Abstract
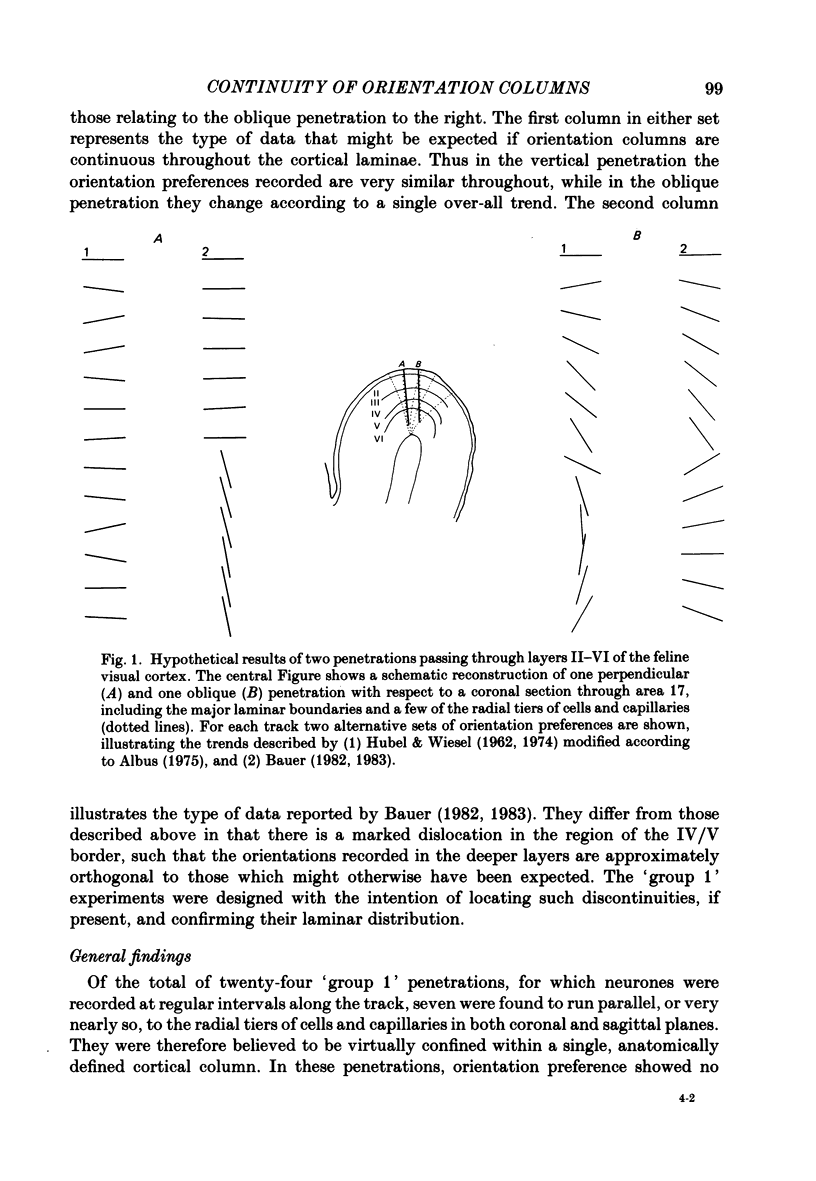
1. Recent reports of a marked and consistent dislocation between orientation columns in the superficial and deep layers of cat striate cortex (Bauer, 1982, 1983) directly contradict the traditional view of the system (Hubel & Wiesel, 1962). This has considerable implications for our current understanding of cortical organization, and in order to clarify the issue we have carried out experiments to test the continuity of the columnar system with depth, in central regions of area 17. 2. In twenty-four penetrations, eighteen of which were placed as perpendicular as possible to the surface of the cortex, orientation preference was assessed at regular intervals both qualitatively and using a randomly interleaved quantitative technique. The distribution of preferred orientations was analysed with reference to a detailed histological reconstruction of the electrode track, including the location of laminar boundaries and the course of radial tiers of cells and capillaries. 3. From a further series of eighteen near-perpendicular penetrations, the change in average orientation between one superficial and one deep layer recording site was compared with the deviation of the track from perpendicular to the surface and hence parallel to the orientation columns. 4. In penetrations perpendicular to the surface of the cortex, orientation preference showed little variation between superficial and deep laminae. In oblique penetrations, preferred orientation generally changed according to a single, smooth trend. Those irregularities that were encountered were confined to oblique penetrations, and were distributed throughout the cortical laminae. 5. In conclusion, our evidence does not support the presence of a systematic discontinuity with depth within the orientation columnar system. It is therefore entirely consistent with earlier evidence on the subject.
