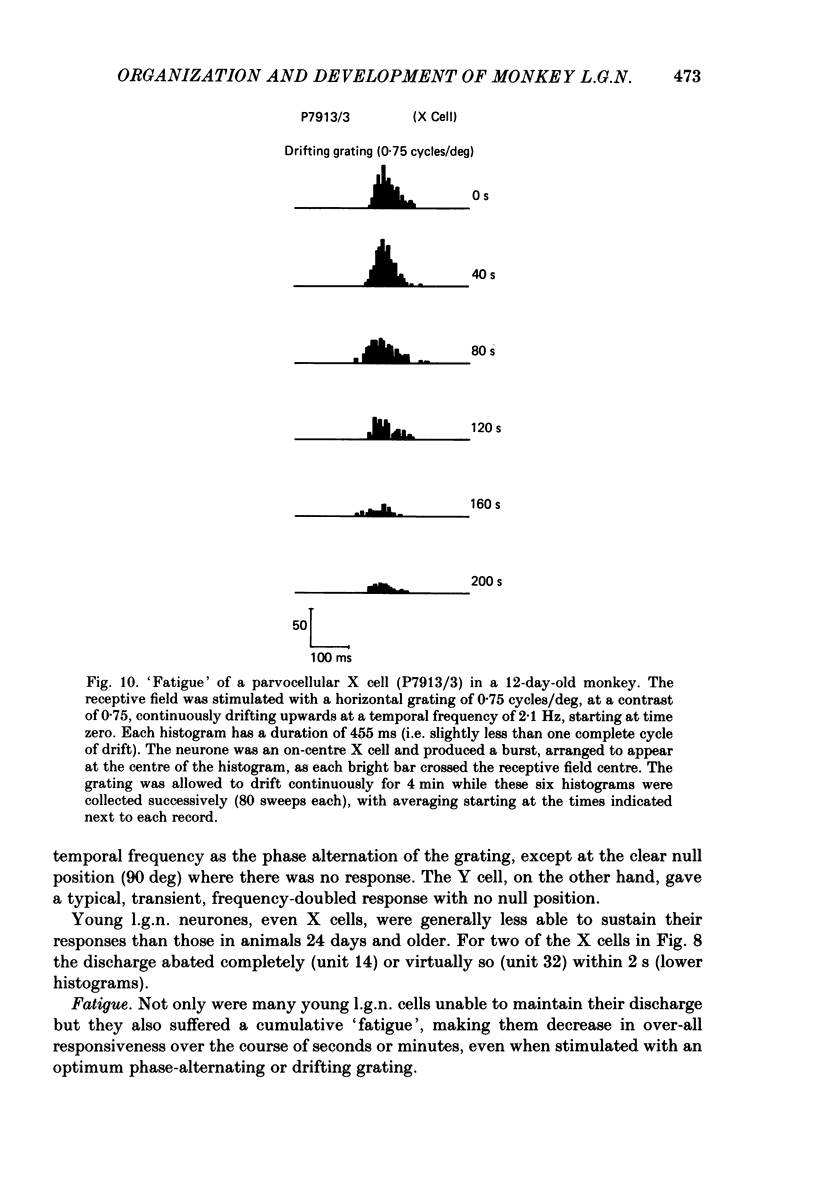
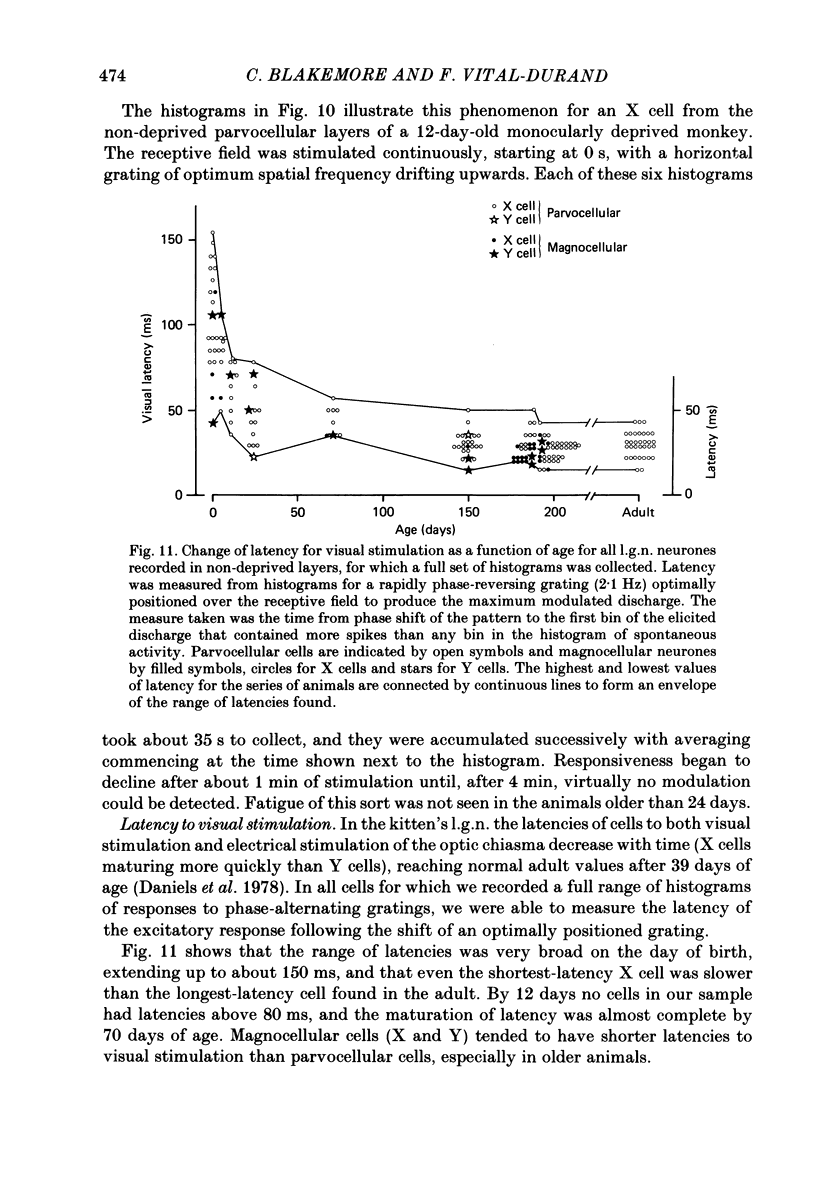
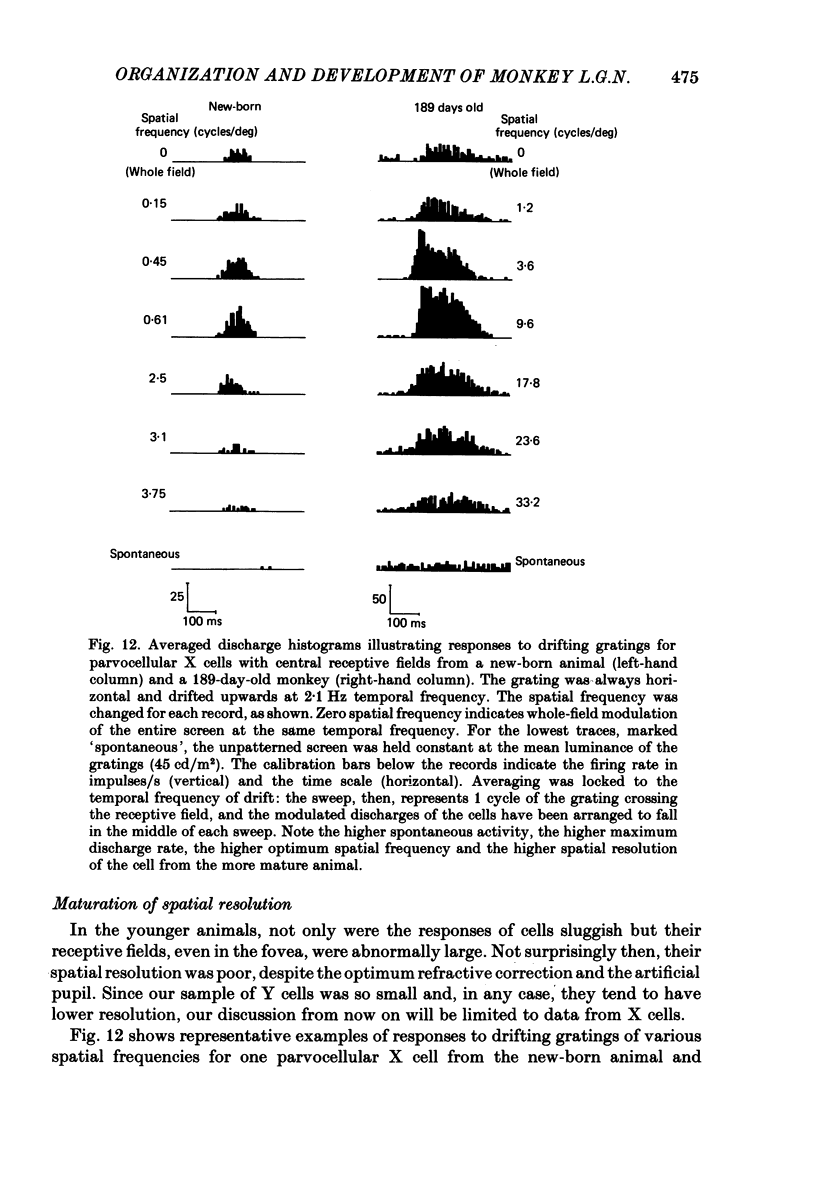
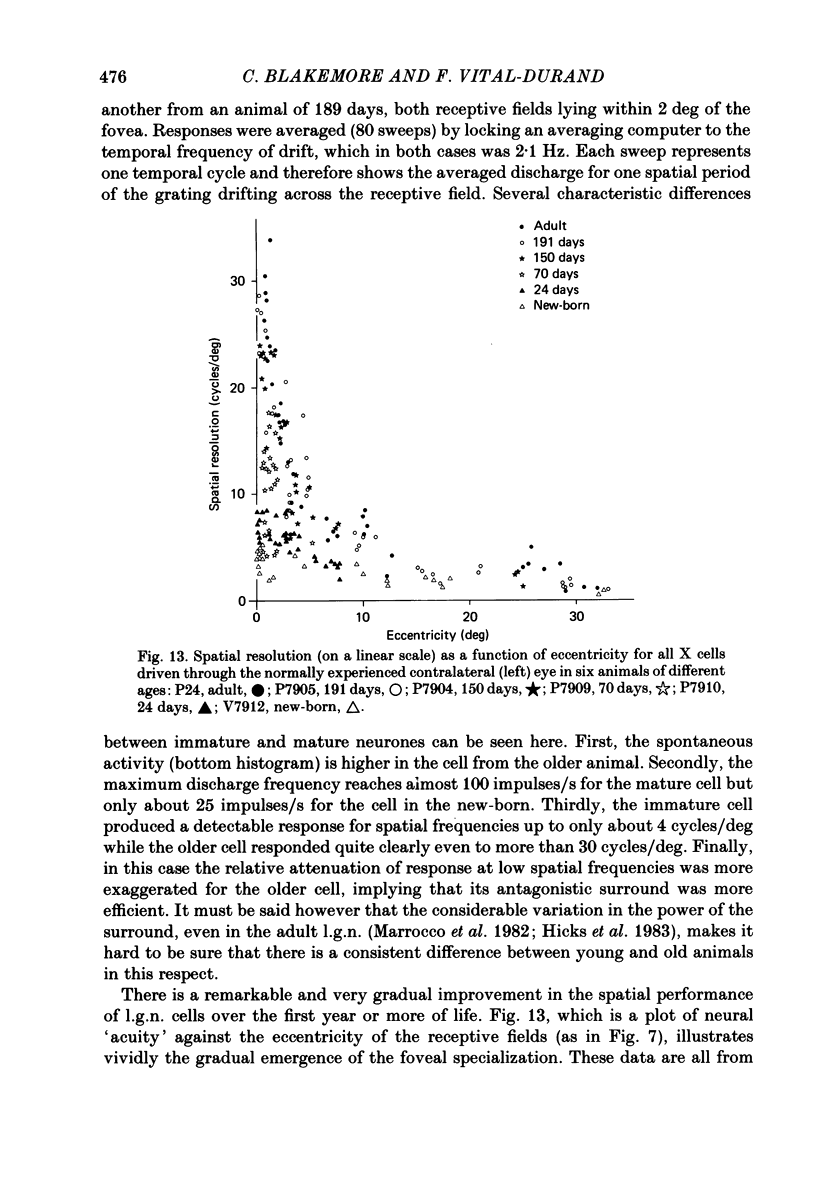
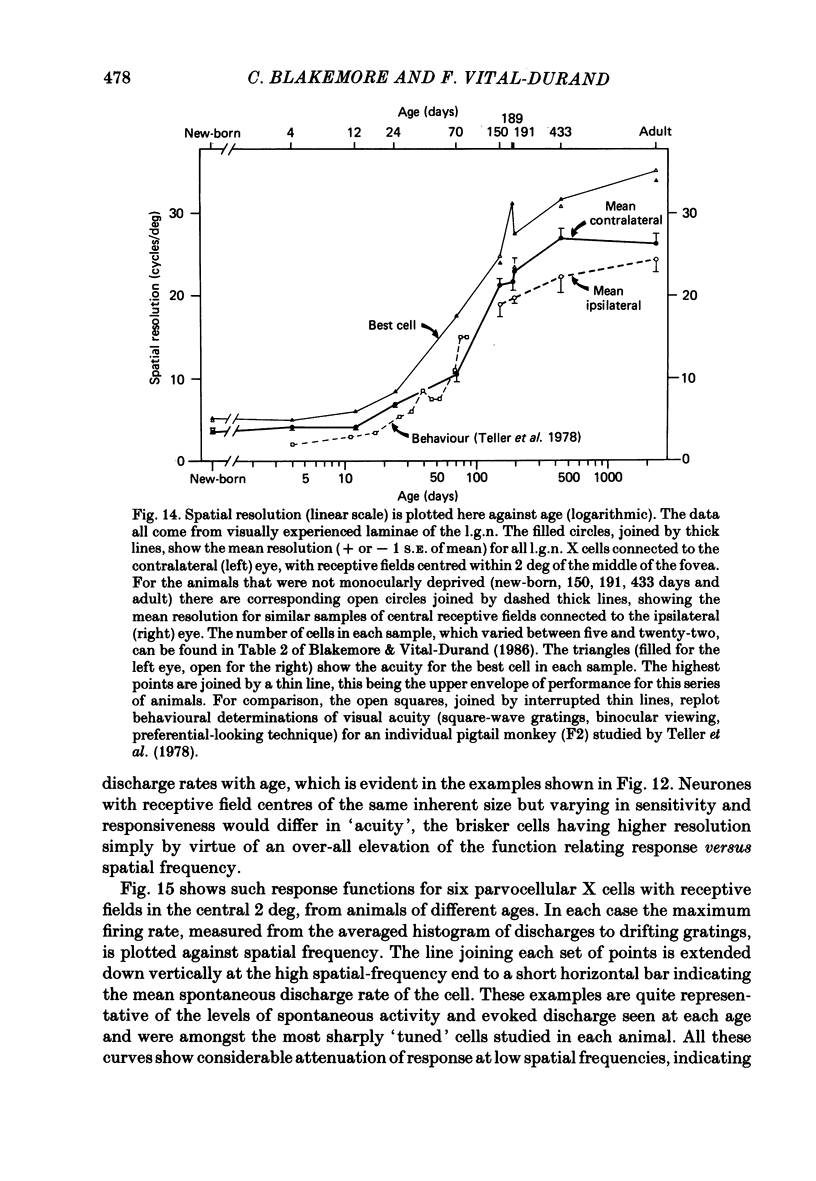
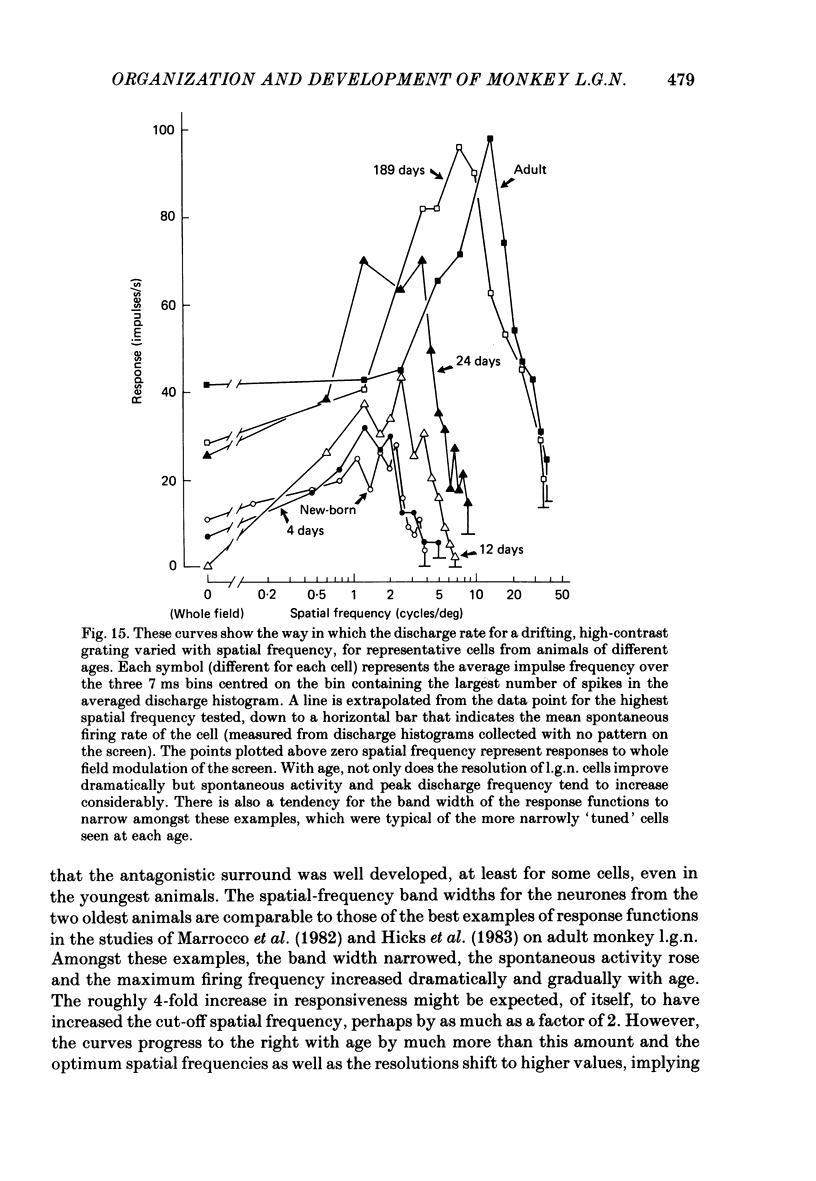
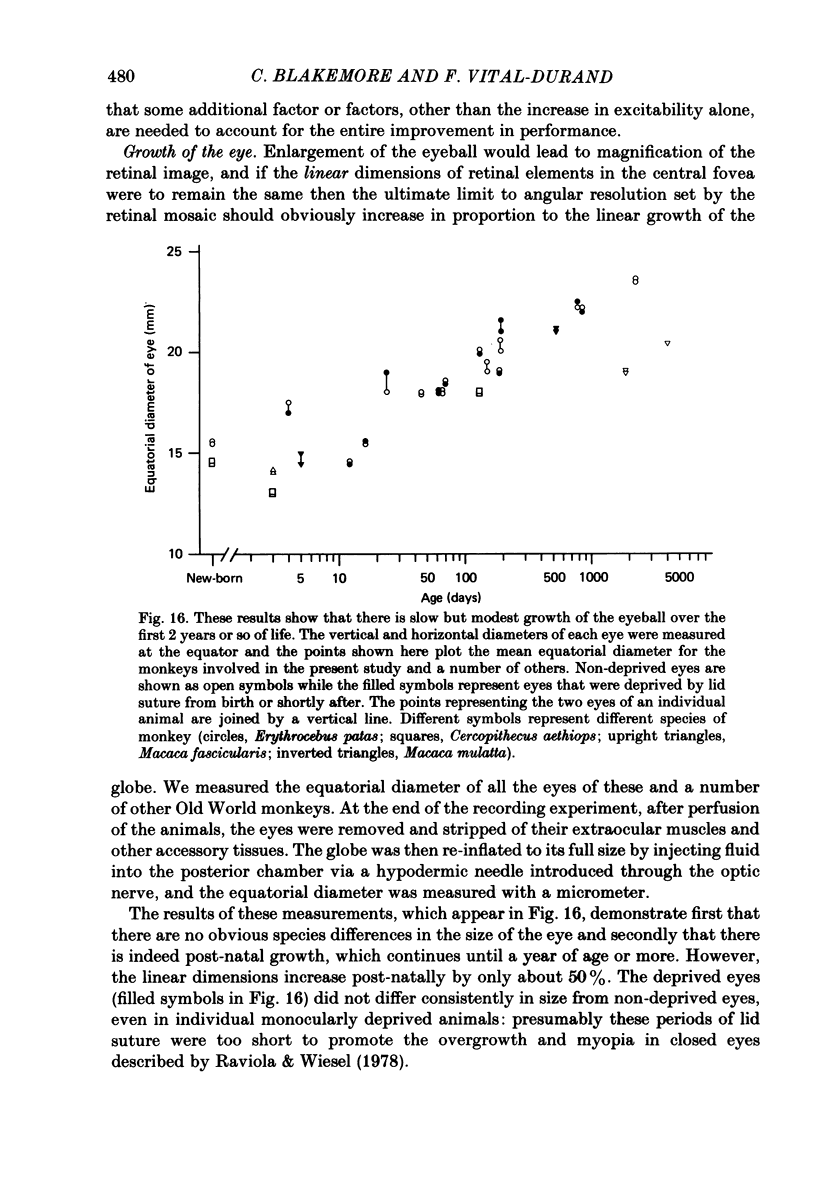
We have studied the properties of neurones in the lateral geniculate nucleus (l.g.n.) of Old World monkeys, both in mature animals and throughout post-natal development. Cells were classified as X (linear) or Y (non-linear) on the basis of their responses to contrast-reversing achromatic gratings ('null position test'). In older animals virtually all parvocellular neurones and the majority of magnocellular units were X cells; only about 15% of magnocellular neurones displayed highly non-linear spatial summation, with no 'null position', typical of Y cells. X cells could not reliably be distinguished from Y cells, nor magnocellular from parvocellular, on the basis of their temporal patterns of discharge. Some Y cells responded transiently to contrast reversal of a grating far from the receptive field but X cells showed little or no such 'shift effect'. The spatial resolution of mature l.g.n. cells varied with the eccentricity of their receptive fields such that the best of them, at each point in the visual field, resolved drifting achromatic gratings about as well as a human observer. X cells in parvocellular and magnocellular layers had similar 'acuities', even in the central foveal representation, but Y cells generally had poorer resolution. Receptive fields in the temporal retina tended to have lower resolution than those at comparable eccentricities in the nasal retina. Even on the day of birth all cells we studied responded to visual stimulation and virtually all could be classified as X or Y. The laminar distribution of cell types and the general morphological appearance of the nucleus seemed very similar to those in the adult, but neurones in very young animals had low spontaneous activity, sluggish responses, and latencies to visual stimulation longer than any we saw in the adult. Until 3 weeks of age or so, many neurones suffered cumulative 'fatigue' when visually stimulated over several minutes. Visual latency was essentially