Abstract
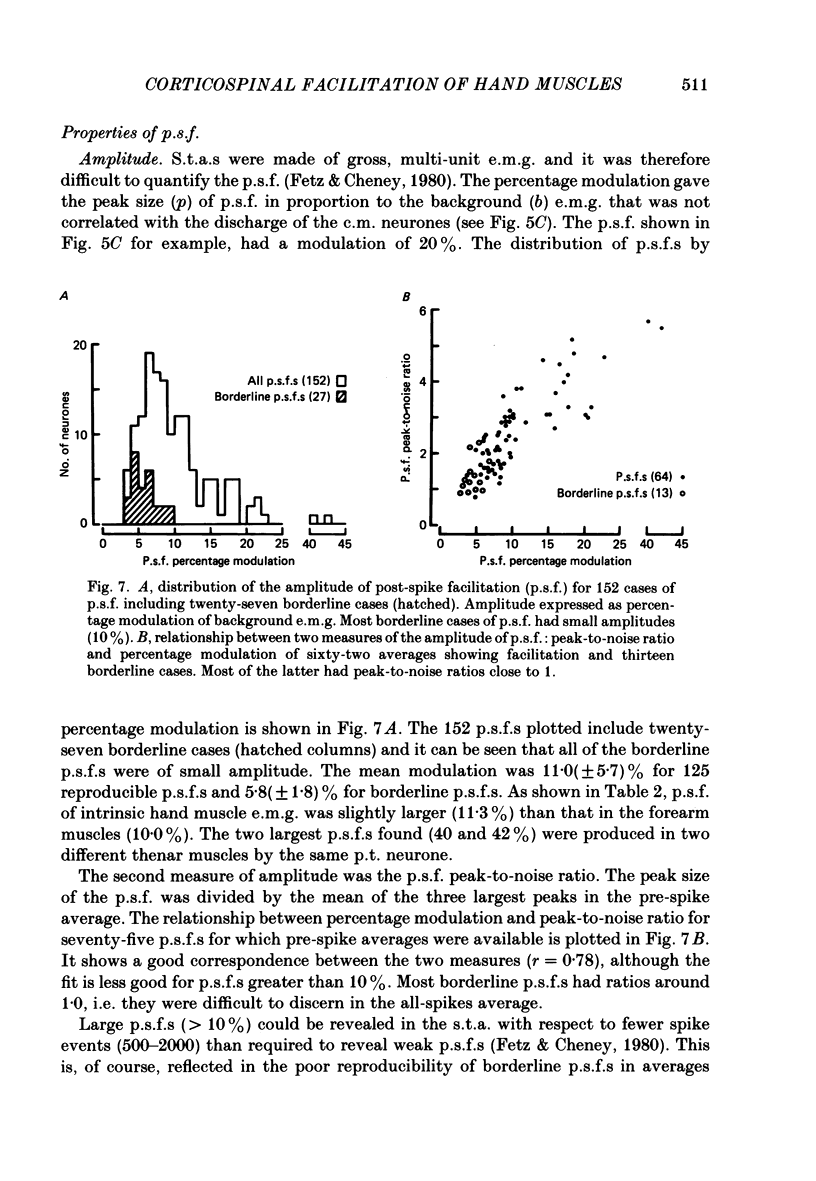
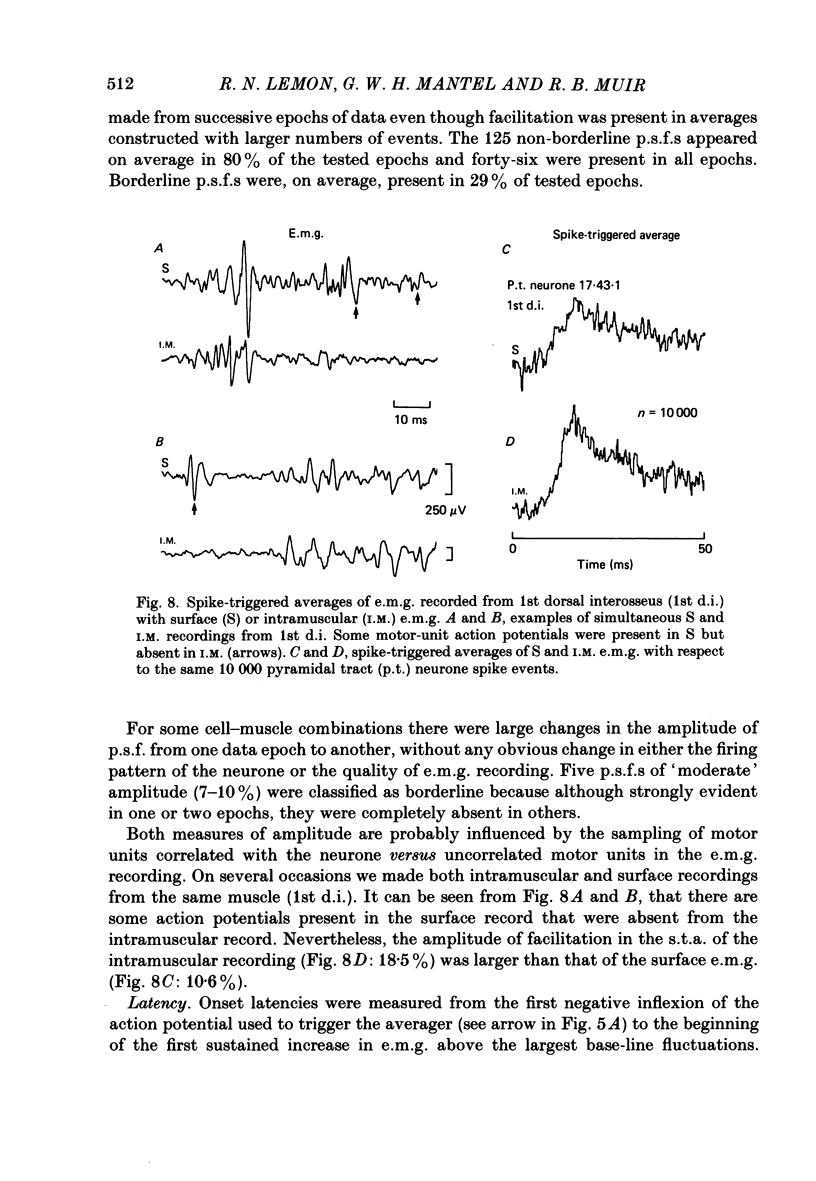
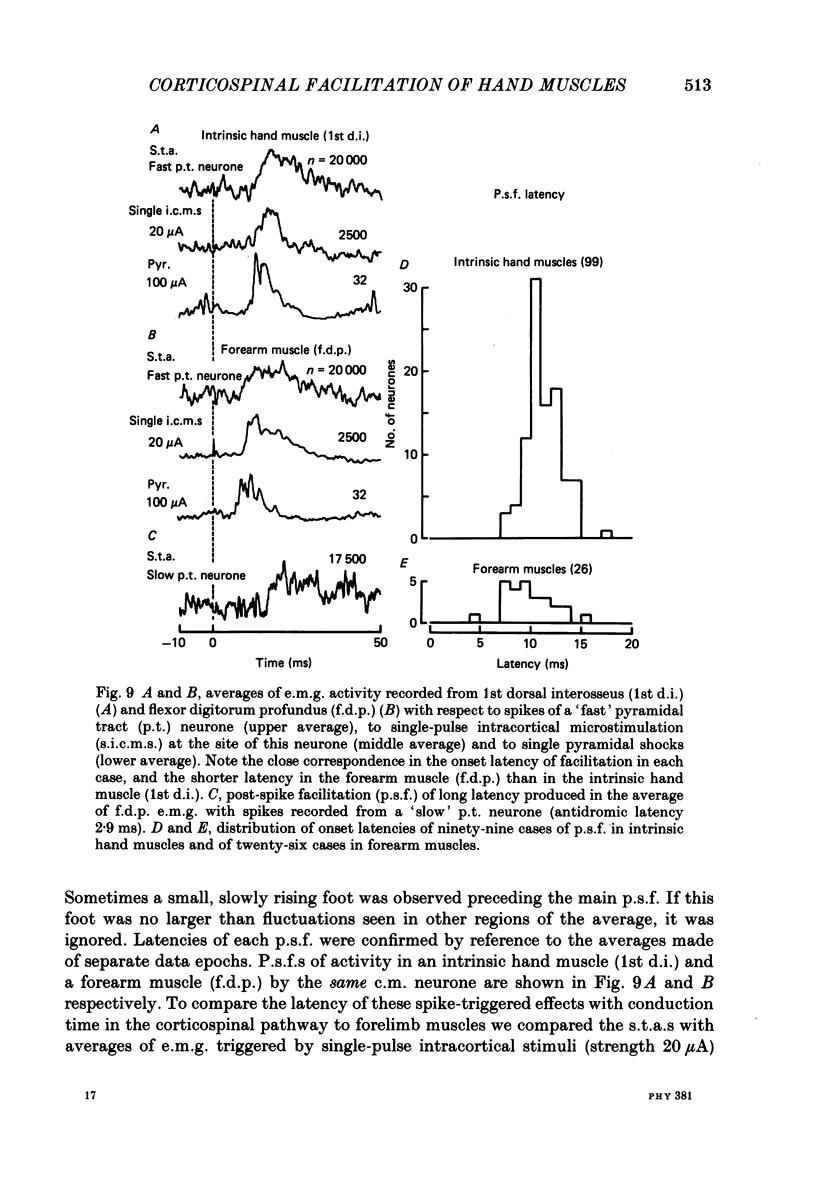
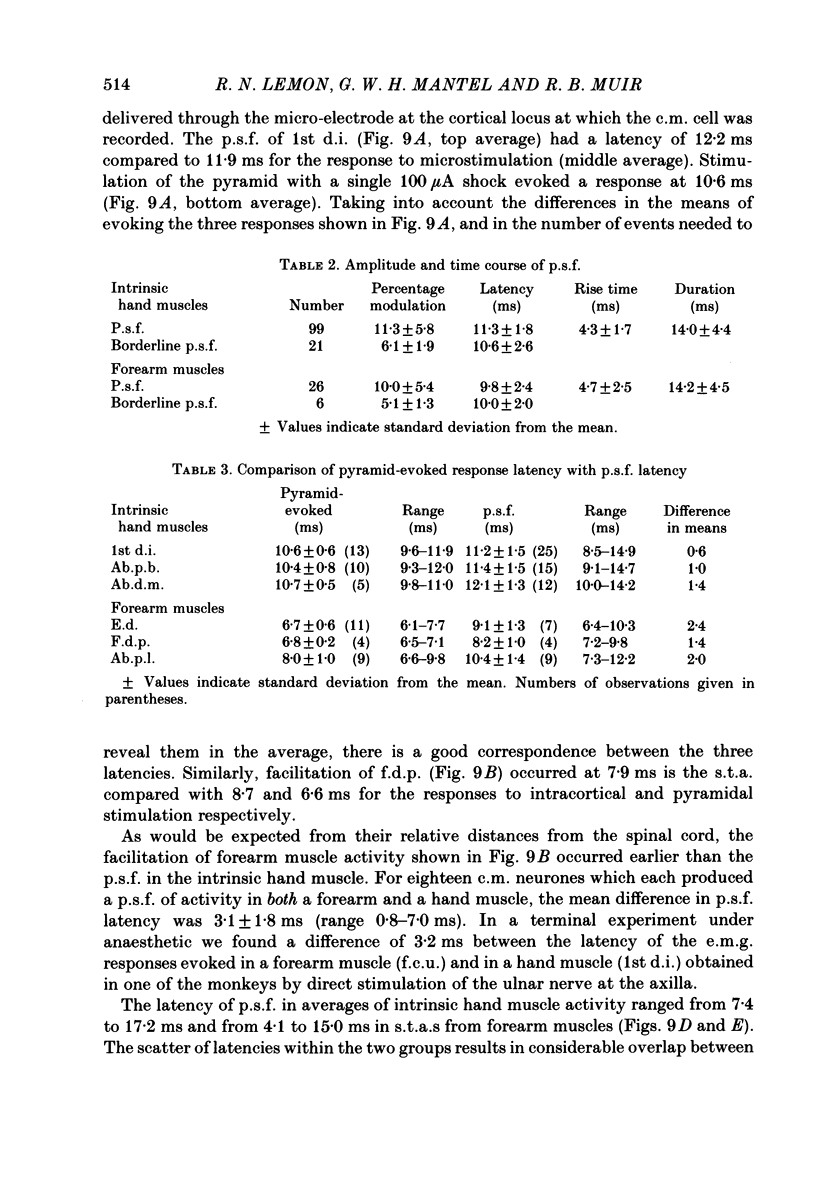
1. The method of spike-triggered averaging has been used to detect a direct influence of pyramidal tract neurones on the activity of hand and forearm muscles in conscious monkeys trained to perform repetitive movements of the hand and fingers. Gross electromyograms (e.m.g.s) from individual muscles were rectified and synchronously averaged with respect to the discharge of single, antidromically identified pyramidal tract cells in the 'hand' area of the pre-central gyrus. 2. The presence in an average of a post-spike facilitation which could be revealed reproducibly from successive epochs of recording and was clearly larger than the biggest fluctuations seen in pseudo-randomly triggered averages of the same e.m.g. data, was taken to indicate a direct cortico-motoneuronal excitatory influence. 3. 55% of cortical neurones analysed showed post-spike facilitation in one or more recorded muscle and 7% showed post-spike suppression. In terms of the total number of muscle-neurone combinations analysed, the