Abstract
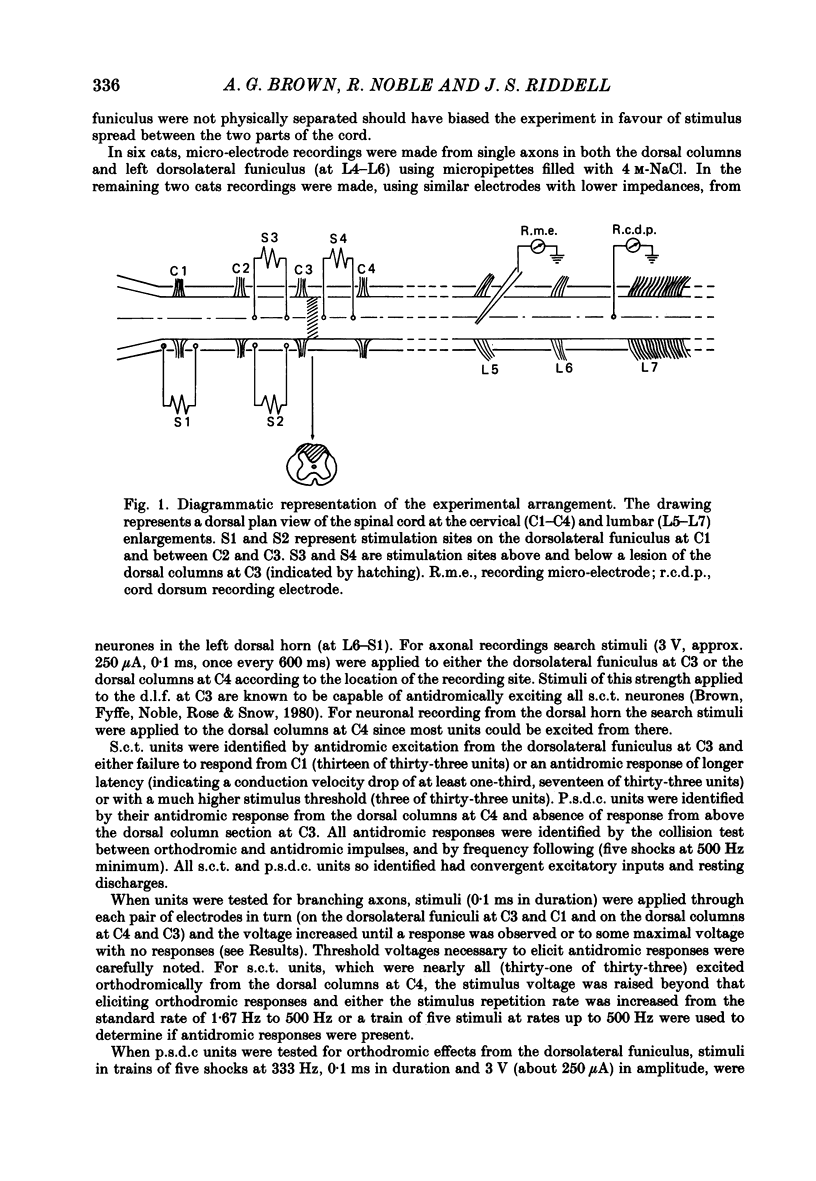
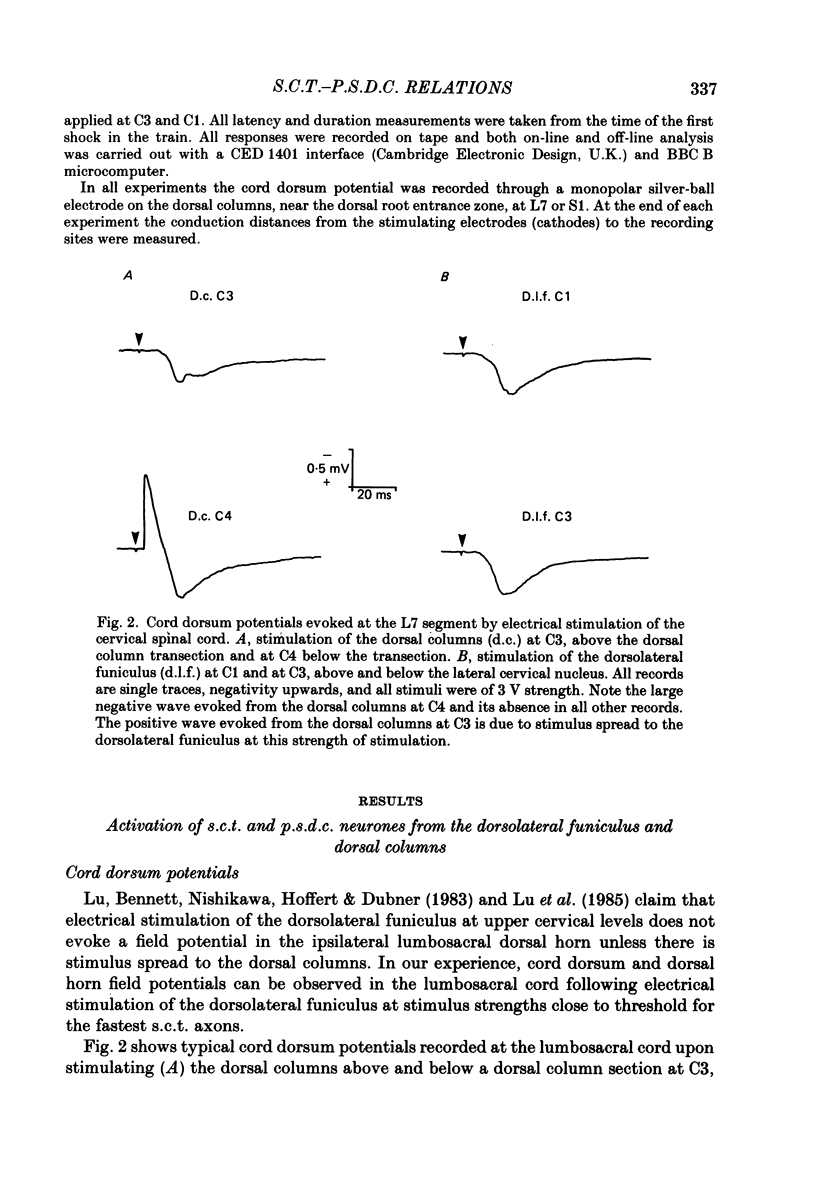
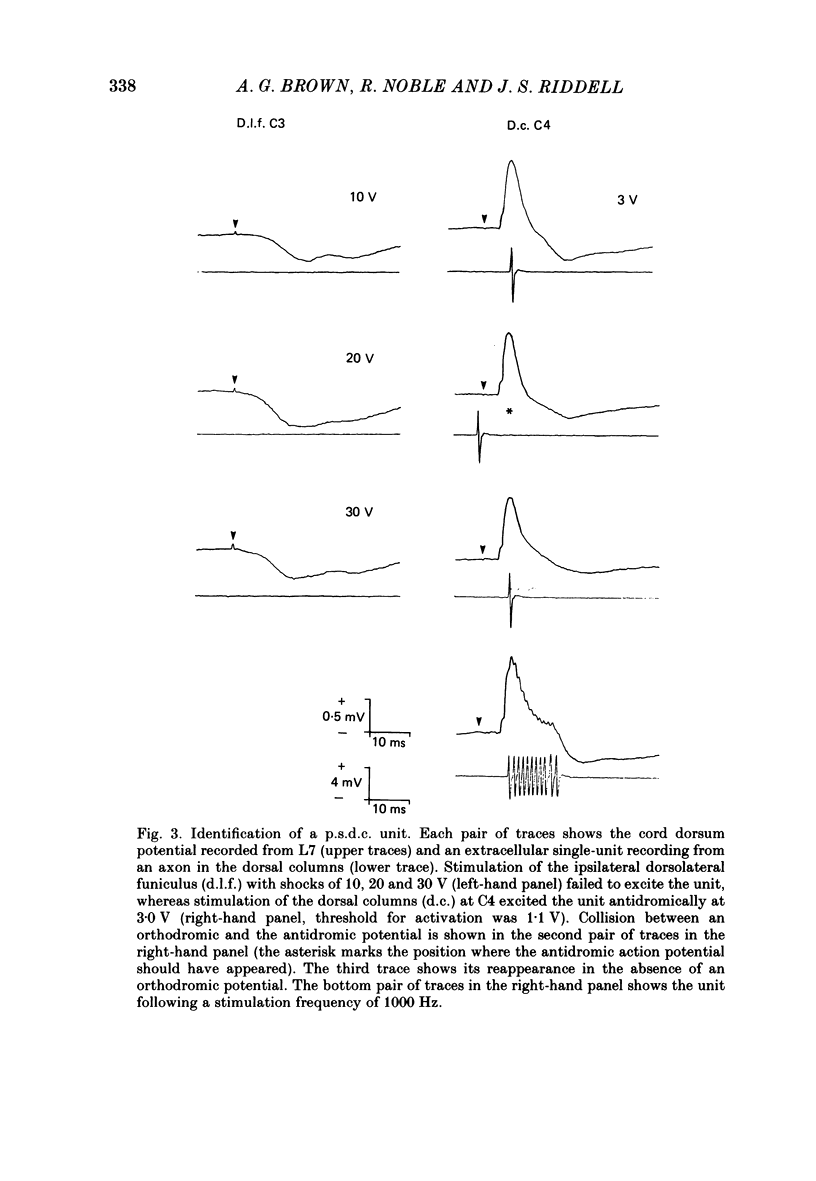
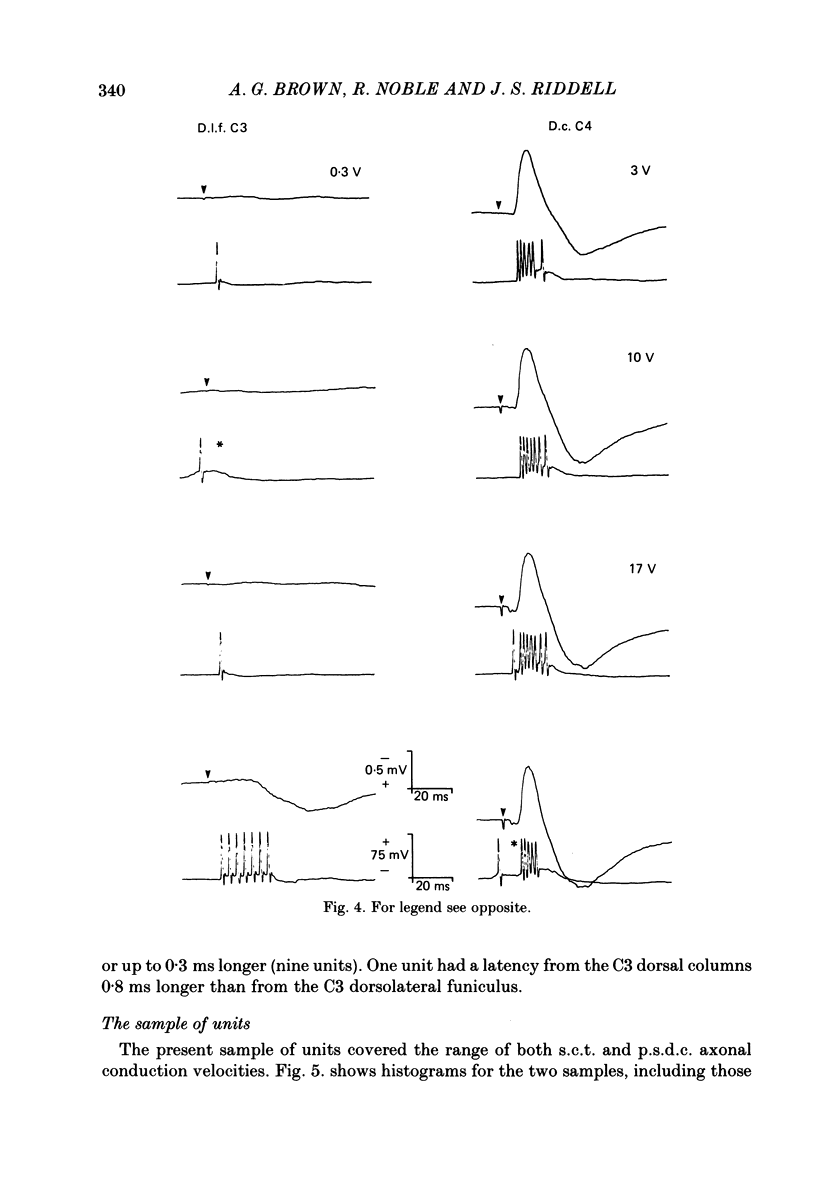
1. In chloralose-anaesthetized cats single-unit micro-electrode recordings were made at the lumbosacral level either from axons in the dorsolateral funiculus and dorsal columns, identified as belonging to the spinocervical tract (s.c.t.) or post-synaptic dorsal column (p.s.d.c.) pathway respectively, or from neurones in the dorsal horn similarly identified. 2. Attempts were made to show that s.c.t. and p.s.d.c. neurones had axons that bifurcated, so that they sent branches into both the ipsilateral dorsolateral funiculus and the dorsal columns. That is, that some, or all, of the presumed s.c.t. or p.s.d.c. axons were common to both populations. In addition, the effects of stimuli applied to the ipsilateral dorsolateral funiculus at C3 and C1 on the resting discharges of p.s.d.c. neurones were examined in order to determine the effectiveness of the link between the s.c.t. and the p.s.d.c. pathway. 