mature by about 10 weeks. In the l.g.n. of the neonatal monkey there was little variation in neuronal 'acuity' with eccentricity: even in the foveal area the best cells could resolve only about 5 cycles/deg. Over the first year or more of life there is a gradual increase in responsiveness and about a 7-fold improvement in spatial resolution for foveal l.g.n. cells, correlating roughly with the behavioural maturation of visual acuity.
Full text
PDF




















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson J., Braddick O., Braddick F. Acuity and contrast sensivity of infant vision. Nature. 1974 Feb 8;247(5440):403–404. doi: 10.1038/247403a0. [DOI] [PubMed] [Google Scholar]
- Barlow H. B., Derrington A. M., Harris L. R., Lennie P. The effects of remote retinal stimulation on the responses of cat retinal ganglion cells. J Physiol. 1977 Jul;269(1):177–194. doi: 10.1113/jphysiol.1977.sp011898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore C., Garey L. J., Vital-Durand F. The physiological effects of monocular deprivation and their reversal in the monkey's visual cortex. J Physiol. 1978 Oct;283:223–262. doi: 10.1113/jphysiol.1978.sp012498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore C., Van Sluyters R. C. Innate and environmental factors in the development of the kitten's visual cortex. J Physiol. 1975 Jul;248(3):663–716. doi: 10.1113/jphysiol.1975.sp010995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore C., Vital-Durand F. Development of the neural basis of visual acuity in monkeys: speculation on the origin of deprivation amblyopia. Trans Ophthalmol Soc U K. 1979;99(3):363–368. [PubMed] [Google Scholar]
- Blakemore C., Vital-Durand F. Effects of visual deprivation on the development of the monkey's lateral geniculate nucleus. J Physiol. 1986 Nov;380:493–511. doi: 10.1113/jphysiol.1986.sp016298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore C., Vital-Durand F., Garey L. J. Recovery from monocular deprivation in the monkey. I. Reversal of physiological effects in the visual cortex. Proc R Soc Lond B Biol Sci. 1981 Nov 24;213(1193):399–423. doi: 10.1098/rspb.1981.0072. [DOI] [PubMed] [Google Scholar]