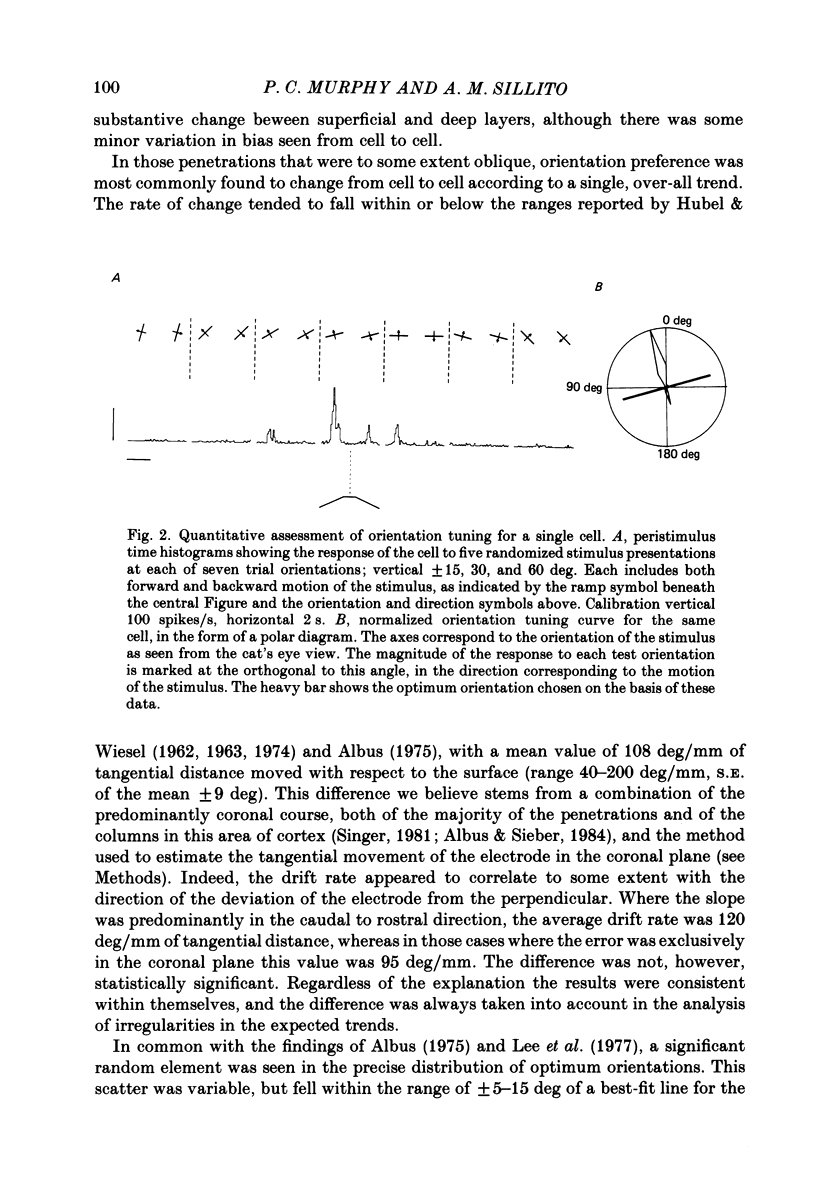
Full text
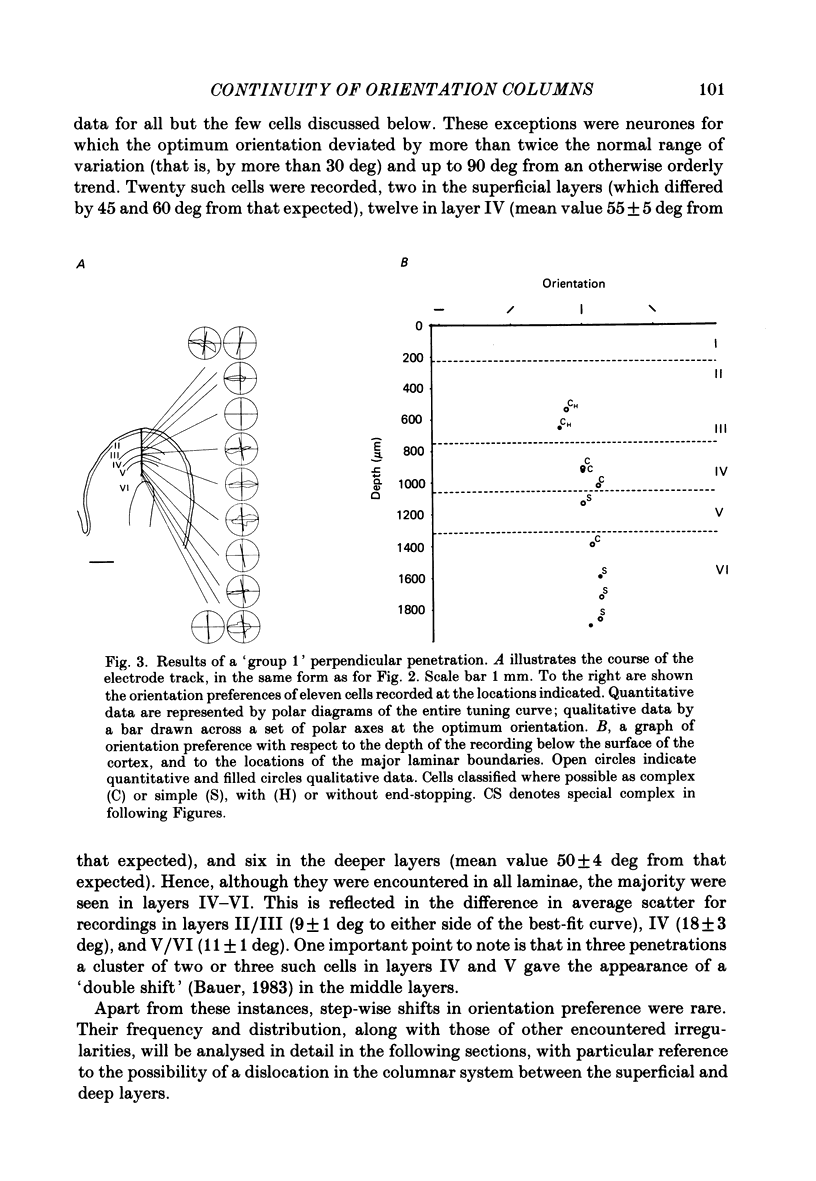
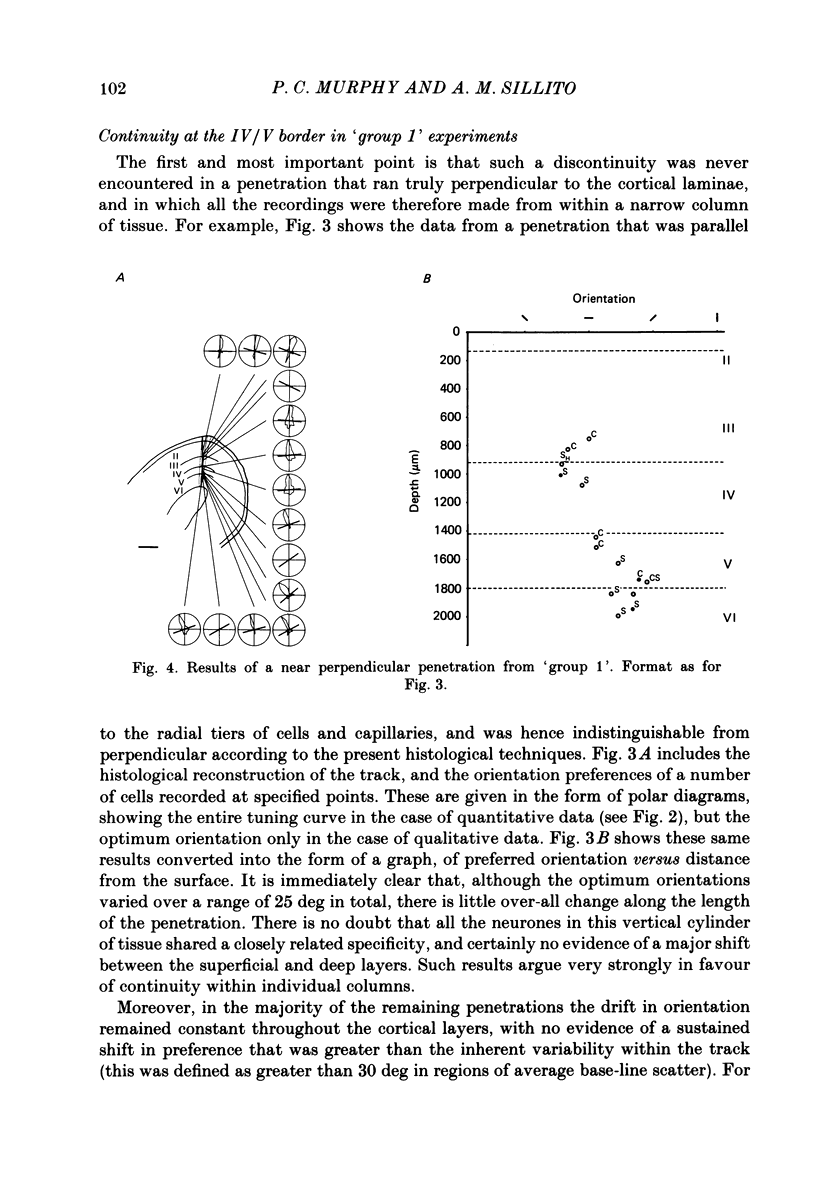
PDF



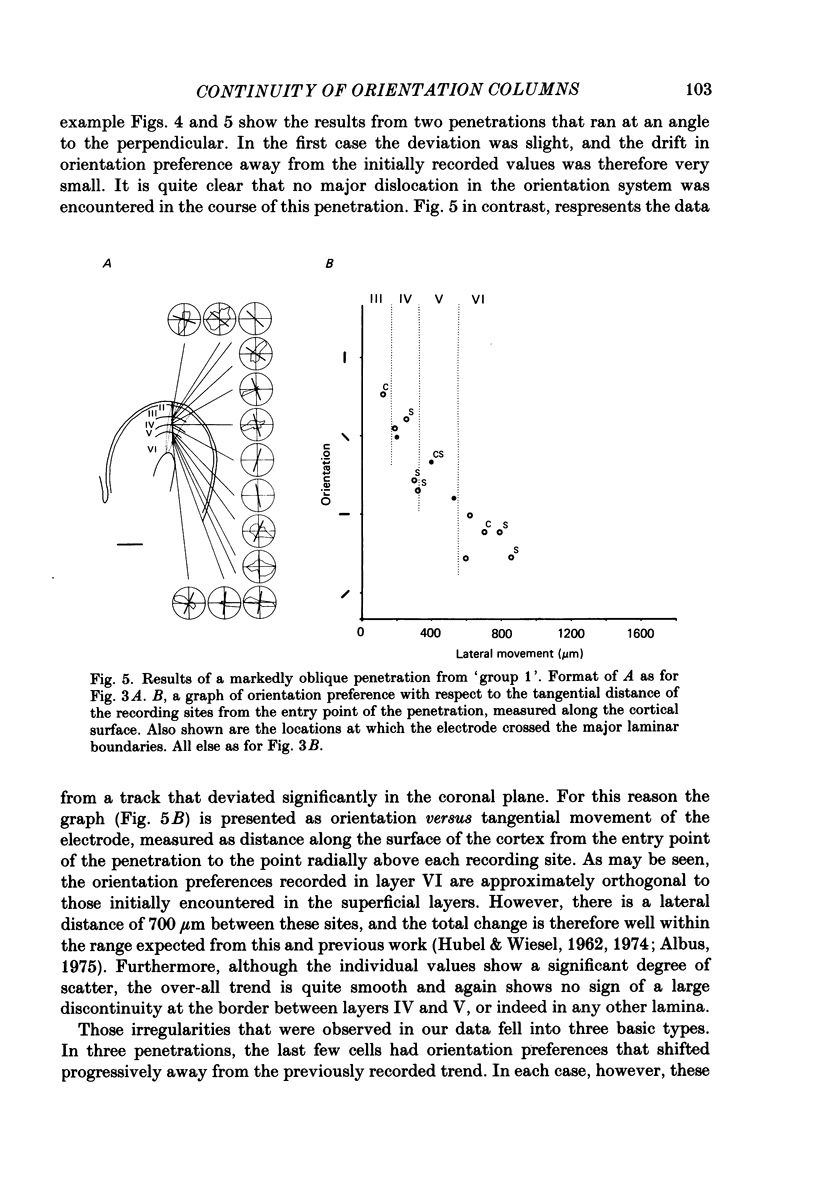
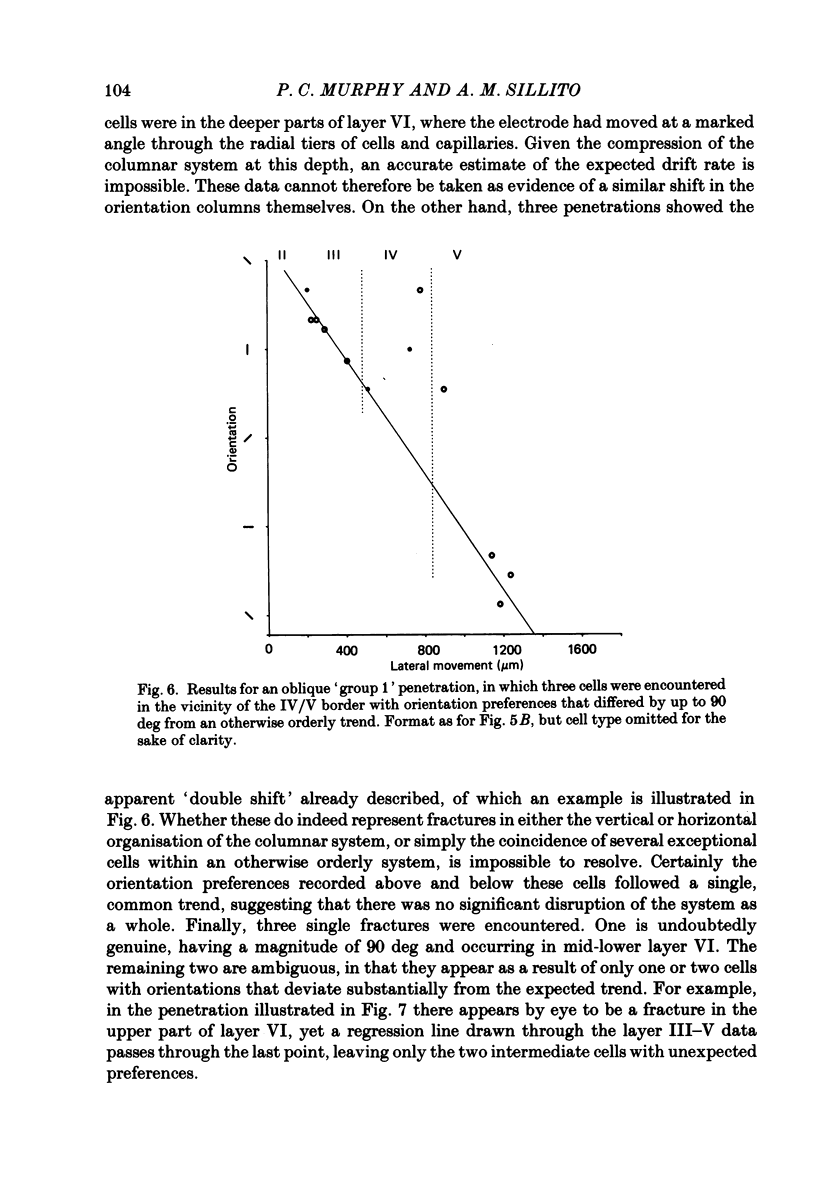
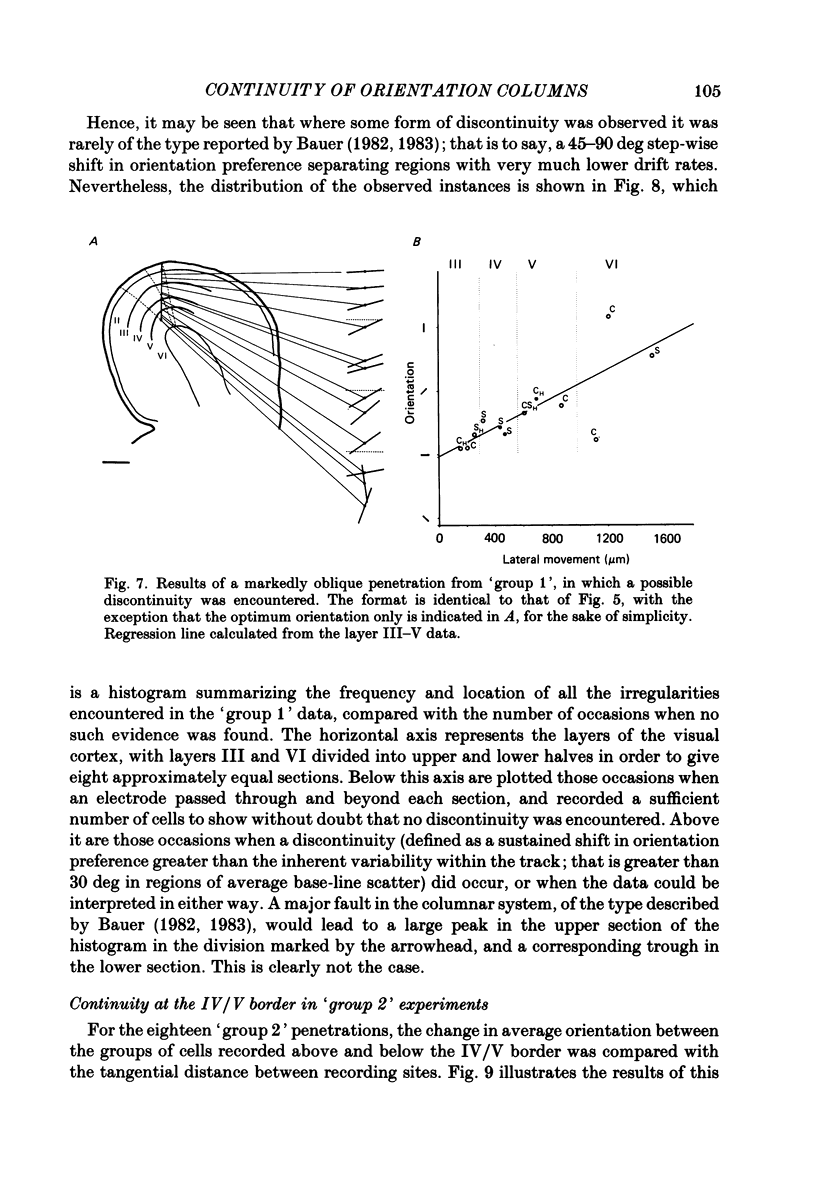
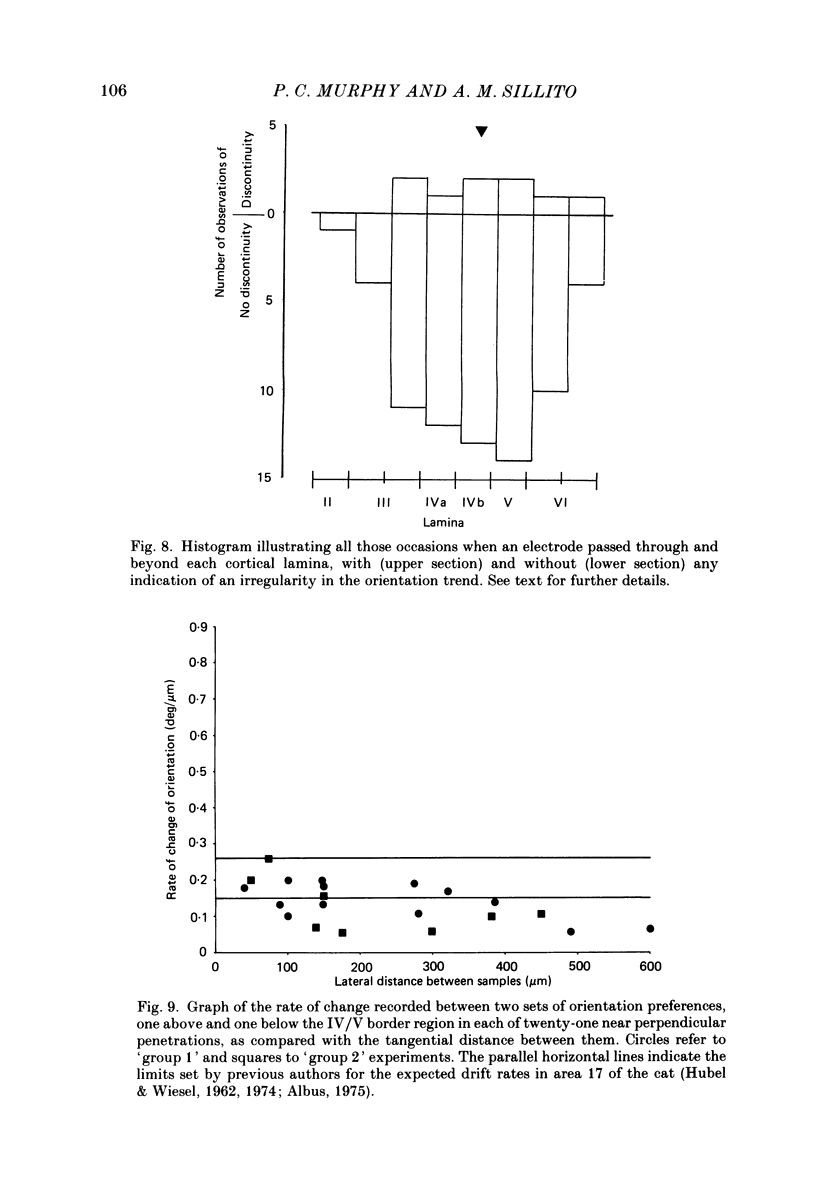




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albus K. 14C-deoxyglucose mapping of orientation subunits in the cats visual cortical areas. Exp Brain Res. 1979;37(3):609–613. doi: 10.1007/BF00236828. [DOI] [PubMed] [Google Scholar]
- Albus K. A quantitative study of the projection area of the central and the paracentral visual field in area 17 of the cat. II. The spatial organization of the orientation domain. Exp Brain Res. 1975 Dec 22;24(2):181–202. doi: 10.1007/BF00234062. [DOI] [PubMed] [Google Scholar]
