Abstract
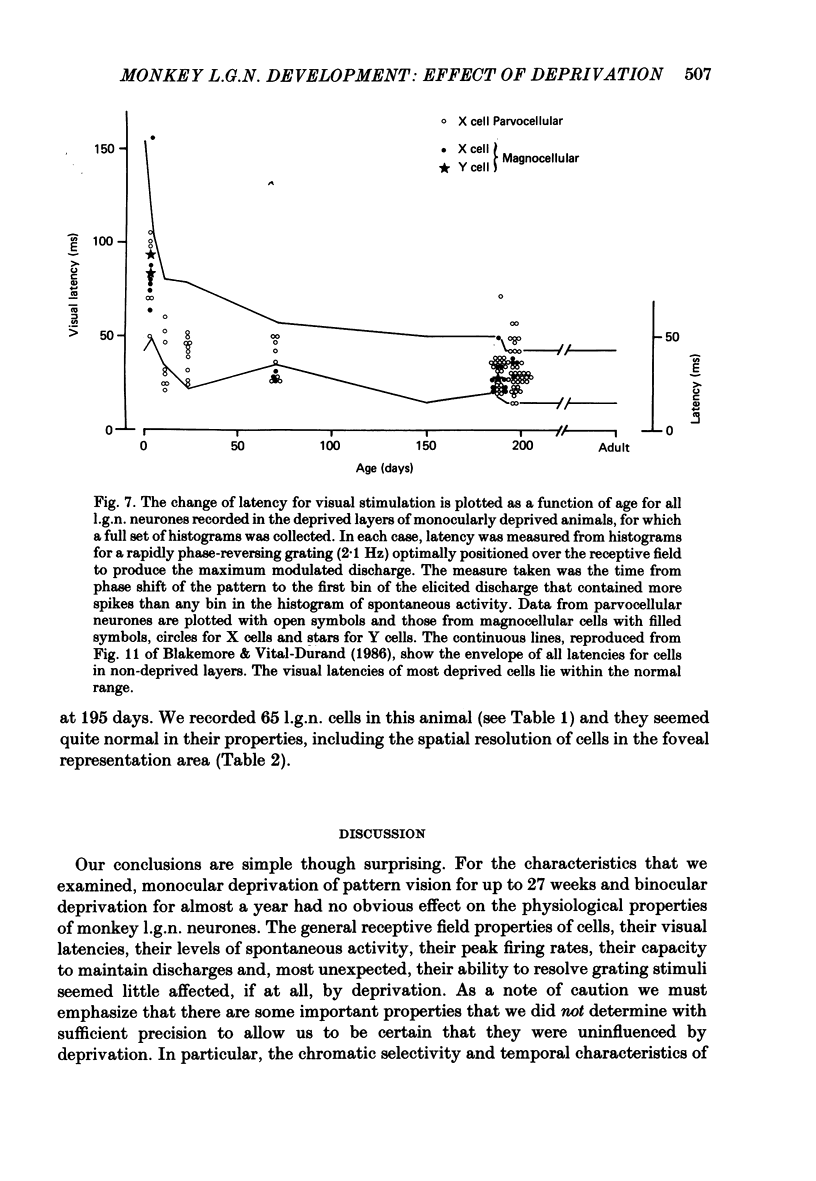
We have studied the physiological properties of cells in the deprived layers of the lateral geniculate nucleus (l.g.n.) in monkeys monocularly deprived from birth for up to 27 weeks, and compared them with results from the non-deprived layers in the same animals and in a series of normal animals. Despite the relative shrinkage of cell bodies in the deprived layers, units were easily isolated, were visually responsive and could readily be classified as linear (X) or non-linear (Y) by means of tests of spatial summation. The laminar distribution of cell types and the proportion of Y cells did not seem to be affected by deprivation. The patterns and latencies of discharge produced by contrast-reversing gratings did not differ grossly between deprived and non-deprived cells. The peak firing frequencies for drifting gratings were also similar. The degree of surround antagonism (though very variable from cell to cell) seemed unaffected by deprivation. Most surprising of all, there was little or no deficit in the spatial resolution of the receptive fields of deprived cells. Recordings were always taken ipsilateral to the deprived eye, and neural 'acuity' tended to be sligtly lower in the deprived laminae than the non-deprived. However, this nasal/temporal asymmetry in spatial resolution was not obviously more pronounced than in normal animals. Neural 'acuity' was not abnormally low in either contralateral or ipsilateral layers in the l.g.n. of an animal binocularly deprived from birth until a year of age. We have not examined chromatic properties or temporal characteristics adequately to say whether they are affected by deprivation. Paradoxically, although the post-natal maturation of visual acuity in normal monkeys seems to be mainly limited by peripheral factors, deprivation (which causes a profound defect of behavioural acuity) does not seem to interfere substantially with physiological development of the retina or the geniculate nucleus.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Awaya S., Sugawara M., Miyake S. Observations in patients with occlusion amblyopia: results of treatment. Trans Ophthalmol Soc U K. 1979;99(3):447–454. [PubMed] [Google Scholar]
- Blakemore C., Garey L. J., Vital-Durand F. The physiological effects of monocular deprivation and their reversal in the monkey's visual cortex. J Physiol. 1978 Oct;283:223–262. doi: 10.1113/jphysiol.1978.sp012498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore C., Van Sluyters R. C. Innate and environmental factors in the development of the kitten's visual cortex. J Physiol. 1975 Jul;248(3):663–716. doi: 10.1113/jphysiol.1975.sp010995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore C., Vital-Durand F. Organization and post-natal development of the monkey's lateral geniculate nucleus. J Physiol. 1986 Nov;380:453–491. doi: 10.1113/jphysiol.1986.sp016297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland B. G., Mitchell D. E., Gillard-Crewther S., Crewther D. P. Visual resolution of retinal ganglion cells in monocularly-deprived cats. Brain Res. 1980 Jun 16;192(1):261–266. doi: 10.1016/0006-8993(80)91026-4. [DOI] [PubMed] [Google Scholar]