- Blasdel G. G., Lund J. S. Termination of afferent axons in macaque striate cortex. J Neurosci. 1983 Jul;3(7):1389–1413. doi: 10.1523/JNEUROSCI.03-07-01389.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boothe R. G., Dobson V., Teller D. Y. Postnatal development of vision in human and nonhuman primates. Annu Rev Neurosci. 1985;8:495–545. doi: 10.1146/annurev.ne.08.030185.002431. [DOI] [PubMed] [Google Scholar]
- Boothe R. G. Optical and neural factors limiting acuity development: evidence obtained from a monkey model. Curr Eye Res. 1982;2(3):211–215. doi: 10.3109/02713688208997696. [DOI] [PubMed] [Google Scholar]
- Boothe R. G., Williams R. A., Kiorpes L., Teller D. Y. Development of contrast sensitivity in infant Macaca nemestrina monkeys. Science. 1980 Jun 13;208(4449):1290–1292. doi: 10.1126/science.6769162. [DOI] [PubMed] [Google Scholar]
- Buisseret P., Imbert M. Visual cortical cells: their developmental properties in normal and dark reared kittens. J Physiol. 1976 Feb;255(2):511–525. doi: 10.1113/jphysiol.1976.sp011293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly M., Van Essen D. The representation of the visual field in parvicellular and magnocellular layers of the lateral geniculate nucleus in the macaque monkey. J Comp Neurol. 1984 Jul 10;226(4):544–564. doi: 10.1002/cne.902260408. [DOI] [PubMed] [Google Scholar]
- Daniels J. D., Pettigrew J. D., Norman J. L. Development of single-neuron responses in kitten's lateral geniculate nucleus. J Neurophysiol. 1978 Nov;41(6):1373–1393. doi: 10.1152/jn.1978.41.6.1373. [DOI] [PubMed] [Google Scholar]
- De Valois R. L., Morgan H., Snodderly D. M. Psychophysical studies of monkey vision. 3. Spatial luminance contrast sensitivity tests of macaque and human observers. Vision Res. 1974 Jan;14(1):75–81. doi: 10.1016/0042-6989(74)90118-7. [DOI] [PubMed] [Google Scholar]
- Derrington A. M., Fuchs A. F. The development of spatial-frequency selectivity in kitten striate cortex. J Physiol. 1981 Jul;316:1–10. doi: 10.1113/jphysiol.1981.sp013767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrington A. M., Lennie P. Spatial and temporal contrast sensitivities of neurones in lateral geniculate nucleus of macaque. J Physiol. 1984 Dec;357:219–240. doi: 10.1113/jphysiol.1984.sp015498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson V., Teller D. Y. Visual acuity in human infants: a review and comparison of behavioral and electrophysiological studies. Vision Res. 1978;18(11):1469–1483. doi: 10.1016/0042-6989(78)90001-9. [DOI] [PubMed] [Google Scholar]
