Abstract
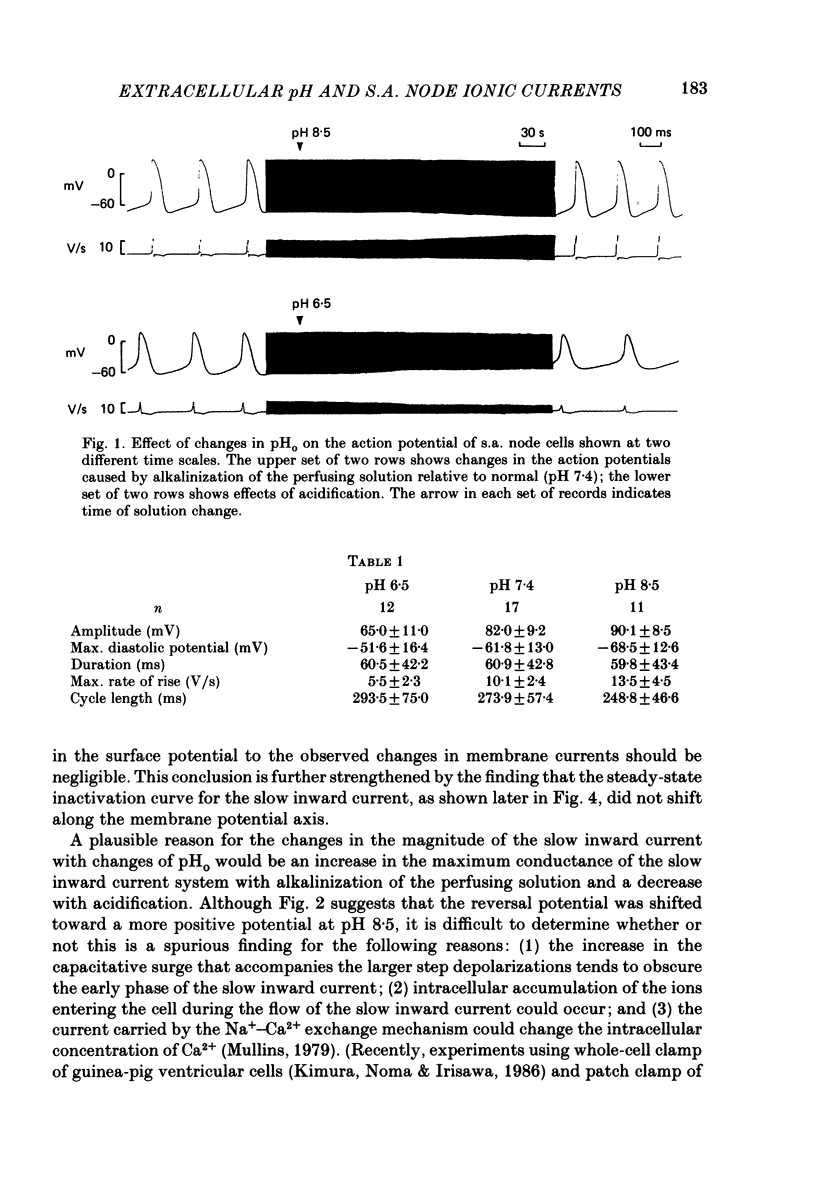
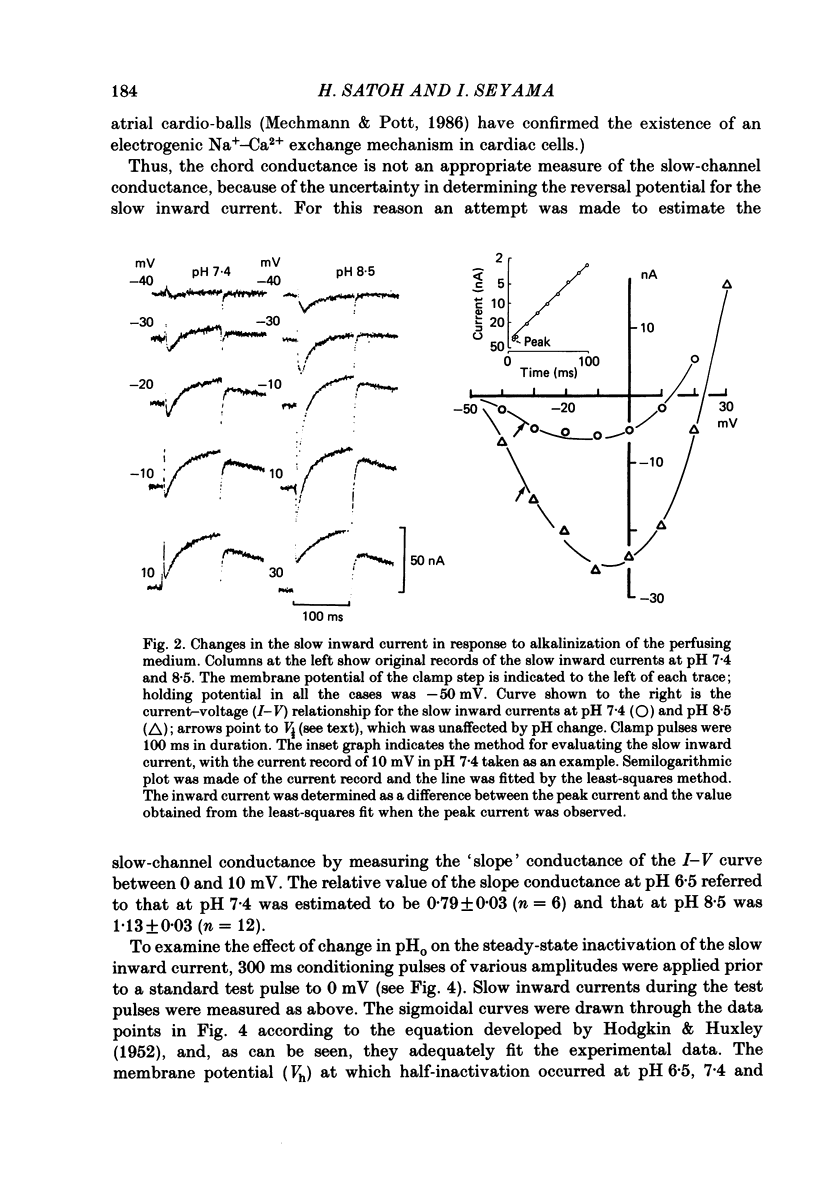
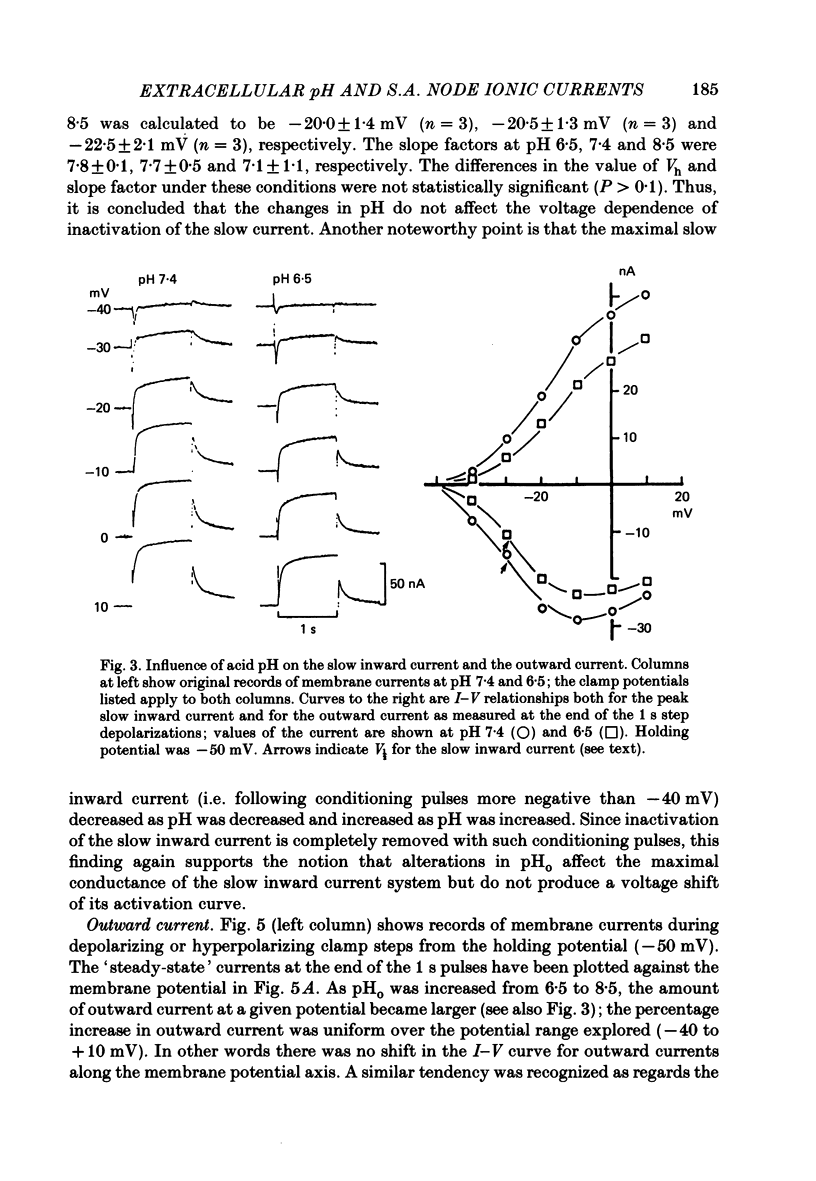
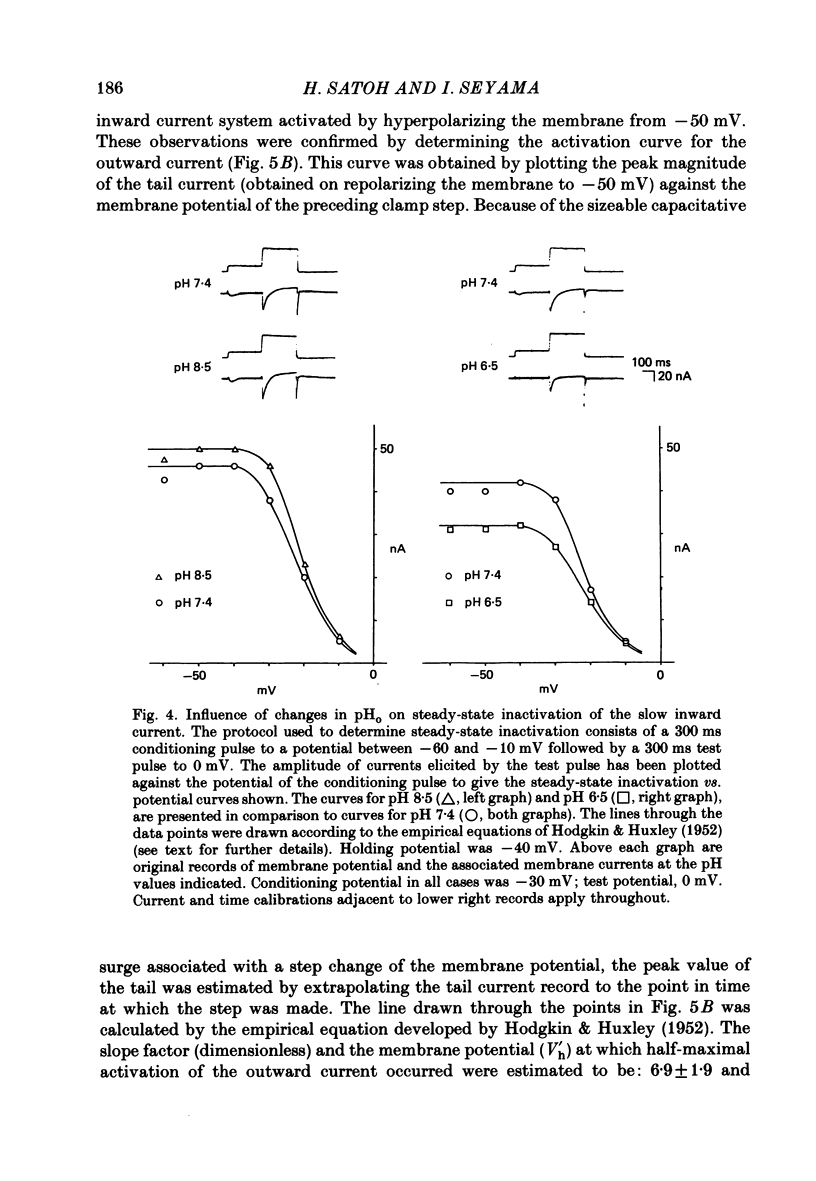
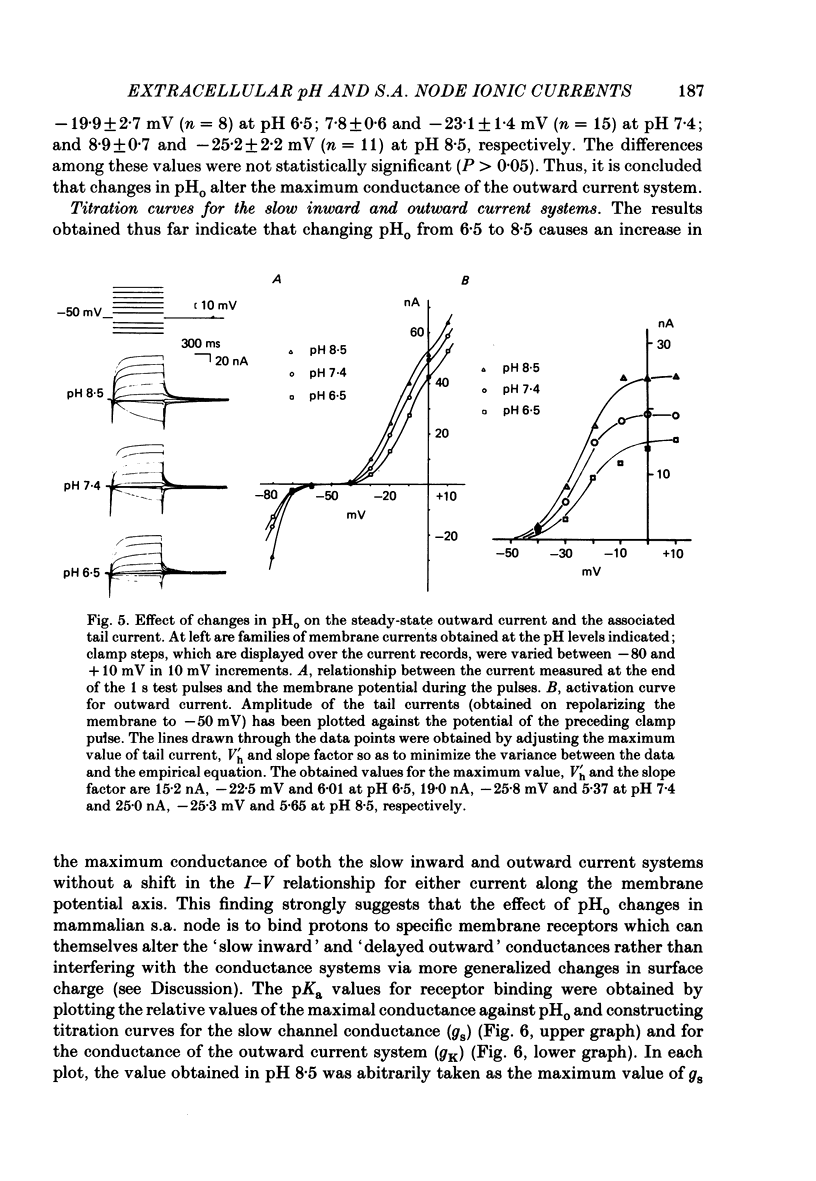
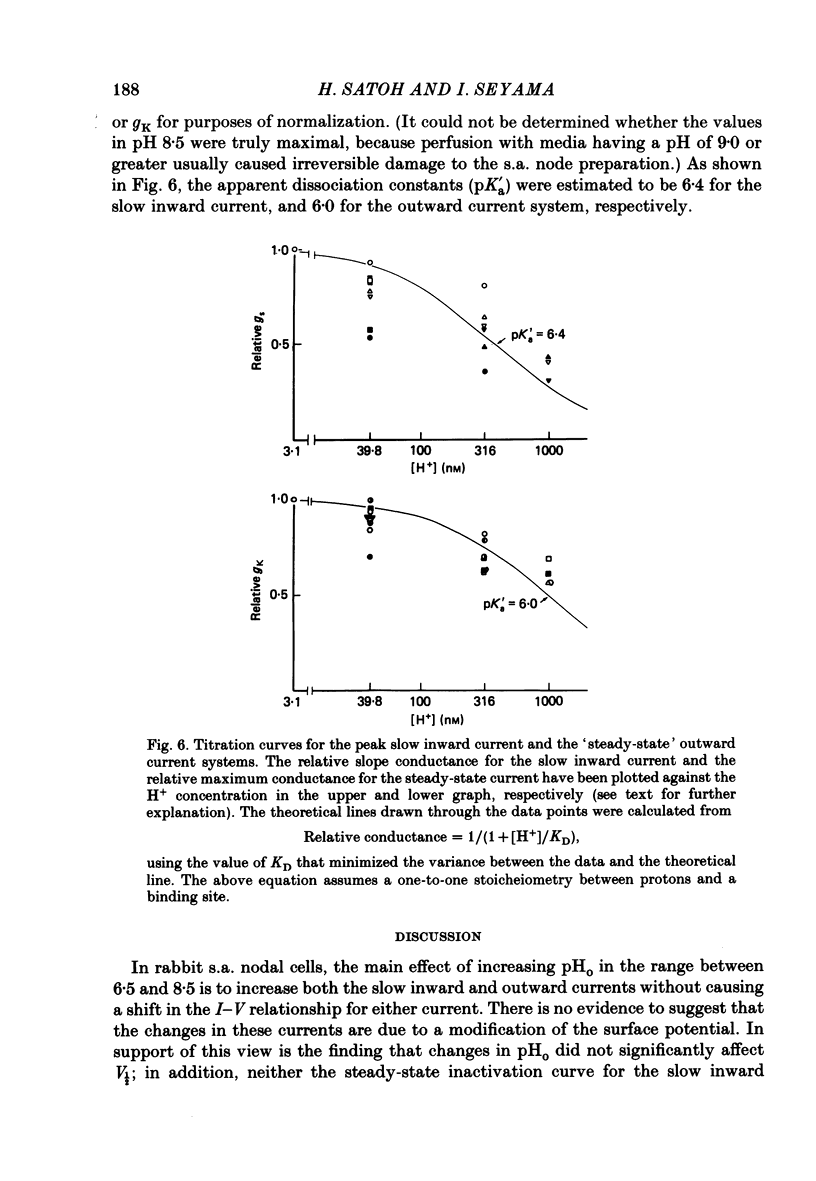
1. The effect of altering the extracellular pH (pHo) on the electrical activity of the isolated rabbit sino-atrial (s.a.) node was studied using the two-micro-electrode voltage-clamp technique. 2. Alkalinization of the perfusate increased the maximum rate of rise and amplitude of the nodal action potential, and also the frequency of spontaneous beating of the s.a. node; acidification produced opposite effects. 3. A change in the extracellular pH from a value of 6.5 to 8.5 caused increases of the maximal conductance for both the slow inward current and the steady-state outward current systems without affecting the gating processes of the channels. 4. H+ could modify the electrical activity of s.a. nodal cells, not through altering the membrane surface potential but through the protonation of the ionic channels themselves. From the titration curves, the apparent pKa values for the slow inward current and the steady-state outward current were estimated to be 6.4 and 6.0, respectively.