- Derrington A. M., Hawken M. J. Spatial and temporal properties of cat geniculate neurones after prolonged deprivation. J Physiol. 1981 May;314:107–120. doi: 10.1113/jphysiol.1981.sp013694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrington A. M., Lennie P. Spatial and temporal contrast sensitivities of neurones in lateral geniculate nucleus of macaque. J Physiol. 1984 Dec;357:219–240. doi: 10.1113/jphysiol.1984.sp015498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eysel U. T., Grüsser O. J., Hoffmann K. P. Monocular deprivation and the signal transmission by X- and Y-neurons of the cat lateral geniculate nucleus. Exp Brain Res. 1979 Feb 15;34(3):521–539. doi: 10.1007/BF00239147. [DOI] [PubMed] [Google Scholar]
- Friedlander M. J., Stanford L. R. Effects of monocular deprivation on the distribution of cell types in the LGNd: a sampling study with fine-tipped micropipettes. Exp Brain Res. 1984;53(2):451–461. doi: 10.1007/BF00238175. [DOI] [PubMed] [Google Scholar]
- Friedlander M. J., Stanford L. R., Sherman S. M. Effects of monocular deprivation on the structure-function relationship of individual neurons in the cat's lateral geniculate nucleus. J Neurosci. 1982 Mar;2(3):321–330. doi: 10.1523/JNEUROSCI.02-03-00321.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frégnac Y., Imbert M. Development of neuronal selectivity in primary visual cortex of cat. Physiol Rev. 1984 Jan;64(1):325–434. doi: 10.1152/physrev.1984.64.1.325. [DOI] [PubMed] [Google Scholar]
- Garey L. J., Blakemore C. The effects of monocular deprivation on different neuronal classes in the lateral geniculate nucleus of the cat. Exp Brain Res. 1977 Jun 27;28(3-4):259–278. doi: 10.1007/BF00235708. [DOI] [PubMed] [Google Scholar]
- Geisert E. E., Spear P. D., Zetlan S. R., Langsetmo A. Recovery of Y-cells in the lateral geniculate nucleus of monocularly deprived cats. J Neurosci. 1982 May;2(5):577–588. doi: 10.1523/JNEUROSCI.02-05-00577.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillery R. W., Stelzner D. J. The differential effects of unilateral lid closure upon the monocular and binocular segments of the dorsal lateral geniculate nucleus in the cat. J Comp Neurol. 1970 Aug;139(4):413–421. doi: 10.1002/cne.901390403. [DOI] [PubMed] [Google Scholar]
- Harwerth R. S., Smith E. L., 3rd, Boltz R. L., Crawford M. L., von Noorden G. K. Behavioral studies on the effect of abnormal early visual experience in monkeys: spatial modulation sensitivity. Vision Res. 1983;23(12):1501–1510. doi: 10.1016/0042-6989(83)90162-1. [DOI] [PubMed] [Google Scholar]
- Headon M. P., Powell T. P. Cellular changes in the lateral geniculate nucleus of infant monkeys after suture of the eyelids. J Anat. 1973 Oct;116(Pt 1):135–145. [PMC free article] [PubMed] [Google Scholar]
- Headon M. P., Sloper J. J., Hiorns R. W., Powell T. P. Effects of monocular closure at different ages on deprived and undeprived cells in the primate lateral geniculate nucleus. Brain Res. 1985 Feb;350(1-2):57–78. doi: 10.1016/0165-3806(85)90250-0. [DOI] [PubMed] [Google Scholar]
- Headon M. P., Sloper J. J., Powell T. P. Initial hypertrophy of cells in undeprived laminae of the lateral geniculate nucleus of the monkey following early monocular visual deprivation. Brain Res. 1982 Apr 29;238(2):439–444. doi: 10.1016/0006-8993(82)90120-2. [DOI] [PubMed] [Google Scholar]