3. Thirty-three s.c.t. units (twenty-six axonal recordings and seven soma-dendritic recordings) and thirty p.s.d.c. units (twenty-four axonal and six soma-dendritic recordings) were examined for bifurcating axons by electrically stimulating the dorsolateral funiculus at C3 and the dorsal columns at C4. None of the p.s.d.c. units could be antidromically activated from the ipsilateral dorsolateral funiculus with stimulus strengths up to 40 V or seventy times threshold for antidromic activation from the dorsal columns. Similarly, twenty s.c.t. units could not be activated antidromically from the dorsal columns at stimulus strengths up to 30 V or thirty times threshold for their antidromic excitation from the dorsolateral funiculus. Thirteen s.c.t. units were antidromically activated from the cervical dorsal columns, eight at seventeen or more times threshold for their activation from the dorsolateral funiculus and five at between two and nine times threshold. All s.c.t. units that were activated antidromically from both the cervical dorsal columns and the dorsolateral funiculus showed similar latencies for the two responses. 4. Twenty-five p.s.d.c. units were examined for the effects of ipsilateral dorsolateral funiculus stimulation on their resting activity. In thirteen, clear evidence of facilitatory effects from C3 were observed, whereas similar results were seen in only six of these units when C1 was stimulated and the effects were less. The facilitation had a latency of 3-16 ms and lasted for 6-22 ms. In all but one of the twenty-five units, stimulation at both C1 and C3 produced profound inhibition of the resting discharge that began at between 8 and 26 ms and lasted for up to 300 ms.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
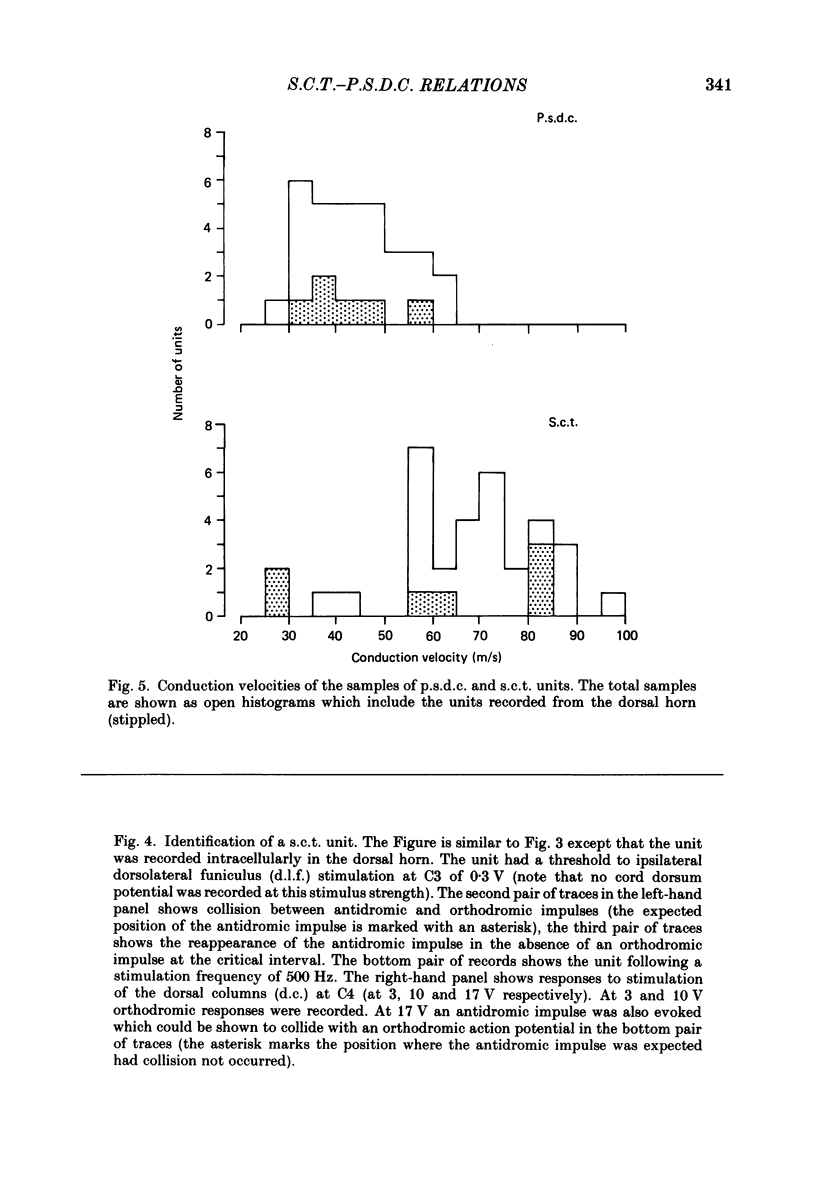
PDF




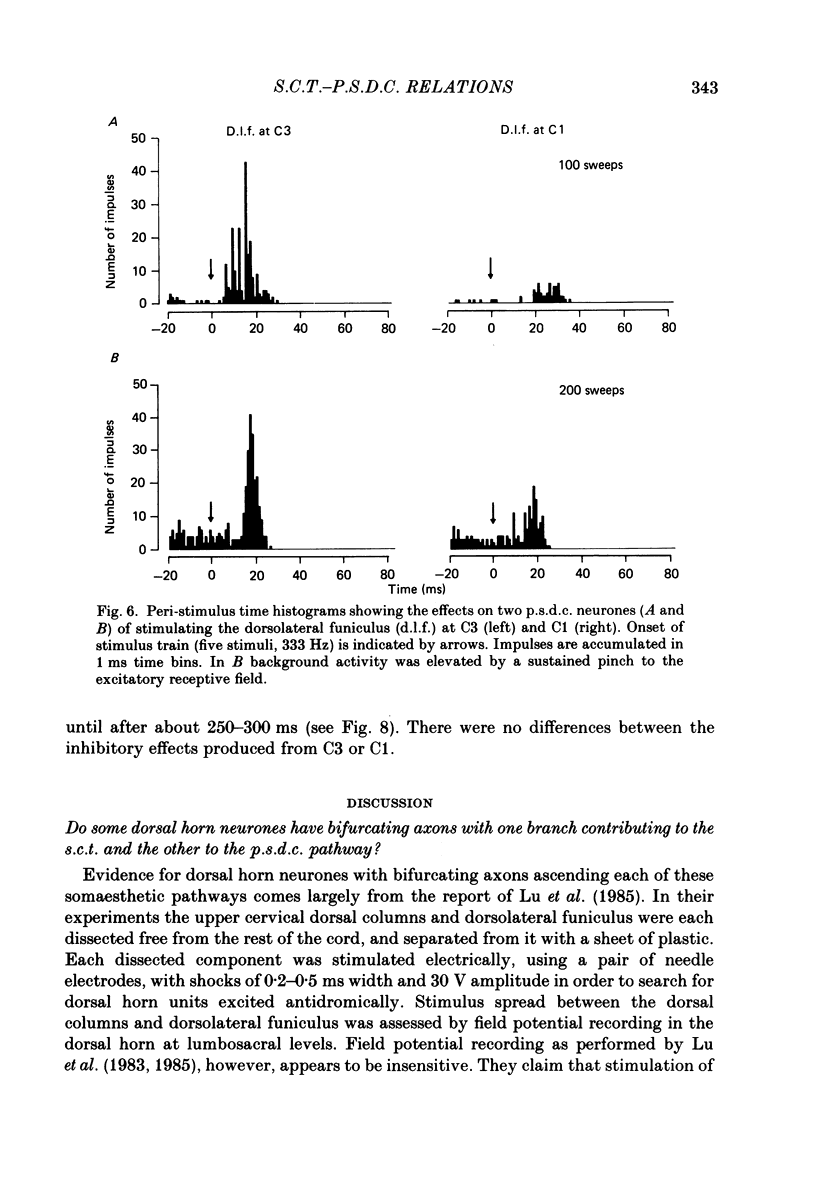
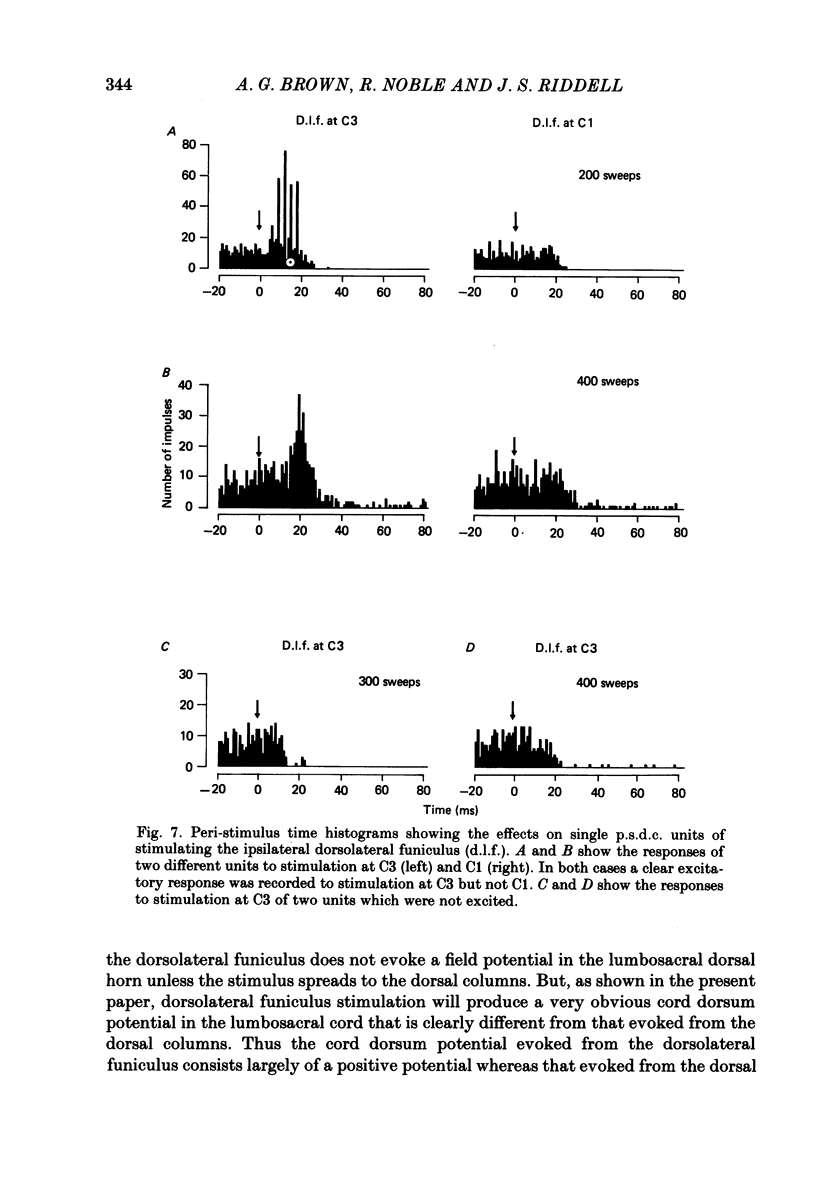
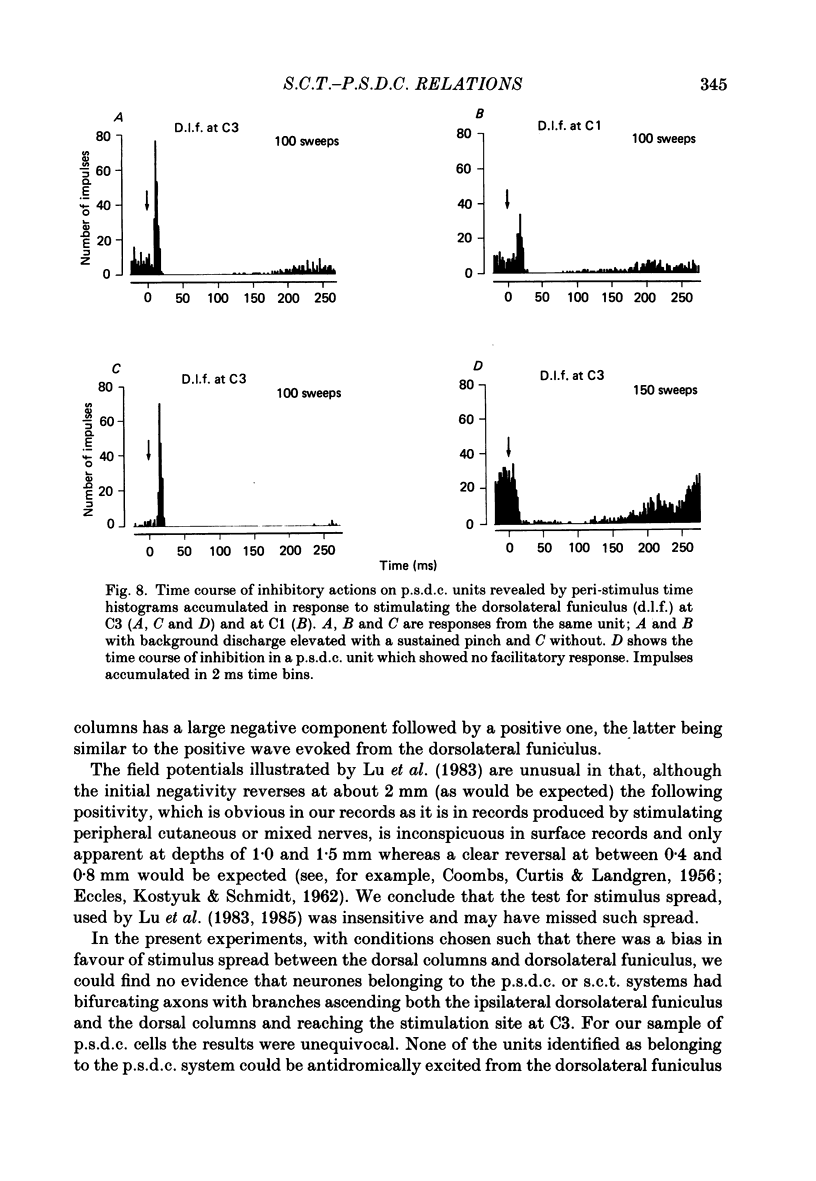




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angaut-Petit D. The dorsal column system: II. Functional properties and bulbar relay of the postsynaptic fibres of the cat's fasciculus gracilis. Exp Brain Res. 1975 May 22;22(5):471–493. doi: 10.1007/BF00237349. [DOI] [PubMed] [Google Scholar]
- Bennett G. J., Nishikawa N., Lu G. W., Hoffert M. J., Dubner R. The morphology of dorsal column postsynaptic spinomedullary neurons in the cat. J Comp Neurol. 1984 Apr 20;224(4):568–578. doi: 10.1002/cne.902240406. [DOI] [PubMed] [Google Scholar]