- Dreher B., Fukada Y., Rodieck R. W. Identification, classification and anatomical segregation of cells with X-like and Y-like properties in the lateral geniculate nucleus of old-world primates. J Physiol. 1976 Jun;258(2):433–452. doi: 10.1113/jphysiol.1976.sp011429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge J. L. A reversible ophthalmoscope using a corner-cube [proceedings]. J Physiol. 1979 Oct;295:1P–2P. [PubMed] [Google Scholar]
- Enroth-Cugell C., Robson J. G. The contrast sensitivity of retinal ganglion cells of the cat. J Physiol. 1966 Dec;187(3):517–552. doi: 10.1113/jphysiol.1966.sp008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman D. N., Marg E. Visual acuity development coincides with the sensitive period in kittens. Nature. 1975 Apr 17;254(5501):614–615. doi: 10.1038/254614a0. [DOI] [PubMed] [Google Scholar]
- Garey L. J., Saini K. D. Golgi studies of the normal development of neurons in the lateral geniculate nucleus of the monkey. Exp Brain Res. 1981;44(2):117–128. doi: 10.1007/BF00237332. [DOI] [PubMed] [Google Scholar]
- Gottlieb M. D., Pasik P., Pasik T. Early postnatal development of the monkey visual system. I. Growth of the lateral geniculate nucleus and striate cortex. Brain Res. 1985 Jan;349(1-2):53–62. doi: 10.1016/0165-3806(85)90131-2. [DOI] [PubMed] [Google Scholar]
- Gwiazda J., Brill S., Mohindra I., Held R. Preferential looking acuity in infants from two to fifty-eight weeks of age. Am J Optom Physiol Opt. 1980 Jul;57(7):428–432. doi: 10.1097/00006324-198007000-00004. [DOI] [PubMed] [Google Scholar]
- Harris L., Atkinson J., Braddick O. Visual contrast sensitivity of a 6-month-old infant measured by the evoked potential. Nature. 1976 Dec 9;264(5586):570–571. doi: 10.1038/264570a0. [DOI] [PubMed] [Google Scholar]
- Headon M. P., Sloper J. J., Hiorns R. W., Powell T. P. Sizes of neurons in the primate lateral geniculate nucleus during normal development. Brain Res. 1985 Feb;350(1-2):51–56. doi: 10.1016/0165-3806(85)90249-4. [DOI] [PubMed] [Google Scholar]