- Albus K., Sieber B. On the spatial arrangement of iso-orientation bands in the cat's visual cortical areas 17 and 18: a 14C-deoxyglucose study. Exp Brain Res. 1984;56(2):384–388. doi: 10.1007/BF00236295. [DOI] [PubMed] [Google Scholar]
- Bauer R. A high probability of an orientation shift between layers 4 and 5 in central parts of the cat striate cortex. Exp Brain Res. 1982;48(2):245–255. doi: 10.1007/BF00237220. [DOI] [PubMed] [Google Scholar]
- Bauer R. Differences in orientation and receptive field position between supra- and infragranular cells of cat striate cortex and their possible functional implications. Biol Cybern. 1983;49(2):137–148. doi: 10.1007/BF00320394. [DOI] [PubMed] [Google Scholar]
- Bauer R., Dow B. M., Snyder A. Z., Vautin R. Orientation shift between upper and lower layers in monkey visual cortex. Exp Brain Res. 1983;50(1):133–145. doi: 10.1007/BF00238240. [DOI] [PubMed] [Google Scholar]
- Bauer R., Dow B. M., Vautin R. G. Laminar distribution of preferred orientations in foveal striate cortex of the monkey. Exp Brain Res. 1980;41(1):54–60. doi: 10.1007/BF00236679. [DOI] [PubMed] [Google Scholar]
- Dow B. M., Bauer R. Retinotopy and orientation columns in the monkey: a new model. Biol Cybern. 1984;49(3):189–200. doi: 10.1007/BF00334465. [DOI] [PubMed] [Google Scholar]
- Gilbert C. D. Laminar differences in receptive field properties of cells in cat primary visual cortex. J Physiol. 1977 Jun;268(2):391–421. doi: 10.1113/jphysiol.1977.sp011863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. RECEPTIVE FIELDS AND FUNCTIONAL ARCHITECTURE IN TWO NONSTRIATE VISUAL AREAS (18 AND 19) OF THE CAT. J Neurophysiol. 1965 Mar;28:229–289. doi: 10.1152/jn.1965.28.2.229. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields of single neurones in the cat's striate cortex. J Physiol. 