proportions showing post-spike effects were 18 and 1% respectively. These figures have been influenced by the pre-selection of neurones for analysis according to restrictive criteria. The neurones selected (a) were recorded at cortical loci where weak intracortical microstimulation could evoke finger movements, (b) could be activated antidromically at short latency by medullary pyramidal tract stimulation, (c) showed natural discharge activity which was clearly modulated in relation to voluntary finger movements, and (d) were located in the anterior bank of the central sulcus. The results provide some evidence to vindicate these criteria. 4. The strongest post-spike facilitation observed had a peak which was 42% higher than the average pre-spike level of e.m.g. activity, but most were within the range 5-20%. Facilitation peaks below about 3% could not have been resolved from the 'noise' in the averages. The mean latency from cell discharge in the cortex to the start of the post-spike facilitation was 11.2 ms (range 7.4-17.2) for intrinsic hand muscles and 9.8 ms (range 4.1-15.0) for forearm muscles. These latencies were compared with the latencies of responses to intracortical microstimulation and to stimulation of the medullary pyramidal tract. 5. Evidence was obtained suggesting that the latency for cortico-motoneuronal activation of an individual motor unit was commonly subject to considerable variability and that different motor units of a muscle could be facilitated by the one cortical neurone at different latencies. These factors are thought to contribute to an elongation of the time course of post-spike facilitation.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
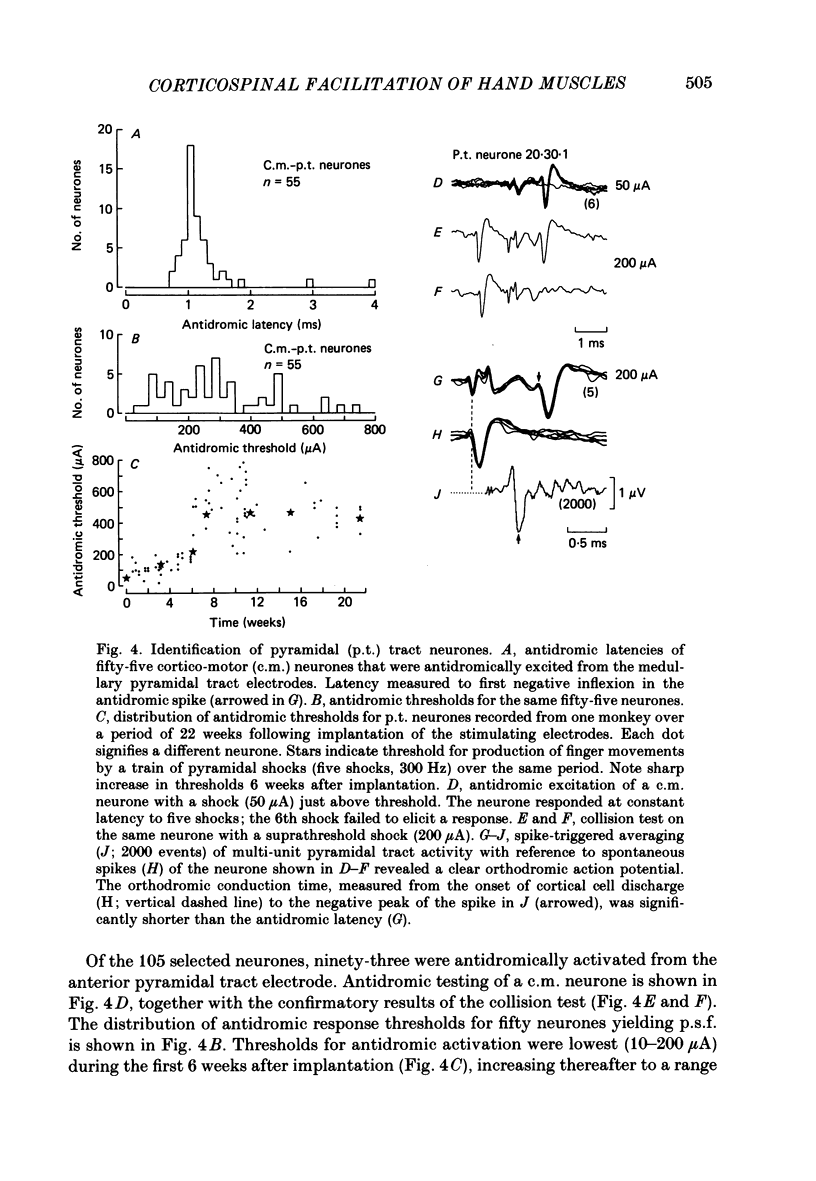
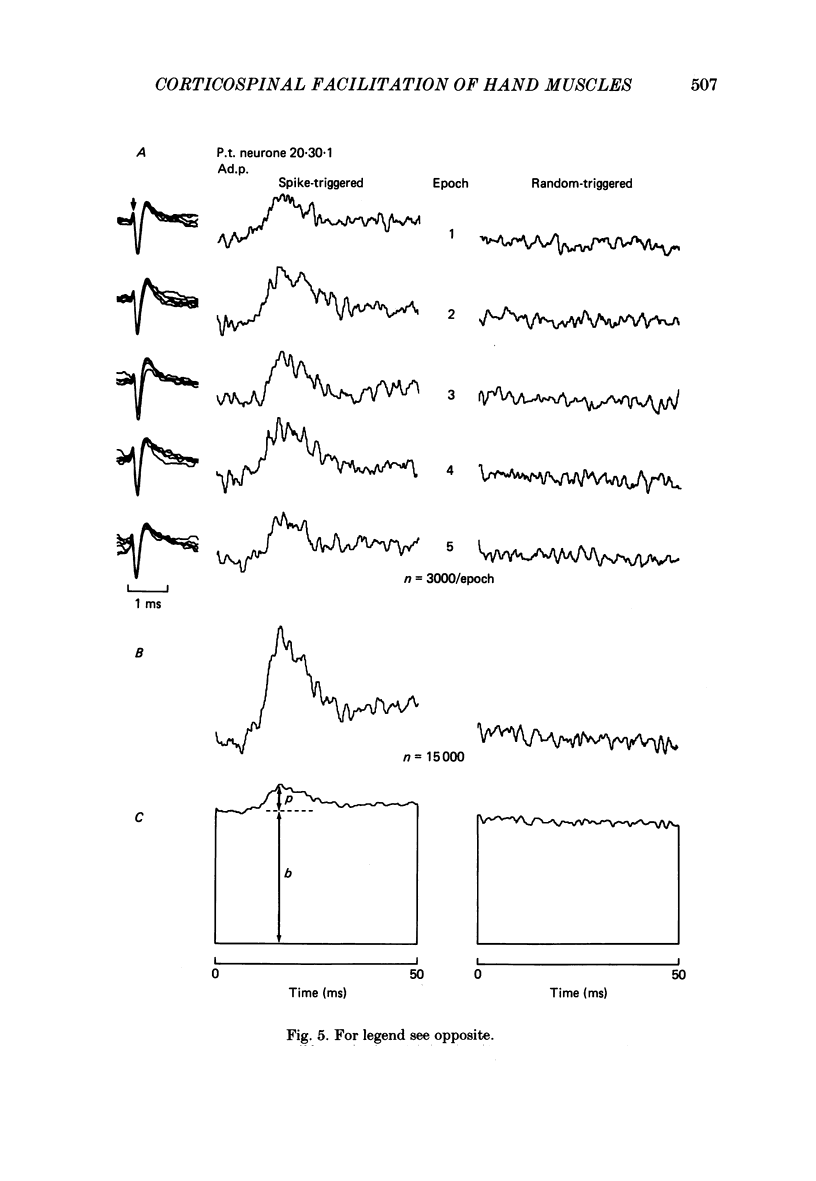
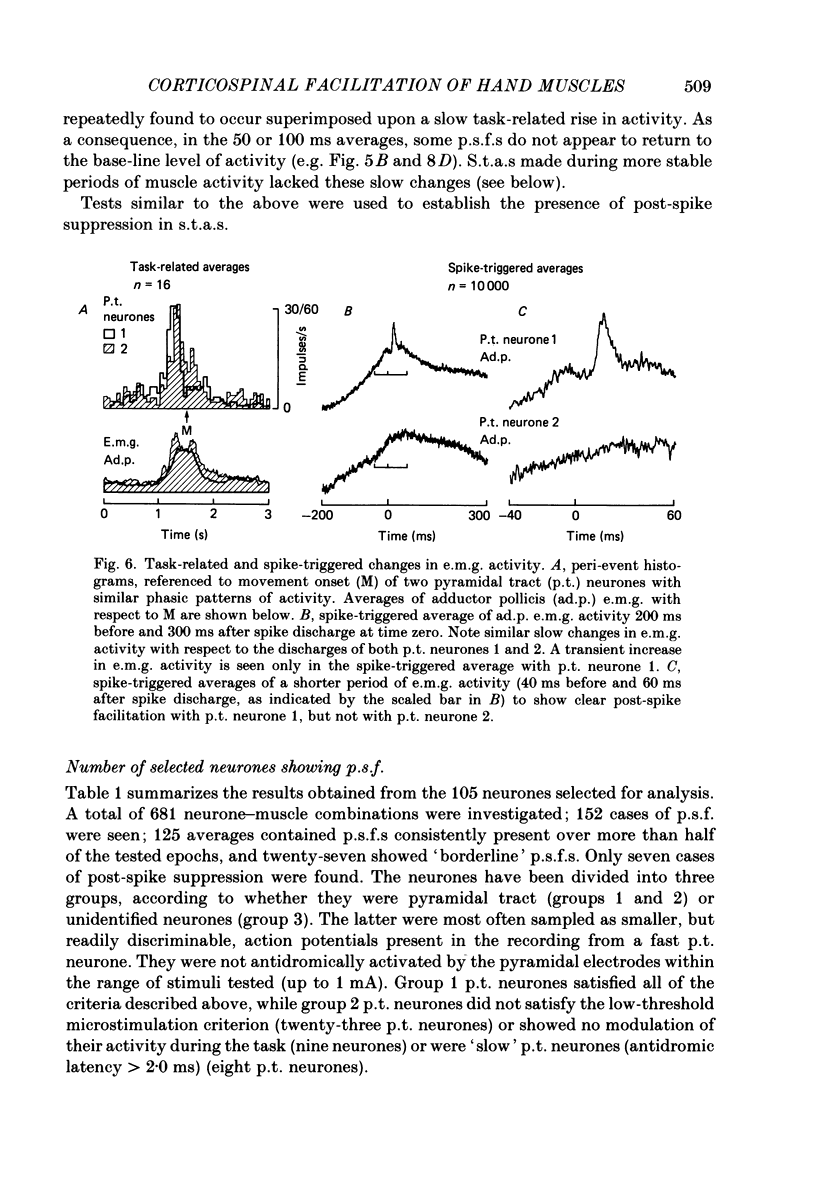
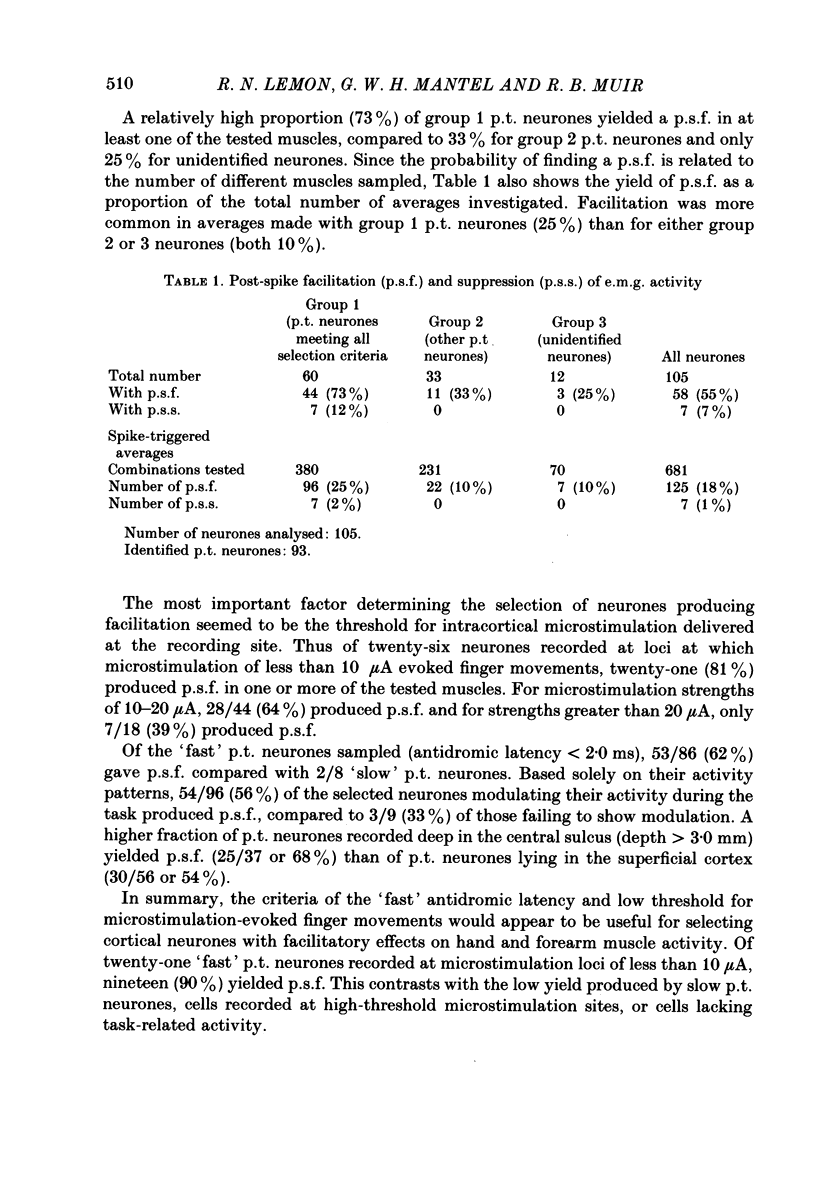
PDF







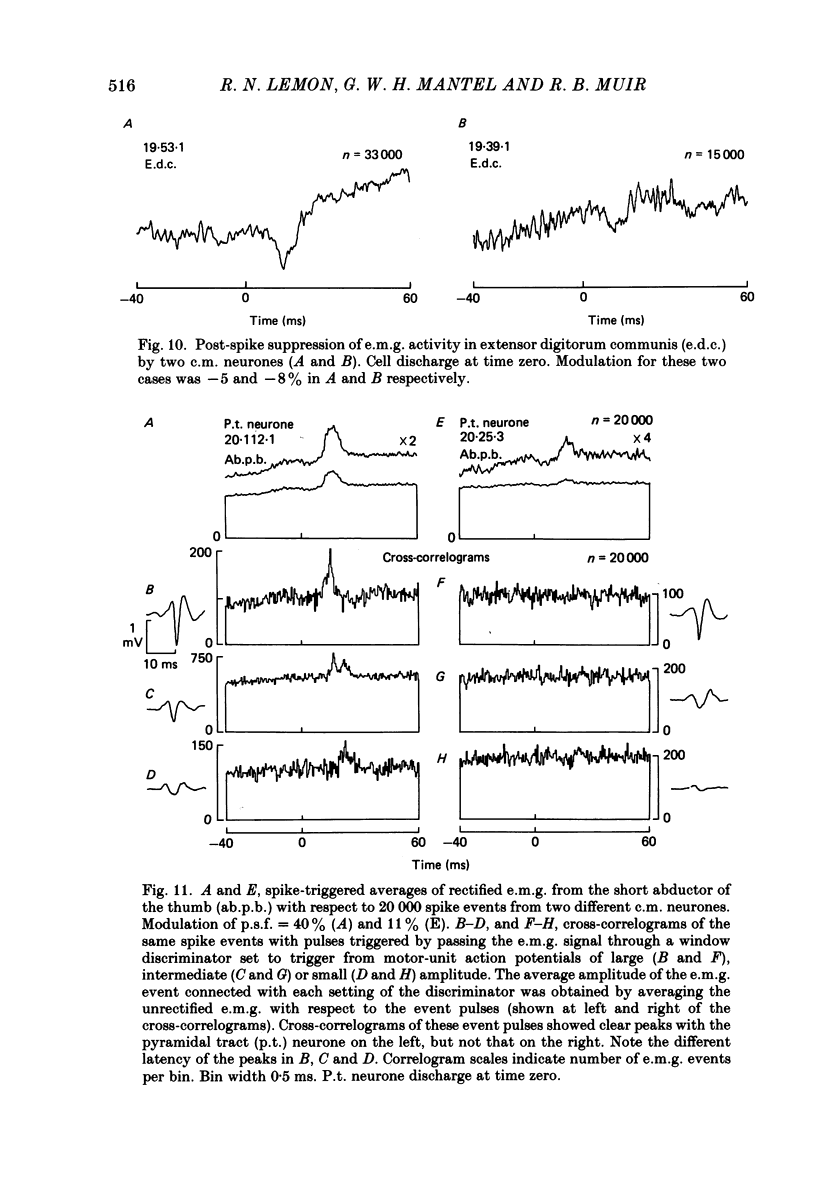

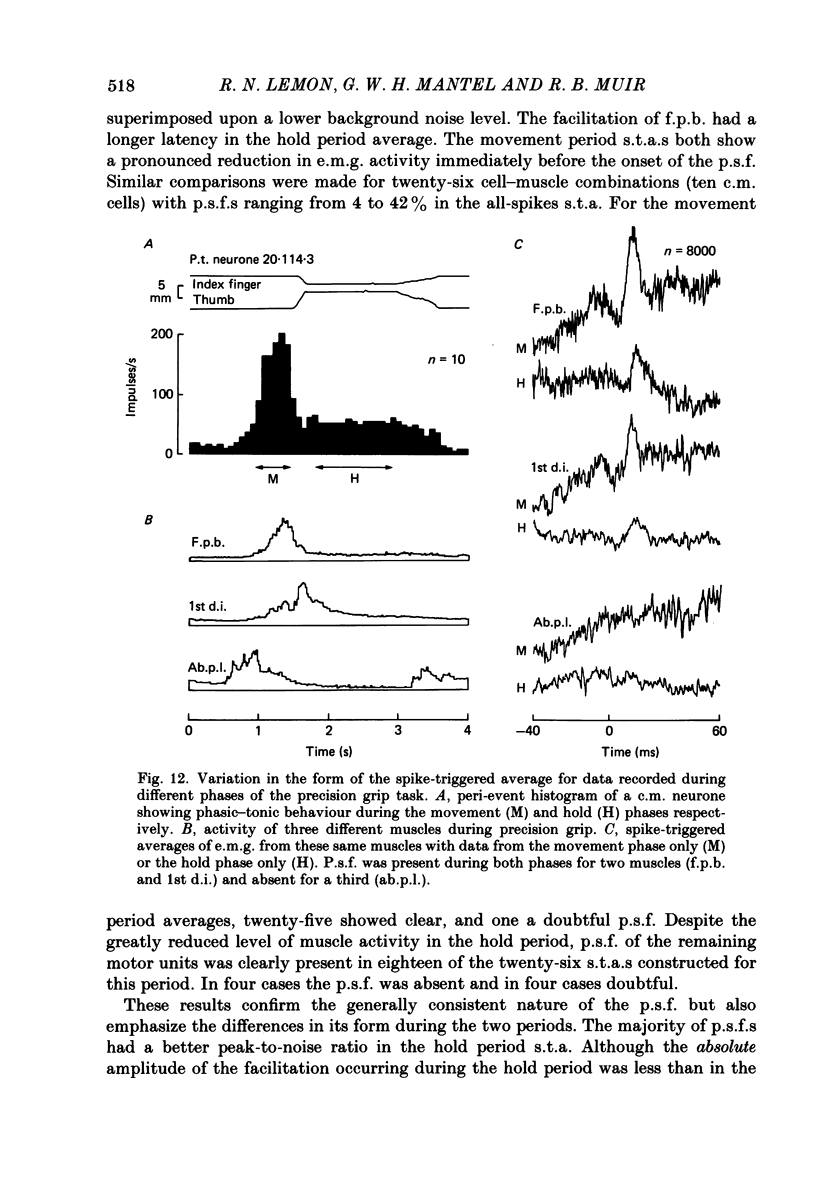









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allum J. H., Hepp-Reymond M. C., Gysin R. Cross-correlation analysis of interneuronal connectivity in the motor cortex of the monkey. Brain Res. 1982 Jan 14;231(2):325–334. doi: 10.1016/0006-8993(82)90369-9. [DOI] [PubMed] [Google Scholar]