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrus E. C., Carter E. P. THE MECHANISM OF THE ACTION OF THE HYDROGEN ION UPON THE CARDIAC RHYTHM. J Clin Invest. 1927 Feb;3(3):555–564. doi: 10.1172/JCI100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown H. F., DiFrancesco D., Noble S. J. How does adrenaline accelerate the heart? Nature. 1979 Jul 19;280(5719):235–236. doi: 10.1038/280235a0. [DOI] [PubMed] [Google Scholar]
- Brown R. H., Jr, Noble D. Displacement of activator thresholds in cardiac muscle by protons and calcium ions. J Physiol. 1978 Sep;282:333–343. doi: 10.1113/jphysiol.1978.sp012466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell D. T. Do protons block Na+ channels by binding to a site outside the pore? Nature. 1982 Jul 8;298(5870):165–167. doi: 10.1038/298165a0. [DOI] [PubMed] [Google Scholar]
- Campbell D. T., Hille B. Kinetic and pharmacological properties of the sodium channel of frog skeletal muscle. J Gen Physiol. 1976 Mar;67(3):309–323. doi: 10.1085/jgp.67.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesnais J. M., Coraboeuf E., Sauviat M. P., Vassas J. M. Sensitivity to H, Li and Mg ions of the slow inward sodium current in frog atrial fibres. J Mol Cell Cardiol. 