- Bennett G. J., Seltzer Z., Lu G. W., Nishikawa N., Dubner R. The cells of origin of the dorsal column postsynaptic projection in the lumbosacral enlargements of cats and monkeys. Somatosens Res. 1983;1(2):131–149. doi: 10.3109/07367228309144545. [DOI] [PubMed] [Google Scholar]
- Brown A. G., Brown P. B., Fyffe R. E., Pubols L. M. Receptive field organization and response properties of spinal neurones with axons ascending the dorsal columns in the cat. J Physiol. 1983 Apr;337:575–588. doi: 10.1113/jphysiol.1983.sp014643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G. Effects of descending impulses on transmission through the spinocervical tract. J Physiol. 1971 Dec;219(1):103–125. doi: 10.1113/jphysiol.1971.sp009652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., Franz D. N. Responses of spinocervical tract neurones to natural stimulation of identified cutaneous receptors. Exp Brain Res. 1969;7(3):231–249. doi: 10.1007/BF00239031. [DOI] [PubMed] [Google Scholar]
- Brown A. G., Fyffe R. E. Form and function of dorsal horn neurones with axons ascending the dorsal columns in cat. J Physiol. 1981 Dec;321:31–47. doi: 10.1113/jphysiol.1981.sp013970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., Fyffe R. E., Noble R., Rose P. K., Snow P. J. The density, distribution and topographical organization of spinocervical tract neurones in the cat. J Physiol. 1980 Mar;300:409–428. doi: 10.1113/jphysiol.1980.sp013169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., Fyffe R. E., Noble R., Rowe M. J. Effects of hind limb nerve section on lumbosacral dorsal horn neurones in the cat. J Physiol. 1984 Sep;354:375–394. doi: 10.1113/jphysiol.1984.sp015382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., Rose P. K., Snow P. J. The morphology of spinocervical tract neurones revealed by intracellular injection of horseradish peroxidase. J Physiol. 1977 Sep;270(3):747–764. doi: 10.1113/jphysiol.1977.sp011980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOMBS J. S., CURTIS D. R., LANDGREN S. Spinal cord potentials generated by impulses in muscle and cutaneous afferent fibres. J Neurophysiol. 1956 Sep;19(5):452–467. doi: 10.1152/jn.1956.19.5.452. [DOI] [PubMed] [Google Scholar]
- Craig A. D., Jr, Tapper D. N. Lateral cervical nucleus in the cat: functional organization and characteristics. J Neurophysiol. 1978 Nov;41(6):1511–1534. doi: 10.1152/jn.1978.41.6.1511. [DOI] [PubMed] [Google Scholar]