- Hendrickson A., Boothe R. Morphology of the retina and dorsal lateral geniculate nucleus in dark-reared monkeys (Macaca nemestrina). Vision Res. 1976;16(5):517–521. doi: 10.1016/0042-6989(76)90033-x. [DOI] [PubMed] [Google Scholar]
- Hicks T. P., Lee B. B., Vidyasagar T. R. The responses of cells in macaque lateral geniculate nucleus to sinusoidal gratings. J Physiol. 1983 Apr;337:183–200. doi: 10.1113/jphysiol.1983.sp014619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda H., Plant G. T., Tremain K. E. Nasal field loss in kittens reared with convergent squint: neurophysiological and morphological studies of the lateral geniculate nucleus. J Physiol. 1977 Sep;270(2):345–366. doi: 10.1113/jphysiol.1977.sp011956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan E., Shapley R. M. X and Y cells in the lateral geniculate nucleus of macaque monkeys. J Physiol. 1982 Sep;330:125–143. doi: 10.1113/jphysiol.1982.sp014333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kratz K. E., Mangel S. C., Lehmkuhle S., Sherman M. Retinal X- and Y-cells in monocularly lid-sutured cats: normality of spatial and temporal properties. Brain Res. 1979 Aug 31;172(3):545–551. doi: 10.1016/0006-8993(79)90586-9. [DOI] [PubMed] [Google Scholar]
- LeVay S., Wiesel T. N., Hubel D. H. The development of ocular dominance columns in normal and visually deprived monkeys. J Comp Neurol. 1980 May 1;191(1):1–51. doi: 10.1002/cne.901910102. [DOI] [PubMed] [Google Scholar]
- Lehmkuhle S., Kratz K. E., Mangel S. C., Sherman S. M. An effect of early monocular lid suture upon the development of X-cells in the cat's lateral geniculate nucleus. Brain Res. 1978 Nov 24;157(2):346–350. doi: 10.1016/0006-8993(78)90039-2. [DOI] [PubMed] [Google Scholar]
- Lehmkuhle S., Kratz K. E., Mangel S. C., Sherman S. M. Effects of early monocular lid suture on spatial and temporal sensitivity of neurons in dorsal lateral geniculate nucleus of the cat. J Neurophysiol. 1980 Feb;43(2):542–556. doi: 10.1152/jn.1980.43.2.542. [DOI] [PubMed] [Google Scholar]
- Leventhal A. G., Hirsch H. V. Effects of visual deprivation upon the morphology of retinal ganglion cells projecting to the dorsal lateral geniculate nucleus of the cat. J Neurosci. 1983 Feb;3(2):332–344. doi: 10.1523/JNEUROSCI.03-02-00332.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei L., Fiorentini A. Monocular deprivation in kittens impairs the spatial resolution of geniculate neurones. Nature. 1976 Dec 23;264(5588):754–755. doi: 10.1038/264754a0. [DOI] [PubMed] [Google Scholar]
- Movshon J. A., Van Sluyters R. C. Visual neural development. Annu Rev Psychol. 1981;32:477–522. doi: 10.1146/annurev.ps.32.020181.002401. [DOI] [PubMed] [Google Scholar]
- Mower G. D., Christen W. G. Effects of early monocular deprivation on the acuity of lateral geniculate neurons in the cat. Brain Res. 1982 Mar;255(3):475–480. doi: 10.1016/0165-3806(82)90012-8. [DOI] [PubMed] [Google Scholar]
- Perry V. H., Oehler R., Cowey A. Retinal ganglion cells that project to the dorsal lateral geniculate nucleus in the macaque monkey. Neuroscience. 1984 Aug;12(4):1101–1123. doi: 10.1016/0306-4522(84)90006-x. [DOI] [PubMed] [Google Scholar]
- Raviola E., Wiesel T. N. Effect of dark-rearing on experimental myopia in monkeys. Invest Ophthalmol Vis Sci. 1978 Jun;17(6):485–488. [PubMed] [Google Scholar]