- Hendrickson A. E., Wilson J. R., Ogren M. P. The neuroanatomical organization of pathways between the dorsal lateral geniculate nucleus and visual cortex in Old World and New World primates. J Comp Neurol. 1978 Nov 1;182(1):123–136. doi: 10.1002/cne.901820108. [DOI] [PubMed] [Google Scholar]
- Hendrickson A., Kupfer C. The histogenesis of the fovea in the macaque monkey. Invest Ophthalmol Vis Sci. 1976 Sep;15(9):746–756. [PubMed] [Google Scholar]
- Hicks T. P., Lee B. B., Vidyasagar T. R. The responses of cells in macaque lateral geniculate nucleus to sinusoidal gratings. J Physiol. 1983 Apr;337:183–200. doi: 10.1113/jphysiol.1983.sp014619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochstein S., Shapley R. M. Linear and nonlinear spatial subunits in Y cat retinal ganglion cells. J Physiol. 1976 Nov;262(2):265–284. doi: 10.1113/jphysiol.1976.sp011595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochstein S., Shapley R. M. Quantitative analysis of retinal ganglion cell classifications. J Physiol. 1976 Nov;262(2):237–264. doi: 10.1113/jphysiol.1976.sp011594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howland H., Boothe R. G., Kiorpes L. Accomodative defocus does not limit development of acuity in infant Macaca nemestrina monkeys. Science. 1982 Mar 12;215(4538):1409–1411. doi: 10.1126/science.7063852. [DOI] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. Laminar and columnar distribution of geniculo-cortical fibers in the macaque monkey. J Comp Neurol. 1972 Dec;146(4):421–450. doi: 10.1002/cne.901460402. [DOI] [PubMed] [Google Scholar]
- Ikeda H., Tremain K. E. The development of spatial resolving power of lateral geniculate neurones in kittens. Exp Brain Res. 1978 Feb 15;31(2):193–206. doi: 10.1007/BF00237599. [DOI] [PubMed] [Google Scholar]
- Kaas J. H., Huerta M. F., Weber J. T., Harting J. K. Patterns of retinal terminations and laminar organization of the lateral geniculate nucleus of primates. J Comp Neurol. 1978 Dec 1;182(3):517–553. doi: 10.1002/cne.901820308. [DOI] [PubMed] [Google Scholar]