- Dart A. M., Gordon G. Some properties of spinal connections of the cat's dorsal column nuclei which do not involve the dorsal columns. Brain Res. 1973 Aug 17;58(1):61–68. doi: 10.1016/0006-8993(73)90823-8. [DOI] [PubMed] [Google Scholar]
- ECCLES J. C., KOSTYUK P. G., SCHMIDT R. F. Central pathways responsible for depolarization of primary afferent fibres. J Physiol. 1962 May;161:237–257. doi: 10.1113/jphysiol.1962.sp006884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon G., Grant G. Dorsolateral spinal afferents to some medullary sensory nuclei. An anatomical study in the cat. Exp Brain Res. 1982;46(1):12–23. doi: 10.1007/BF00238093. [DOI] [PubMed] [Google Scholar]
- Hongo T., Jankowska E., Lundberg A. Post-synaptic excitation and inhibition from primary afferents in neurones of the spinocervical tract. J Physiol. 1968 Dec;199(3):569–592. doi: 10.1113/jphysiol.1968.sp008669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E., Rastad J., Zarzecki P. Segmental and supraspinal input to cells of origin of non-primary fibres in the feline dorsal columns. J Physiol. 1979 May;290(2):185–200. doi: 10.1113/jphysiol.1979.sp012767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu G. W., Bennett G. J., Nishikawa N., Dubner R. Spinal neurons with branched axons traveling in both the dorsal and dorsolateral funiculi. Exp Neurol. 1985 Mar;87(3):571–577. doi: 10.1016/0014-4886(85)90185-2. [DOI] [PubMed] [Google Scholar]
- Lu G. W., Bennett G. J., Nishikawa N., Hoffert M. J., Dubner R. Extra- and intracellular recordings from dorsal column postsynaptic spinomedullary neurons in the cat. Exp Neurol. 1983 Nov;82(2):456–477. doi: 10.1016/0014-4886(83)90417-x. [DOI] [PubMed] [Google Scholar]
- Maxwell D. J., Fyffe R. E., Brown A. G. Fine structure of normal and degenerating primary afferent boutons associated with characterized spinocervical tract neurons in the cat. Neuroscience. 1984 May;12(1):151–163. doi: 10.1016/0306-4522(84)90144-1. [DOI] [PubMed] [Google Scholar]
- Maxwell D. J., Fyffe R. E., Brown A. G. Fine structure of spinocervical tract neurones and the synaptic boutons in contact with them. Brain Res. 1982 Feb 11;233(2):394–399. doi: 10.1016/0006-8993(82)91212-4. [DOI] [PubMed] [Google Scholar]