- Sesma M. A., Irvin G. E., Kuyk T. K., Norton T. T., Casagrande V. A. Effects of monocular deprivation on the lateral geniculate nucleus in a primate. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2255–2259. doi: 10.1073/pnas.81.7.2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapley R., So Y. T. Is there an effect of monocular deprivation on the proportions of X and Y cells in the cat lateral geniculate nucleus? Exp Brain Res. 1980;39(1):41–48. doi: 10.1007/BF00237068. [DOI] [PubMed] [Google Scholar]
- Sherman S. M., Hoffmann K. P., Stone J. Loss of a specific cell type from dorsal lateral geniculate nucleus in visually deprived cats. J Neurophysiol. 1972 Jul;35(4):532–541. doi: 10.1152/jn.1972.35.4.532. [DOI] [PubMed] [Google Scholar]
- Sherman S. M., Spear P. D. Organization of visual pathways in normal and visually deprived cats. Physiol Rev. 1982 Apr;62(2):738–855. doi: 10.1152/physrev.1982.62.2.738. [DOI] [PubMed] [Google Scholar]
- Sherman S. M., Stone J. Physiological normality of the retinal in visually deprived cats. Brain Res. 1973 Sep 28;60(1):224–230. doi: 10.1016/0006-8993(73)90861-5. [DOI] [PubMed] [Google Scholar]
- Sireteanu R., Hoffmann K. P. Relative frequency and visual resolution of X- and Y-cells in the LGN of normal and monocularly deprived cats: interlaminar differences. Exp Brain Res. 1979 Feb 15;34(3):591–603. doi: 10.1007/BF00239151. [DOI] [PubMed] [Google Scholar]
- Sur M., Humphrey A. L., Sherman S. M. Monocular deprivation affects X- and Y-cell retinogeniculate terminations in cats. Nature. 1982 Nov 11;300(5888):183–185. doi: 10.1038/300183a0. [DOI] [PubMed] [Google Scholar]
- Swindale N. V., Vital-Durand F., Blakemore C. Recovery from monocular deprivation in the monkey. III. Reversal of anatomical effects in the visual cortex. Proc R Soc Lond B Biol Sci. 1981 Nov 24;213(1193):435–450. doi: 10.1098/rspb.1981.0074. [DOI] [PubMed] [Google Scholar]
- Vaegan, Taylor D. Critical period for deprivation amblyopia in children. Trans Ophthalmol Soc U K. 1979;99(3):432–439. [PubMed] [Google Scholar]
- WIESEL T. N., HUBEL D. H. EFFECTS OF VISUAL DEPRIVATION ON MORPHOLOGY AND PHYSIOLOGY OF CELLS IN THE CATS LATERAL GENICULATE BODY. J Neurophysiol. 1963 Nov;26:978–993. doi: 10.1152/jn.1963.26.6.978. [DOI] [PubMed] [Google Scholar]
- Wilson J. R., Hendrickson A. E. Neuronal and synaptic structure of the dorsal lateral geniculate nucleus in normal and monocularly deprived Macaca monkeys. J Comp Neurol. 1981 Apr 10;197(3):517–539. doi: 10.1002/cne.901970311. [DOI] [PubMed] [Google Scholar]
- Winfield D. A., Hiorns R. W., Powell T. P. A quantitative electron-microscopical study of the postnatal development of the lateral geniculate nucleus in normal kittens and in kittens with eyelid suture. Proc R Soc Lond B Biol Sci. 1980 Nov 19;210(1179):211–234. doi: 10.1098/rspb.1980.0130. [DOI] [PubMed] [Google Scholar]
- Winfield D. A., Powell T. P. An electron-microscopical study of the postnatal development of the lateral geniculate nucleus in the normal kitten and after eyelid suture. Proc R Soc Lond B Biol Sci. 1980 Nov 19;210(1179):197–210. doi: 10.1098/rspb.1980.0129. [DOI] [PubMed] [Google Scholar]
- von Noorden G. K. Experimental amblyopia in monkeys. Further behavioral observations and clinical correlations. Invest Ophthalmol. 1973 Oct;12(10):721–726. [PubMed] [Google Scholar]