1975 Sep;7(9):627–642. doi: 10.1016/0022-2828(75)90140-6. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D., Noble D. A model of cardiac electrical activity incorporating ionic pumps and concentration changes. Philos Trans R Soc Lond B Biol Sci. 1985 Jan 10;307(1133):353–398. doi: 10.1098/rstb.1985.0001. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D. The cardiac hyperpolarizing-activated current, if. Origins and developments. Prog Biophys Mol Biol. 1985;46(3):163–183. doi: 10.1016/0079-6107(85)90008-2. [DOI] [PubMed] [Google Scholar]
- Ellis D., MacLeod K. T. Sodium-dependent control of intracellular pH in Purkinje fibres of sheep heart. J Physiol. 1985 Feb;359:81–105. doi: 10.1113/jphysiol.1985.sp015576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis D., Thomas R. C. Direct measurement of the intracellular pH of mammalian cardiac muscle. J Physiol. 1976 Nov;262(3):755–771. doi: 10.1113/jphysiol.1976.sp011619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. The dual effect of membrane potential on sodium conductance in the giant axon of Loligo. J Physiol. 1952 Apr;116(4):497–506. doi: 10.1113/jphysiol.1952.sp004719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Charges and potentials at the nerve surface. Divalent ions and pH. J Gen Physiol. 1968 Feb;51(2):221–236. doi: 10.1085/jgp.51.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura J., Noma A., Irisawa H. Na-Ca exchange current in mammalian heart cells. Nature. 1986 Feb 13;319(6054):596–597. doi: 10.1038/319596a0. [DOI] [PubMed] [Google Scholar]
- Kohlhardt M., Haap K., Figulla H. R. Influence of low extracellular pH upon the Ca inward current and isometric contractile force in mammalian ventricular myocardium. Pflugers Arch. 1976 Oct 15;366(1):31–38. doi: 10.1007/BF02486557. [DOI] [PubMed] [Google Scholar]
- Kurachi Y. The effects of intracellular protons on the electrical activity of single ventricular cells. Pflugers Arch. 1982 Sep;394(3):264–270. doi: 10.1007/BF00589102. [DOI] [PubMed] [Google Scholar]
- McDonald T. F., Trautwein W. Membrane currents in cat myocardium: separation of inward and outward components. J Physiol. 1978 Jan;274:193–216. doi: 10.1113/jphysiol.1978.sp012143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechmann S., Pott L. Identification of Na-Ca exchange current in single cardiac myocytes. Nature. 1986 Feb 13;319(6054):597–599. doi: 10.1038/319597a0. [DOI] [PubMed] [Google Scholar]