- Kaplan E., Shapley R. M. X and Y cells in the lateral geniculate nucleus of macaque monkeys. J Physiol. 1982 Sep;330:125–143. doi: 10.1113/jphysiol.1982.sp014333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krüger J., Fischer B. Strong periphery effect in cat retinal ganglion cells. Excitatory responses in ON- and OFF- center neurones to single grid displacements. Exp Brain Res. 1973 Oct 26;18(3):316–318. doi: 10.1007/BF00234601. [DOI] [PubMed] [Google Scholar]
- Krüger J. The shift-effect in the lateral geniculate body of the rhesus monkey. Exp Brain Res. 1977 Sep 28;29(3-4):387–392. doi: 10.1007/BF00236177. [DOI] [PubMed] [Google Scholar]
- Leuba G., Garey L. J. Development of dendritic patterns in the lateral geniculate nucleus of monkey: a quantitative Golgi study. Brain Res. 1984 Nov;318(2):285–299. doi: 10.1016/0165-3806(84)90033-6. [DOI] [PubMed] [Google Scholar]
- Malpeli J. G., Baker F. H. The representation of the visual field in the lateral geniculate nucleus of Macaca mulatta. J Comp Neurol. 1975 Jun 15;161(4):569–594. doi: 10.1002/cne.901610407. [DOI] [PubMed] [Google Scholar]
- Marrocco R. T., McClurkin J. W. Binocular interaction in the lateral geniculate nucleus of the monkey. Brain Res. 1979 Jun 8;168(3):633–637. doi: 10.1016/0006-8993(79)90319-6. [DOI] [PubMed] [Google Scholar]
- Marrocco R. T., McClurkin J. W., Young R. A. Spatial summation and conduction latency classification of cells of the lateral geniculate nucleus of macaques. J Neurosci. 1982 Sep;2(9):1275–1291. doi: 10.1523/JNEUROSCI.02-09-01275.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill E. G., Ainsworth A. Glass-coated platinum-plated tungsten microelectrodes. Med Biol Eng. 1972 Sep;10(5):662–672. doi: 10.1007/BF02476084. [DOI] [PubMed] [Google Scholar]
- Mitchell D. E., Giffin F., Wilkinson F., Anderson P., Smith M. L. Visual resolution in young kittens. Vision Res. 1976;16(4):363–366. doi: 10.1016/0042-6989(76)90197-8. [DOI] [PubMed] [Google Scholar]
- Mullen K. T. The contrast sensitivity of human colour vision to red-green and blue-yellow chromatic gratings. J Physiol. 1985 Feb;359:381–400. doi: 10.1113/jphysiol.1985.sp015591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pease P. L. Eye movements in systemically paralyzed macaque monkeys. Vision Res. 1973 Feb;13(2):483–487. doi: 10.1016/0042-6989(73)90128-4. [DOI] [PubMed] [Google Scholar]
