Abstract
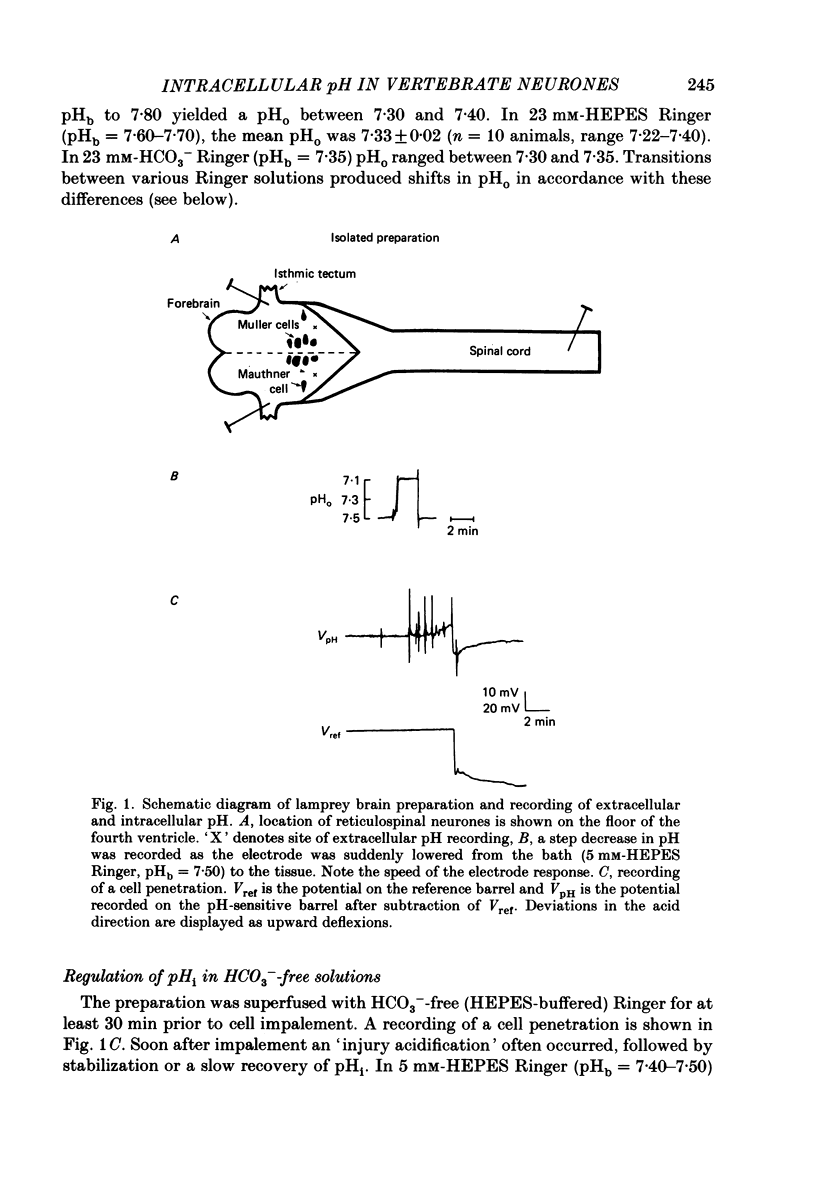
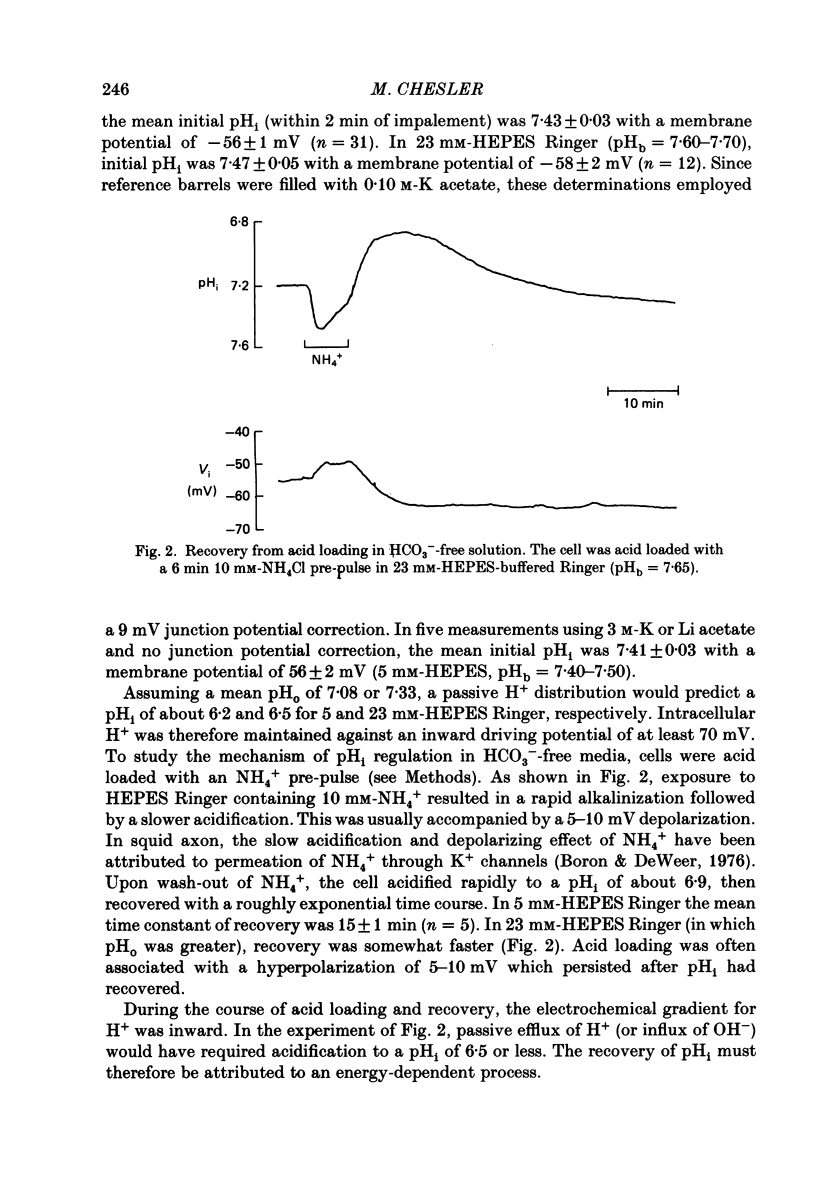
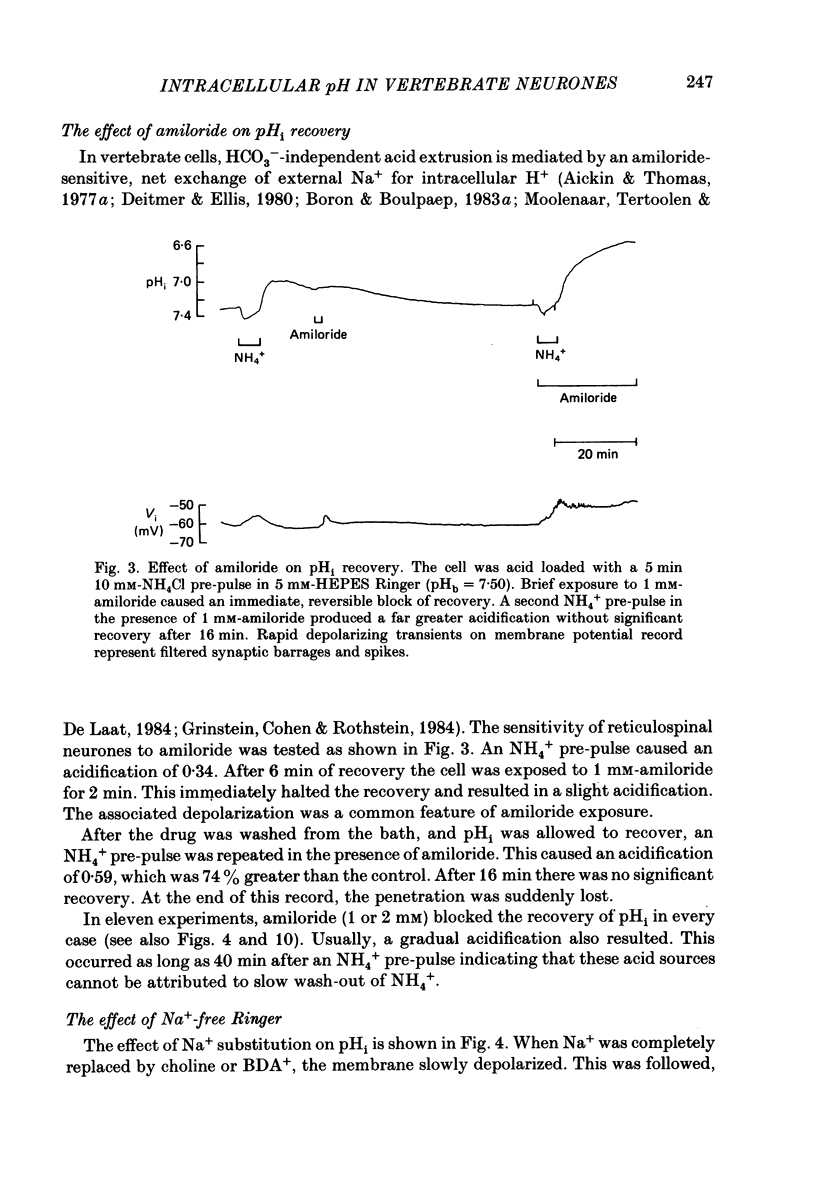
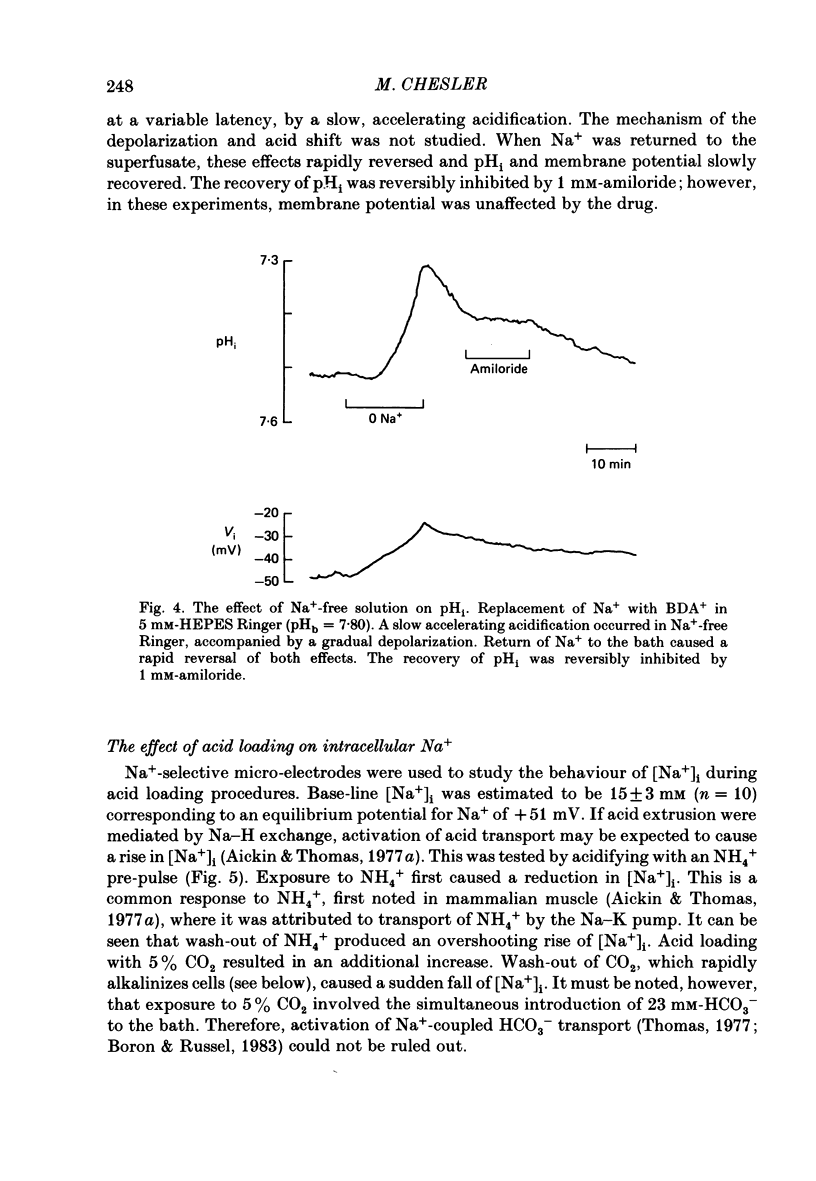
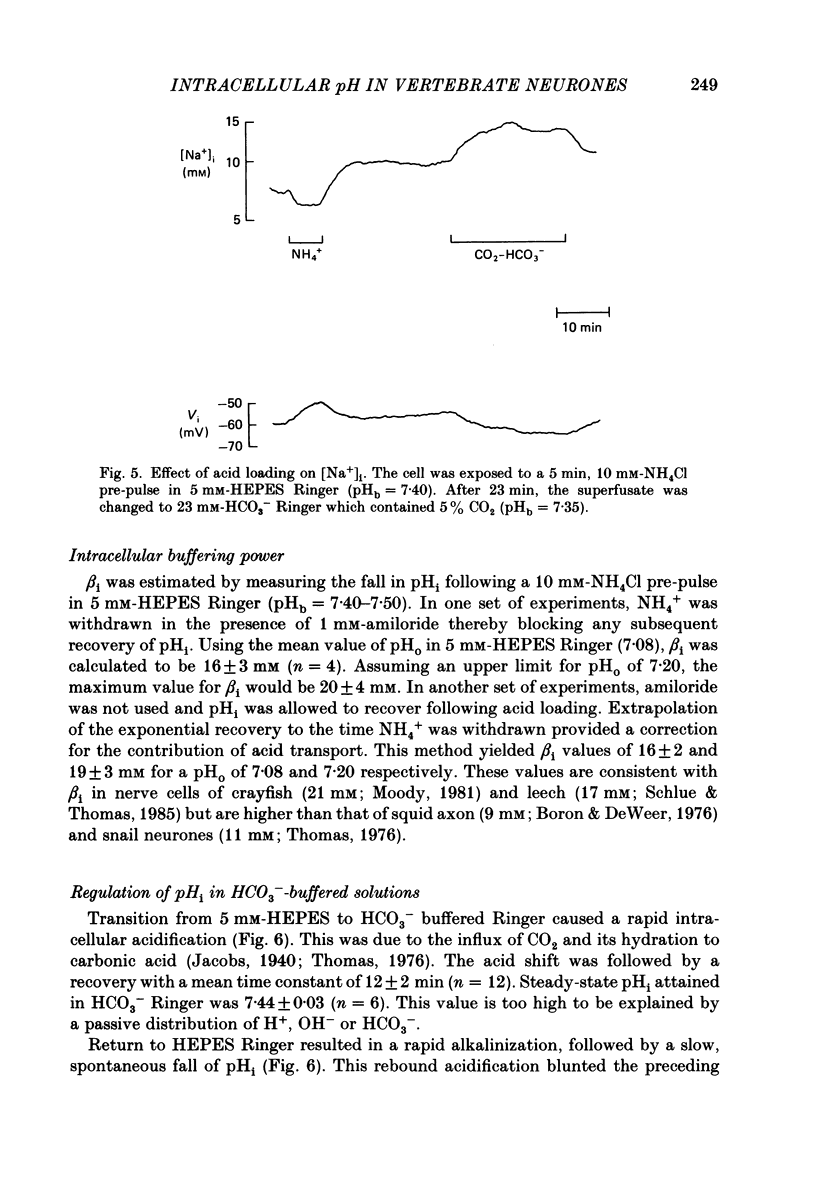
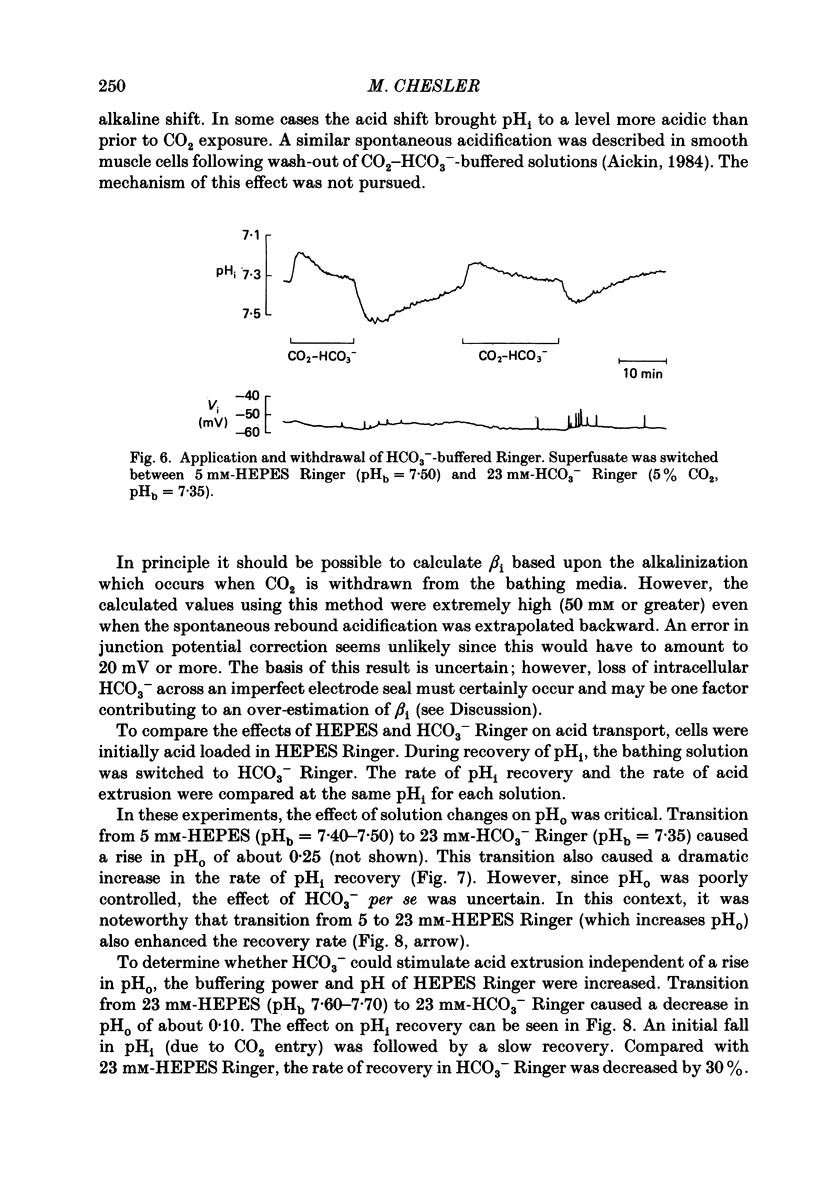
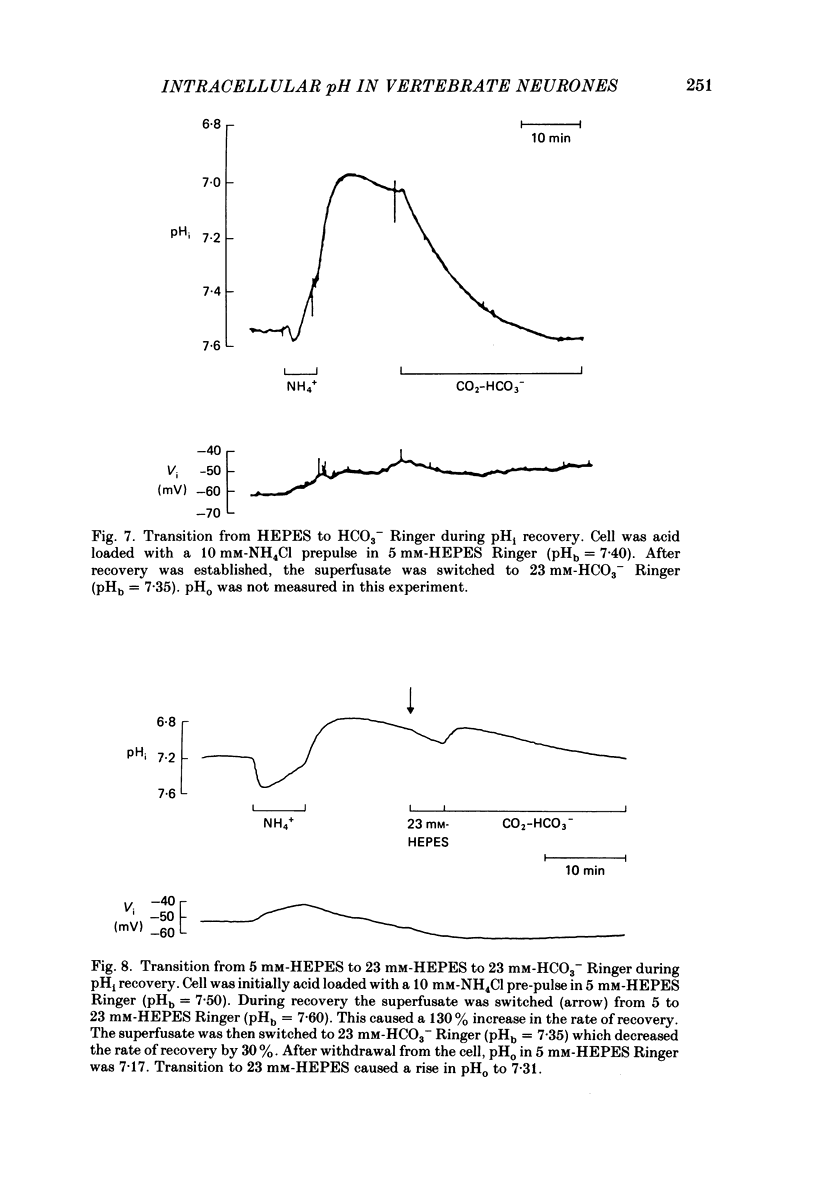
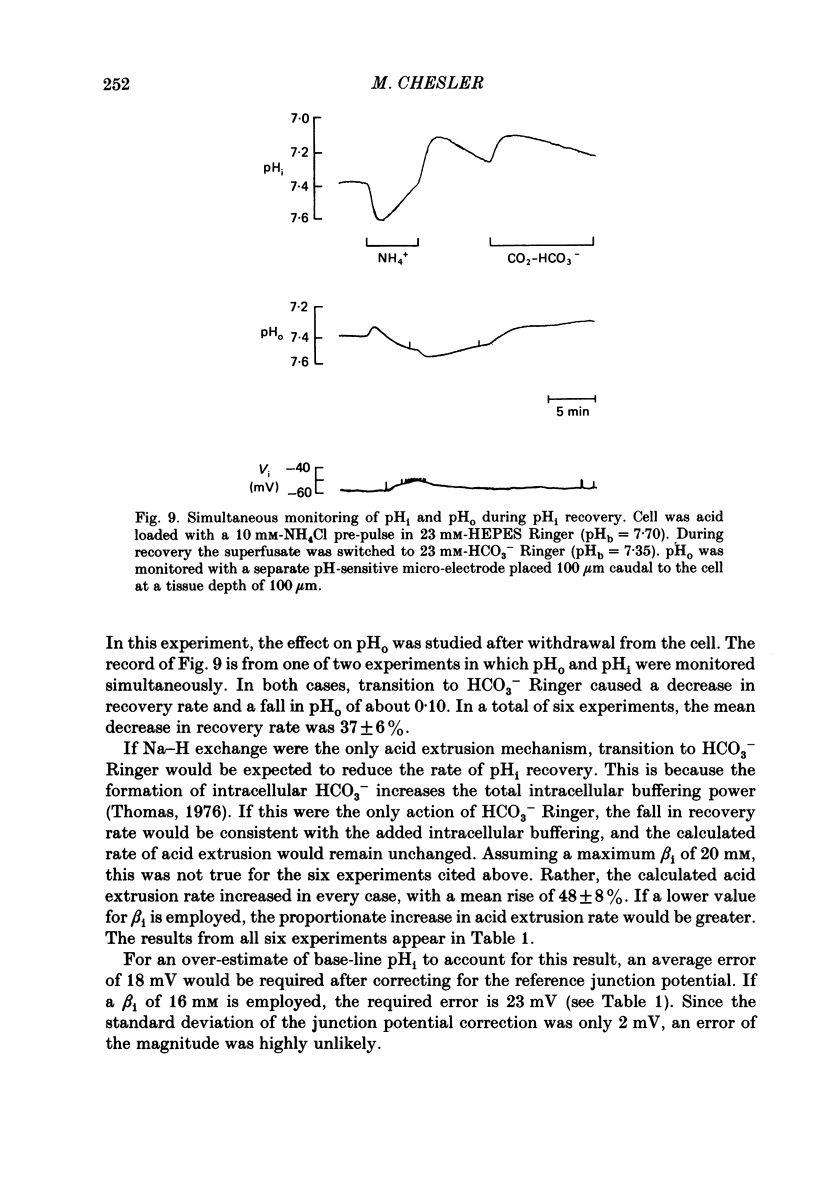
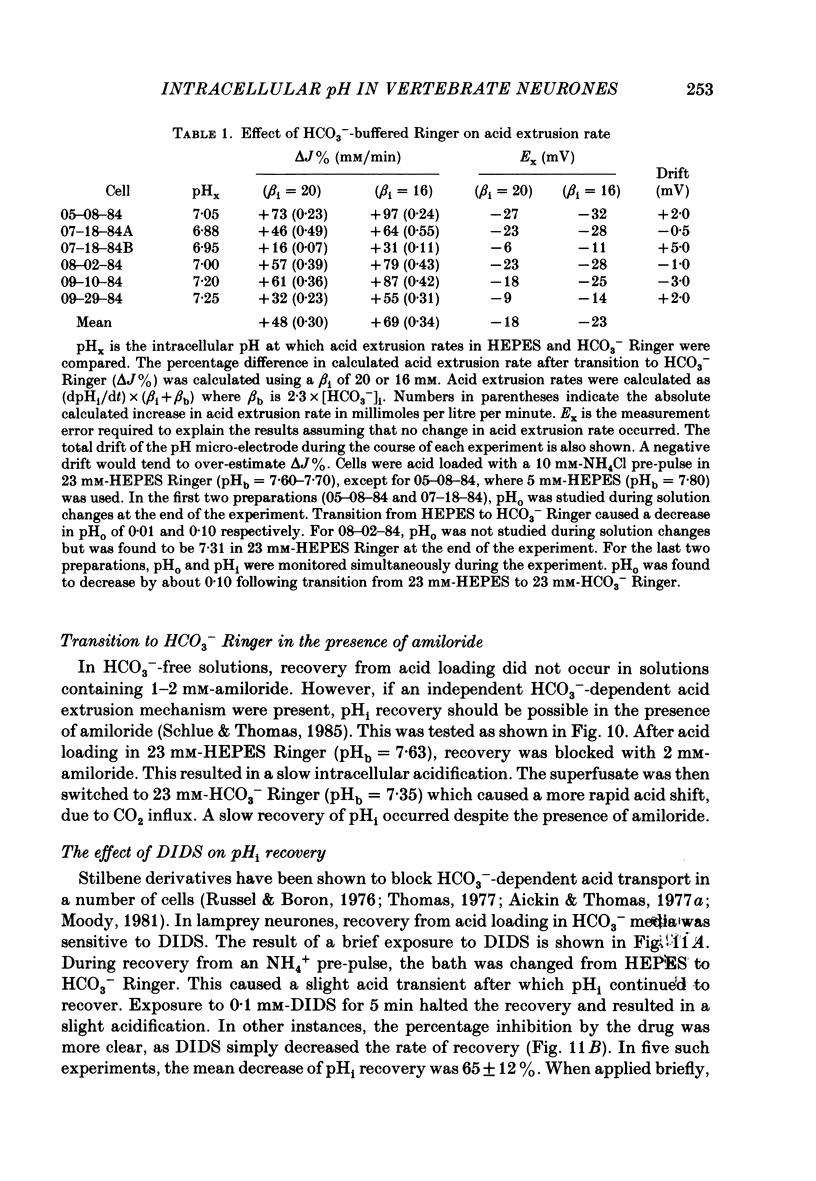
1. The regulation of intracellular pH (pHi) in lamprey reticulospinal neurones was investigated with pH-sensitive