- Andersen P., Hagan P. J., Phillips C. G., Powell T. P. Mapping by microstimulation of overlapping projections from area 4 to motor units of the baboon's hand. Proc R Soc Lond B Biol Sci. 1975 Jan 21;188(1090):31–36. doi: 10.1098/rspb.1975.0002. [DOI] [PubMed] [Google Scholar]
- Armstrong D. M., Drew T. Locomotor-related neuronal discharges in cat motor cortex compared with peripheral receptive fields and evoked movements. J Physiol. 1984 Jan;346:497–517. doi: 10.1113/jphysiol.1984.sp015037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asanuma H., Zarzecki P., Jankowska E., Hongo T., Marcus S. Projection of individual pyramidal tract neurons to lumbar motor nuclei of the monkey. Exp Brain Res. 1979 Jan 2;34(1):73–89. doi: 10.1007/BF00238342. [DOI] [PubMed] [Google Scholar]
- BISHOP P. O., BURKE W., DAVIS R. The identification of single units in central visual pathways. J Physiol. 1962 Aug;162:409–431. doi: 10.1113/jphysiol.1962.sp006942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betts R. P., Johnston D. M., Brown B. H. Nerve fibre velocity and refractory period distributions in nerve trunks. J Neurol Neurosurg Psychiatry. 1976 Jul;39(7):694–700. doi: 10.1136/jnnp.39.7.