- Maxwell D. J., Koerber H. R. Fine structure of collateral axons originating from feline spinocervical tract neurons. Brain Res. 1986 Jan 15;363(1):199–203. doi: 10.1016/0006-8993(86)90680-3. [DOI] [PubMed] [Google Scholar]
- Nishikawa N., Bennett G. J., Ruda M. A., Lu G. W., Dubner R. Immunocytochemical evidence for a serotoninergic innervation of dorsal column postsynaptic neurons in cat and monkey: light- and electron-microscopic observations. Neuroscience. 1983 Dec;10(4):1333–1340. doi: 10.1016/0306-4522(83)90115-x. [DOI] [PubMed] [Google Scholar]
- Rustioni A., Kaufman A. B. Identification of cells or origin of non-primary afferents to the dorsal column nuclei of the cat. Exp Brain Res. 1977 Jan 18;27(1):1–14. doi: 10.1007/BF00234821. [DOI] [PubMed] [Google Scholar]
- Svensson B. A., Westman J., Rastad J. Light and electron microscopic study of neurones in the feline lateral cervical nucleus with a descending projection. Brain Res. 1985 Dec 30;361(1-2):114–124. doi: 10.1016/0006-8993(85)91281-8. [DOI] [PubMed] [Google Scholar]
- Uddenberg N. Functional organization of long, second-order afferents in the dorsal funiculus. Exp Brain Res. 1968;4(4):377–382. doi: 10.1007/BF00235702. [DOI] [PubMed] [Google Scholar]