1959 Oct;148:574–591. doi: 10.1113/jphysiol.1959.sp006308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962 Jan;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Shape and arrangement of columns in cat's striate cortex. J Physiol. 1963 Mar;165:559–568. doi: 10.1113/jphysiol.1963.sp007079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond P., Andrews D. P. Orientation tuning of cells in areas 17 and 18 of the cat's visual cortex. Exp Brain Res. 1978 Mar 15;31(3):341–351. doi: 10.1007/BF00237294. [DOI] [PubMed] [Google Scholar]
- Henry G. H., Dreher B., Bishop P. O. Orientation specificity of cells in cat striate cortex. J Neurophysiol. 1974 Nov;37(6):1394–1409. doi: 10.1152/jn.1974.37.6.1394. [DOI] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. Receptive fields and functional architecture of monkey striate cortex. J Physiol. 1968 Mar;195(1):215–243. doi: 10.1113/jphysiol.1968.sp008455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. Sequence regularity and geometry of orientation columns in the monkey striate cortex. J Comp Neurol. 1974 Dec 1;158(3):267–293. doi: 10.1002/cne.901580304. [DOI] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N., Stryker M. P. Anatomical demonstration of orientation columns in macaque monkey. J Comp Neurol. 1978 Feb 1;177(3):361–380. doi: 10.1002/cne.901770302. [DOI] [PubMed] [Google Scholar]
- Krüger J., Bach M. Independent systems of orientation columns in upper and lower layers of monkey visual cortex. Neurosci Lett. 1982 Aug 31;31(3):225–230. doi: 10.1016/0304-3940(82)90024-6. [DOI] [PubMed] [Google Scholar]
- Lang W., Henn V. Columnar pattern in the cat's visual cortex after optokinetic stimulation. Brain Res. 1980 Jan 27;182(2):446–450. doi: 10.1016/0006-8993(80)91201-9. [DOI] [PubMed] [Google Scholar]