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buys E. J., Lemon R. N., Mantel G. W., Muir R. B. Selective facilitation of different hand muscles by single corticospinal neurones in the conscious monkey. J Physiol. 1986 Dec;381:529–549. doi: 10.1113/jphysiol.1986.sp016342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheney P. D., Fetz E. E. Comparable patterns of muscle facilitation evoked by individual corticomotoneuronal (CM) cells and by single intracortical microstimuli in primates: evidence for functional groups of CM cells. J Neurophysiol. 1985 Mar;53(3):786–804. doi: 10.1152/jn.1985.53.3.786. [DOI] [PubMed] [Google Scholar]
- Cheney P. D., Fetz E. E. Corticomotoneuronal cells contribute to long-latency stretch reflexes in the rhesus monkey. J Physiol. 1984 Apr;349:249–272. doi: 10.1113/jphysiol.1984.sp015155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheney P. D., Fetz E. E., Palmer S. S. Patterns of facilitation and suppression of antagonist forelimb muscles from motor cortex sites in the awake monkey. J Neurophysiol. 1985 Mar;53(3):805–820. doi: 10.1152/jn.1985.53.3.805. [DOI] [PubMed] [Google Scholar]
- Clough J. F., Kernell D., Phillips C. G. The distribution of monosynaptic excitation from the pyramidal tract and from primary spindle afferents to motoneurones of the baboon's hand and forearm. J Physiol. 1968 Sep;198(1):145–166. doi: 10.1113/jphysiol.1968.sp008598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devanandan M. S., Ghosh S., Simoes E. A. Myelinated fibers of the deep branch of the ulnar nerve at the wrist in bonnet monkeys (Macaca radiata) and some of its branches to the hand. Anat Rec. 1980 Aug;197(4):387–396. doi: 10.1002/ar.1091970403. [DOI] [PubMed] [Google Scholar]
- Fetz E. E., Cheney P. D., German D. C. Corticomotoneuronal connections of precentral cells detected by postspike averages of EMG activity in behaving monkeys. Brain Res. 1976 Sep 24;114(3):505–510. doi: 10.1016/0006-8993(76)90973-2. [DOI] [PubMed] [Google Scholar]
- Fetz E. E., Cheney P. D. Postspike facilitation of forelimb muscle activity by primate corticomotoneuronal cells. J Neurophysiol. 1980 Oct;44(4):751–772. doi: 10.1152/jn.1980.44.4.751. [DOI] [PubMed] [Google Scholar]
- Fetz E. E., Gustafsson B. Relation between shapes of post-synaptic potentials and changes in firing probability of cat motoneurones. J Physiol. 