micro-electrodes based on a neutral carrier liquid membrane. Experiments were performed using an in vitro brain-stem preparation. 2. In HEPES-buffered solutions, extracellular pH (pHo) was consistently more acidic than the pH of the bathing solution (pHb). In HCO3(-)-buffered solutions, the brain was also relatively acidic, but the brain pH gradient was smaller. 3. In HEPES- and HCO3(-)-buffered solutions, mean pHi was 7.40-7.50. This range was too high to be explained by a passive distribution of H+, OH- or HCO3-. 4. In nominally HCO3(-)-free, HEPES-buffered solution, cells were acid loaded by addition and subsequent withdrawal of NH4+ from the superfusate. pHi recovered from acid loading by an energy-dependent process in 10-20 min. Recovery from acid loading in HEPES-buffered solutions was blocked by exposure to amiloride. 5. Removal of extracellular Na+ caused a slow, accelerating fall of pHi. Return of Na+ to the bath caused an immediate reversal of this acidification, followed by a slow recovery of pHi. Measurement with Na+-sensitive micro-electrodes during acid loading showed a rapid rise in the intracellular Na+ activity [( Na+]i). 6. Following acid loading, transition from HEPES- to HCO3(-)-buffered solutions caused an increase in the acid extrusion rate of at least 48%. The effect of these solution changes was dependent on pHo. After blocking pHi recovery with amiloride, transition from HEPES- to HCO3(-)-buffered Ringer plus amiloride produced a slow recovery of pHi. 7. Recovery from acid loading in HCO3(-)-buffered solutions was inhibited 65% by the anion transport blocker DIDS (4,4'-diisothiocyanostilbene-2,2'-disulphonic acid). Recovery from acid loading after incubation in Cl(-)-free solution was slower than recovery after replenishment of Cl-. 8. It is concluded that in HCO3(-)-free solutions, pHi regulation is accomplished by a Na-H exchange mechanism. In the presence of extracellular HCO3- an additional mechanism can operate to extrude intracellular acid.