- Lee B. B., Albus K., Heggelund P., Hulme M. J., Creutzfeldt O. D. The depth distribution of optimal stimulus orientations for neurones in cat area 17. Exp Brain Res. 1977 Mar 30;27(3-4):301–314. doi: 10.1007/BF00235505. [DOI] [PubMed] [Google Scholar]
- Schoppmann A., Stryker M. P. Physiological evidence that the 2-deoxyglucose method reveals orientation columns in cat visual cortex. Nature. 1981 Oct 15;293(5833):574–576. doi: 10.1038/293574a0. [DOI] [PubMed] [Google Scholar]
- Sillito A. M. Inhibitory mechanisms influencing complex cell orientation selectivity and their modification at high resting discharge levels. J Physiol. 1979 Apr;289:33–53. doi: 10.1113/jphysiol.1979.sp012723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillito A. M., Kemp J. A., Milson J. A., Berardi N. A re-evaluation of the mechanisms underlying simple cell orientation selectivity. Brain Res. 1980 Aug 4;194(2):517–520. doi: 10.1016/0006-8993(80)91234-2. [DOI] [PubMed] [Google Scholar]
- Sillito A. M., Kemp J. A. The influence of GABAergic inhibitory processes on the receptive field structure of X and Y cells in cat dorsal lateral geniculate nucleus (dLGN). Brain Res. 1983 Oct 24;277(1):63–77. doi: 10.1016/0006-8993(83)90908-3. [DOI] [PubMed] [Google Scholar]
- Sillito A. M. The contribution of inhibitory mechanisms to the receptive field properties of neurones in the striate cortex of the cat. J Physiol. 1975 Sep;250(2):305–329. doi: 10.1113/jphysiol.1975.sp011056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer W. Topographic organization of orientation columns in the cat visual cortex. A deoxyglucose study. Exp Brain Res. 1981;44(4):431–436. doi: 10.1007/BF00238836. [DOI] [PubMed] [Google Scholar]