1983 Aug;341:387–410. doi: 10.1113/jphysiol.1983.sp014812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson B., McCrea D. Influence of stretch-evoked synaptic potentials on firing probability of cat spinal motoneurones. J Physiol. 1984 Feb;347:431–451. doi: 10.1113/jphysiol.1984.sp015074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffner R., Masterton B. Variation in form of the pyramidal tract and its relationship to digital dexterity. Brain Behav Evol. 1975;12(3):161–200. doi: 10.1159/000124401. [DOI] [PubMed] [Google Scholar]
- Jankowska E., Padel Y., Tanaka R. Disynaptic inhibition of spinal motoneurones from the motor cortex in the monkey. J Physiol. 1976 Jun;258(2):467–487. doi: 10.1113/jphysiol.1976.sp011431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E., Padel Y., Tanaka R. Projections of pyramidal tract cells to alpha-motoneurones innervating hind-limb muscles in the monkey. J Physiol. 1975 Aug;249(3):637–667. doi: 10.1113/jphysiol.1975.sp011035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUYPERS H. G. Central cortical projections to motor and somato-sensory cell groups. An experimental study in the rhesus monkey. Brain. 1960 Mar;83:161–184. doi: 10.1093/brain/83.1.161. [DOI] [PubMed] [Google Scholar]