- Mines G. R. On functional analysis by the action of electrolytes. J Physiol. 1913 Jun 19;46(3):188–235. doi: 10.1113/jphysiol.1913.sp001588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins L. J. The generation of electric currents in cardiac fibers by Na/Ca exchange. Am J Physiol. 1979 Mar;236(3):C103–C110. doi: 10.1152/ajpcell.1979.236.3.C103. [DOI] [PubMed] [Google Scholar]
- Noble D., Noble S. J. A model of sino-atrial node electrical activity based on a modification of the DiFrancesco-Noble (1984) equations. Proc R Soc Lond B Biol Sci. 1984 Sep 22;222(1228):295–304. doi: 10.1098/rspb.1984.0065. [DOI] [PubMed] [Google Scholar]
- Noma A., Irisawa H. A time- and voltage-dependent potassium current in the rabbit sinoatrial node cell. Pflugers Arch. 1976 Nov 5;366(2-3):251–258. doi: 10.1007/BF00585886. [DOI] [PubMed] [Google Scholar]
- Ohmori H., Yoshii M. Surface potential reflected in both gating and permeation mechanisms of sodium and calcium channels of the tunicate egg cell membrane. J Physiol. 1977 May;267(2):429–463. doi: 10.1113/jphysiol.1977.sp011821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piwnica-Worms D., Jacob R., Horres C. R., Lieberman M. Na/H exchange in cultured chick heart cells. pHi regulation. J Gen Physiol. 