Full text
PDF



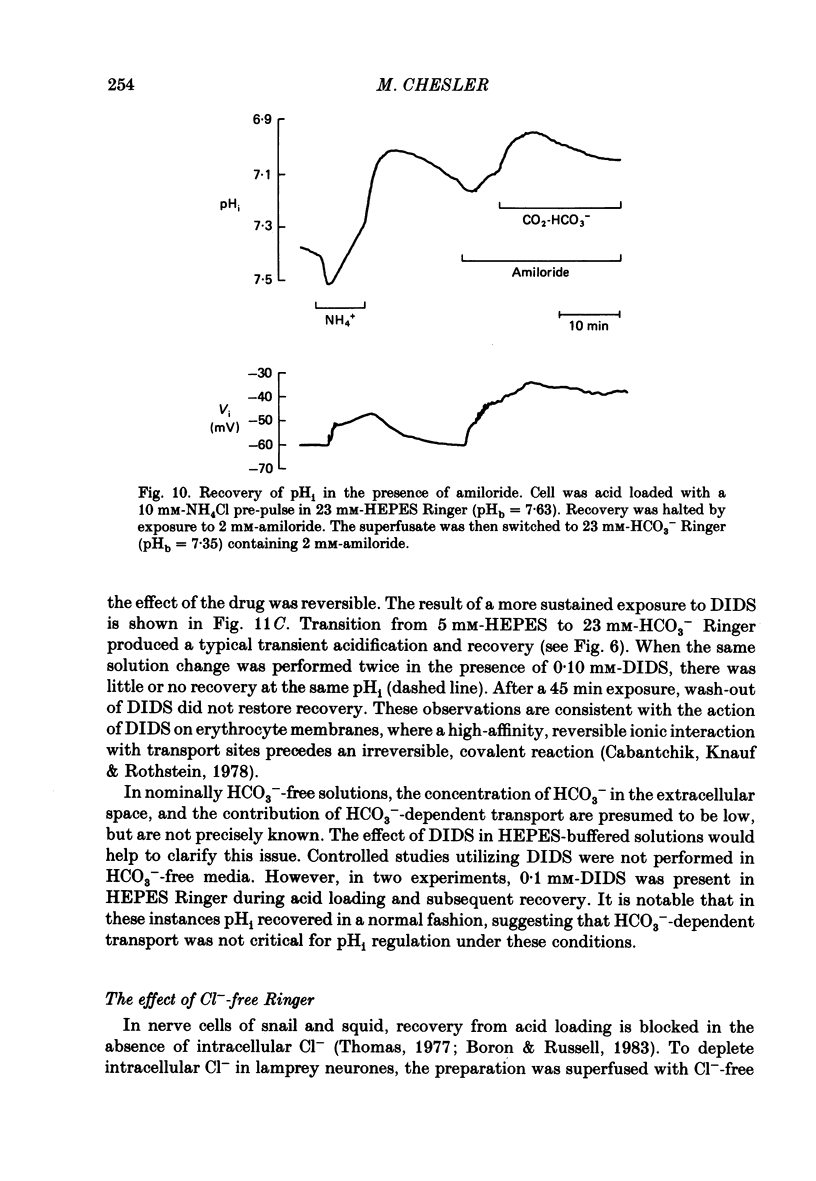





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abercrombie R. F., Putnam R. W., Roos A. The intracellular pH of frog skeletal muscle: its regulation in isotonic solutions. J Physiol. 1983 Dec;345:175–187. doi: 10.1113/jphysiol.1983.sp014973. [DOI] [PMC free article] [PubMed] [Google Scholar]
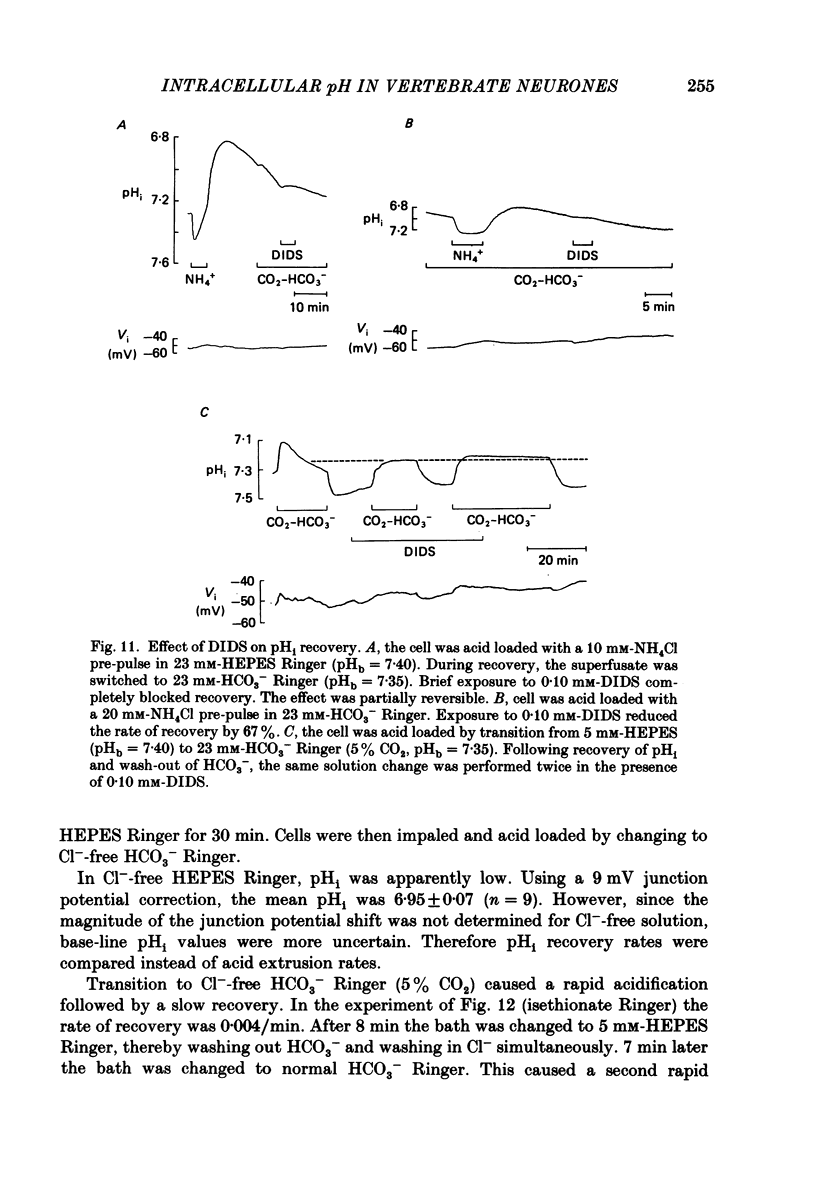
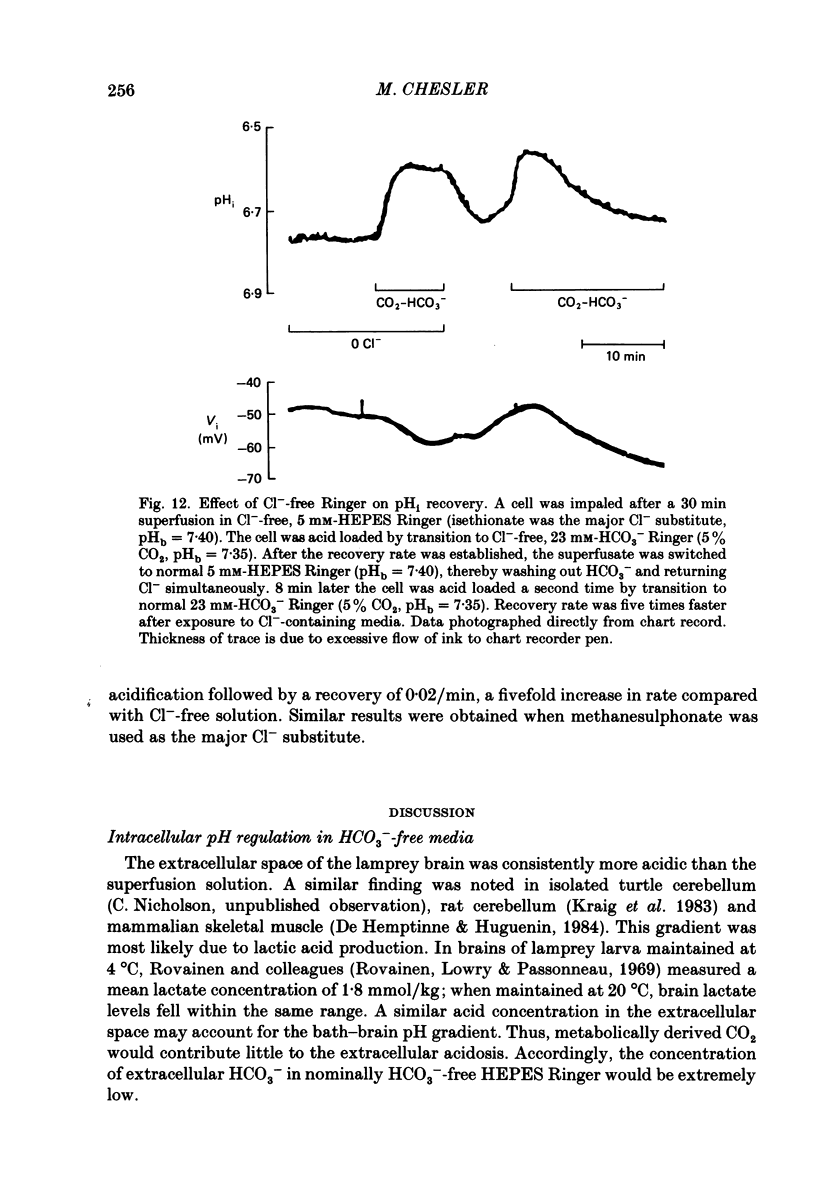
- Aickin C. C. Direct measurement of intracellular pH and buffering power in smooth muscle cells of guinea-pig vas deferens. J Physiol. 1984 Apr;349:571–585. doi: 10.1113/jphysiol.1984.sp015174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aickin C. C., Thomas R. C. An investigation of the ionic mechanism of intracellular pH regulation in mouse soleus muscle fibres. J Physiol. 1977 Dec;273(1):295–316. doi: 10.1113/jphysiol.1977.sp012095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammann D., Lanter F., Steiner R. A., Schulthess P., Shijo Y., Simon W. Neutral carrier based hydrogen ion selective microelectrode for extra- and intracellular studies. Anal Chem. 1981 Dec;53(14):2267–2269. doi: 10.1021/ac00237a031. [DOI] [PubMed] [Google Scholar]
- Bessou P., Joffroy M., Montoya R., Pagès B. Effects of triceps stretch by ankle flexion on intact afferents and efferents of gastrocnemius in the decerebrate cat. J Physiol. 1984 Jan;346:73–91. doi: 10.1113/jphysiol.1984.sp015008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boron W. F., Boulpaep E. L. Intracellular pH regulation in the renal proximal tubule of the salamander. Basolateral HCO3- transport. J Gen Physiol. 1983 Jan;81(1):53–94. doi: 10.1085/jgp.81.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boron W. F., Boulpaep E. L. Intracellular pH regulation in the renal proximal tubule of the salamander. Na-H exchange. J Gen Physiol. 1983 Jan;81(1):29–52. doi: 10.1085/jgp.81.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boron W. F., De Weer P. Intracellular pH transients in squid giant axons caused by CO2, NH3, and metabolic inhibitors. J Gen Physiol. 1976 Jan;67(1):91–112. doi: 10.1085/jgp.67.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boron W. F., McCormick W. C., Roos A. pH regulation in barnacle muscle fibers: dependence on intracellular and extracellular pH. Am J Physiol. 1979 Sep;237(3):C185–C193. doi: 10.1152/ajpcell.1979.237.3.C185. [DOI] [PubMed] [Google Scholar]
- Boron W. F., Russell J. M. Stoichiometry and ion dependencies of the intracellular-pH-regulating mechanism in squid giant axons. J Gen Physiol. 1983 Mar;81(3):373–399. doi: 10.1085/jgp.81.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabantchik Z. I., Knauf P. A., Rothstein A. The anion transport system of the red blood cell. The role of membrane protein evaluated by the use of 'probes'. Biochim Biophys Acta. 1978 Sep 29;515(3):239–302. doi: 10.1016/0304-4157(78)90016-3. [DOI] [PubMed] [Google Scholar]
- Caldwell P. C. Liquid junction potentials and their effect on potential measurements in biological systems. Int Rev Cytol. 1968;24:345–371. doi: 10.1016/s0074-7696(08)61402-3. [DOI] [PubMed] [Google Scholar]