- Perry V. H., Oehler R., Cowey A. Retinal ganglion cells that project to the dorsal lateral geniculate nucleus in the macaque monkey. Neuroscience. 1984 Aug;12(4):1101–1123. doi: 10.1016/0306-4522(84)90006-x. [DOI] [PubMed] [Google Scholar]
- Pettigrew J. D. The effect of visual experience on the development of stimulus specificity by kitten cortical neurones. J Physiol. 1974 Feb;237(1):49–74. doi: 10.1113/jphysiol.1974.sp010469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. Genesis of the dorsal lateral geniculate nucleus in the rhesus monkey: site and time of origin, kinetics of proliferation, routes of migration and pattern of distribution of neurons. J Comp Neurol. 1977 Nov 1;176(1):23–52. doi: 10.1002/cne.901760103. [DOI] [PubMed] [Google Scholar]
- Raviola E., Wiesel T. N. Effect of dark-rearing on experimental myopia in monkeys. Invest Ophthalmol Vis Sci. 1978 Jun;17(6):485–488. [PubMed] [Google Scholar]
- Schiller P. H., Malpeli J. G. Functional specificity of lateral geniculate nucleus laminae of the rhesus monkey. J Neurophysiol. 1978 May;41(3):788–797. doi: 10.1152/jn.1978.41.3.788. [DOI] [PubMed] [Google Scholar]
- Shapley R., Kaplan E., Soodak R. Spatial summation and contrast sensitivity of X and Y cells in the lateral geniculate nucleus of the macaque. Nature. 1981 Aug 6;292(5823):543–545. doi: 10.1038/292543a0. [DOI] [PubMed] [Google Scholar]
- Sherman S. M., Wilson J. R., Kaas J. H., Webb S. V. X- and Y-cells in the dorsal lateral geniculate nucleus of the owl monkey (Aotus trivirgatus). Science. 1976 Apr 30;192(4238):475–477. doi: 10.1126/science.816006. [DOI] [PubMed] [Google Scholar]
- Sireteanu R., Hoffmann K. P. Relative frequency and visual resolution of X- and Y-cells in the LGN of normal and monocularly deprived cats: interlaminar differences. Exp Brain Res. 1979 Feb 15;34(3):591–603. doi: 10.1007/BF00239151. [DOI] [PubMed] [Google Scholar]
- Swindale N. V., Vital-Durand F., Blakemore C. Recovery from monocular deprivation in the monkey. III. Reversal of anatomical effects in the visual cortex. Proc R Soc Lond B Biol Sci. 1981 Nov 24;213(1193):435–450. doi: 10.1098/rspb.1981.0074. [DOI] [PubMed] [Google Scholar]
- Teller D. Y., Boothe R. Development of vision in infant primates. Trans Ophthalmol Soc U K. 1979;99(3):333–337. [PubMed] [Google Scholar]