1985 Jan;85(1):43–64. doi: 10.1085/jgp.85.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh H., Hashimoto K. Effect of pH on the sino-atrial node cells and atrial muscle of dog. Arch Int Pharmacodyn Ther. 1983 Jan;261(1):67–78. [PubMed] [Google Scholar]
- Schauf C. L., Davis F. A. Sensitivity of the sodium and potassium channels of Myxicola giant axons to changes in external pH. J Gen Physiol. 1976 Feb;67(2):185–195. doi: 10.1085/jgp.67.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyama I. Characteristics of the anion channel in the sino-atrial node cell of the rabbit. J Physiol. 1979 Sep;294:447–460. doi: 10.1113/jphysiol.1979.sp012940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrager P. Ionic conductance changes in voltage clamped crayfish axons at low pH. J Gen Physiol. 1974 Dec;64(6):666–690. doi: 10.1085/jgp.64.6.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel S., Sperelakis N. Blockade of myocardial slow inward current at low pH. Am J Physiol. 1977 Sep;233(3):C99–103. doi: 10.1152/ajpcell.1977.233.3.C99. [DOI] [PubMed] [Google Scholar]
- Wada Y., Goto M. Effects of pH on the processes of excitation-contraction coupling of bullfrog atrium. Jpn J Physiol. 1975;25(5):605–620. doi: 10.2170/jjphysiol.25.605. [DOI] [PubMed] [Google Scholar]
- Williams E. M., Whyte J. M. Chemosensitivity of cardiac muscle. J Physiol. 1967 Mar;189(1):119–137. doi: 10.1113/jphysiol.1967.sp008158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodhull A. M. Ionic blockage of sodium channels in nerve. J Gen Physiol. 1973 Jun;61(6):687–708. doi: 10.1085/jgp.61.6.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagihara K., Irisawa H. Inward current activated during hyperpolarization in the rabbit sinoatrial node cell. Pflugers Arch. 1980 May;385(1):11–19. doi: 10.1007/BF00583909. [DOI] [PubMed] [Google Scholar]
- Yanagihara K., Noma A., Irisawa H. Reconstruction of sino-atrial node pacemaker potential based on the voltage clamp experiments. Jpn J Physiol. 1980;30(6):841–857. doi: 10.2170/jjphysiol.30.841. [DOI] [PubMed] [Google Scholar]
- Yatani A., Goto M. The effect of extracellular low pH on the plateau current in isolated, single rat ventricular cells--a voltage clamp study. Jpn J Physiol. 1983;33(3):403–415. doi: 10.2170/jjphysiol.33.403. [DOI] [PubMed] [Google Scholar]