- Chesler M., Nicholson C. Regulation of intracellular pH in vertebrate central neurons. Brain Res. 1985 Jan 28;325(1-2):313–316. doi: 10.1016/0006-8993(85)90330-0. [DOI] [PubMed] [Google Scholar]
- Christoffersen C. R., Skibsted L. H. Calcium ion activity in physiological salt solutions: influence of anions substituted for chloride. Comp Biochem Physiol A Comp Physiol. 1975 Oct 1;52(2):317–322. doi: 10.1016/s0300-9629(75)80094-6. [DOI] [PubMed] [Google Scholar]
- Deitmer J. W., Ellis D. Interactions between the regulation of the intracellular pH and sodium activity of sheep cardiac Purkinje fibres. J Physiol. 1980 Jul;304:471–488. doi: 10.1113/jphysiol.1980.sp013337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EISENMAN G. Cation selective glass electrodes and their mode of operation. Biophys J. 1962 Mar;2(2 Pt 2):259–323. doi: 10.1016/s0006-3495(62)86959-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M. G., Thomas R. C. Acid influx into snail neurones caused by reversal of the normal pHi-regulating system. J Physiol. 1984 Jan;346:143–154. doi: 10.1113/jphysiol.1984.sp015012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold M. R., Martin A. R. Intracellular Cl- accumulation reduces Cl- conductance in inhibitory synaptic channels. Nature. 1982 Oct 28;299(5886):828–830. doi: 10.1038/299828a0. [DOI] [PubMed] [Google Scholar]
- Grinstein S., Cohen S., Rothstein A. Cytoplasmic pH regulation in thymic lymphocytes by an amiloride-sensitive Na+/H+ antiport. J Gen Physiol. 1984 Mar;83(3):341–369. doi: 10.1085/jgp.83.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. The dual effect of membrane potential on sodium conductance in the giant axon of Loligo. J Physiol. 1952 Apr;116(4):497–506. doi: 10.1113/jphysiol.1952.sp004719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen A. J., Zeuthen T. Extracellular ion concentrations during spreading depression and ischemia in the rat brain cortex. Acta Physiol Scand. 1981 Dec;113(4):437–445. doi: 10.1111/j.1748-1716.1981.tb06920.x. [DOI] [PubMed] [Google Scholar]
- Johnson J. D., Epel D. Intracellular pH and activation of sea urchin eggs after fertilisation. Nature. 1976 Aug 19;262(5570):661–664. doi: 10.1038/262661a0. [DOI] [PubMed] [Google Scholar]
- Kenyon J. L., Gibbons W. R. Effects of low-chloride solutions on action potentials of sheep cardiac Purkinje fibers. J Gen Physiol. 1977 Nov;70(5):635–660. doi: 10.1085/jgp.70.5.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraig R. P., Ferreira-Filho C. R., Nicholson C. Alkaline and acid transients in cerebellar microenvironment. J Neurophysiol. 1983 Mar;49(3):831–850. doi: 10.1152/jn.1983.49.3.831. [DOI] [PubMed] [Google Scholar]
- Micro-electrode measurement of the intracellular pH and buffering power of mouse soleus muscle fibres. J Physiol. 1977 Jun;267(3):791–810. doi: 10.1113/jphysiol.1977.sp011838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody W. J., Jr The ionic mechanism of intracellular pH regulation in crayfish neurones. J Physiol. 1981 Jul;316:293–308. doi: 10.1113/jphysiol.1981.sp013788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moolenaar W. H., Boonstra J., van der Saag P. T., de Laat S. W. Sodium/proton exchange in mouse neuroblastoma cells. J Biol Chem. 1981 Dec 25;256(24):12883–12887. [PubMed] [Google Scholar]