- Teller D. Y., Morse R., Borton R., Regal D. Visual acuity for vertical and diagonal gratings in human infants. Vision Res. 1974 Dec;14(12):1433–1439. doi: 10.1016/0042-6989(74)90018-2. [DOI] [PubMed] [Google Scholar]
- Teller D. Y., Regal D. M., Videen T. O., Pulos E. Development of visual acuity in infant monkeys (Macaca nemestrina) during the early postnatal weeks. Vision Res. 1978;18(5):561–566. doi: 10.1016/0042-6989(78)90203-1. [DOI] [PubMed] [Google Scholar]
- Wiesel T. N., Hubel D. H. Ordered arrangement of orientation columns in monkeys lacking visual experience. J Comp Neurol. 1974 Dec 1;158(3):307–318. doi: 10.1002/cne.901580306. [DOI] [PubMed] [Google Scholar]
- Wiesel T. N., Hubel D. H. Spatial and chromatic interactions in the lateral geniculate body of the rhesus monkey. J Neurophysiol. 1966 Nov;29(6):1115–1156. doi: 10.1152/jn.1966.29.6.1115. [DOI] [PubMed] [Google Scholar]
- Williams R. A., Booth R. G. Development of optical quality in the infant monkey (Macaca nemestrina) eye. Invest Ophthalmol Vis Sci. 1981 Nov;21(5):728–736. [PubMed] [Google Scholar]
- Williams R. A., Boothe R. G., Kiorpes L., Teller D. Y. Oblique effects in normally reared monkeys (Macaca nemestrina): meridional variations in contrast sensitivity measured with operant techniques. Vision Res. 1981;21(8):1253–1266. doi: 10.1016/0042-6989(81)90230-3. [DOI] [PubMed] [Google Scholar]
- Yellott J. I., Jr Spectral consequences of photoreceptor sampling in the rhesus retina. Science. 1983 Jul 22;221(4608):382–385. doi: 10.1126/science.6867716. [DOI] [PubMed] [Google Scholar]
- de Monasterio F. M. Center and surround mechanisms of opponent-color X and Y ganglion cells of retina of macaques. J Neurophysiol. 1978 Nov;41(6):1418–1434. doi: 10.1152/jn.1978.41.6.1418. [DOI] [PubMed] [Google Scholar]
- de Monasterio F. M. Properties of concentrically organized X and Y ganglion cells of macaque retina. J Neurophysiol. 1978 Nov;41(6):1394–1417. doi: 10.1152/jn.1978.41.6.1394. [DOI] [PubMed] [Google Scholar]
- de Monasterio F. M. Properties of ganglion cells with atypical receptive-field organization in retina of macaques. J Neurophysiol. 1978 Nov;41(6):1435–1449. doi: 10.1152/jn.1978.41.6.1435. [DOI] [PubMed] [Google Scholar]