- Moolenaar W. H., Tertoolen L. G., de Laat S. W. The regulation of cytoplasmic pH in human fibroblasts. J Biol Chem. 1984 Jun 25;259(12):7563–7569. [PubMed] [Google Scholar]
- Moser H. Intracellular pH regulation in the sensory neurone of the stretch receptor of the crayfish (Astacus fluviatilis). J Physiol. 1985 May;362:23–38. doi: 10.1113/jphysiol.1985.sp015660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovainen C. M., Lowry O. H., Passonneau J. V. Levels of metabolites and production of glucose in the lamprey brain. J Neurochem. 1969 Oct;16(10):1451–1458. doi: 10.1111/j.1471-4159.1969.tb09897.x. [DOI] [PubMed] [Google Scholar]
- Rovainen C. M. Neurobiology of lampreys. Physiol Rev. 1979 Oct;59(4):1007–1077. doi: 10.1152/physrev.1979.59.4.1007. [DOI] [PubMed] [Google Scholar]
- Russell J. M., Boron W. F., Brodwick M. S. Intracellular pH and Na fluxes in barnacle muscle with evidence for reversal of the ionic mechanism of intracellular pH regulation. J Gen Physiol. 1983 Jul;82(1):47–78. doi: 10.1085/jgp.82.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J. M., Boron W. F. Role of choloride transport in regulation of intracellular pH. Nature. 1976 Nov 4;264(5581):73–74. doi: 10.1038/264073a0. [DOI] [PubMed] [Google Scholar]
- Schlue W. R., Thomas R. C. A dual mechanism for intracellular pH regulation by leech neurones. J Physiol. 1985 Jul;364:327–338. doi: 10.1113/jphysiol.1985.sp015748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor P. S., Thomas R. C. The effect of leakage on micro-electrode measurements of intracellular sodium activity in crab muscle fibres. J Physiol. 1984 Jul;352:539–550. doi: 10.1113/jphysiol.1984.sp015309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R. C. Experimental displacement of intracellular pH and the mechanism of its subsequent recovery. J Physiol. 1984 Sep;354:3P–22P. doi: 10.1113/jphysiol.1984.sp015397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R. C. The effect of carbon dioxide on the intracellular pH and buffering power of snail neurones. J Physiol. 1976 Mar;255(3):715–735. doi: 10.1113/jphysiol.1976.sp011305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R. C. The role of bicarbonate, chloride and sodium ions in the regulation of intracellular pH in snail neurones. J Physiol. 1977 Dec;273(1):317–338. doi: 10.1113/jphysiol.1977.sp012096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanheel B., de Hemptinne A., Leusen I. Analysis of Cl- -HCO3(-) exchange during recovery from intracellular acidosis in cardiac Purkinje strands. Am J Physiol. 1984 May;246(5 Pt 1):C391–C400. doi: 10.1152/ajpcell.1984.246.5.C391. [DOI] [PubMed] [Google Scholar]
- Vaughan-Jones R. D., Lederer W. J., Eisner D. A. Ca2+ ions can affect intracellular pH in mammalian cardiac muscle. Nature. 1983 Feb 10;301(5900):522–524. doi: 10.1038/301522a0. [DOI] [PubMed] [Google Scholar]
- de Hemptinne A., Huguenin F. The influence of muscle respiration and glycolysis on surface and intracellular pH in fibres of the rat soleus. J Physiol. 1984 Feb;347:581–592. doi: 10.1113/jphysiol.1984.sp015084. [DOI] [PMC free article] [PubMed] [Google Scholar]