- KUYPERS H. G. Corticospinal connections: postnatal development in the rhesus monkey. Science. 1962 Nov 9;138(3541):678–680. doi: 10.1126/science.138.3541.678. [DOI] [PubMed] [Google Scholar]
- Kasser R. J., Cheney P. D. Characteristics of corticomotoneuronal postspike facilitation and reciprocal suppression of EMG activity in the monkey. J Neurophysiol. 1985 Apr;53(4):959–978. doi: 10.1152/jn.1985.53.4.959. [DOI] [PubMed] [Google Scholar]
- Kirkwood P. A. On the use and interpretation of cross-correlations measurements in the mammalian central nervous system. J Neurosci Methods. 1979 Aug;1(2):107–132. doi: 10.1016/0165-0270(79)90009-8. [DOI] [PubMed] [Google Scholar]
- Kirkwood P. A., Sears T. A. The effects of single afferent impulses on the probability of firing of external intercostal motoneurones in the cat. J Physiol. 1982 Jan;322:315–336. doi: 10.1113/jphysiol.1982.sp014039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan H. C., MacKay W. A., Murphy J. T., Wong Y. C. Spatial organization of precentral cortex in awake primates. II. Motor outputs. J Neurophysiol. 1978 Sep;41(5):1120–1131. doi: 10.1152/jn.1978.41.5.1120. [DOI] [PubMed] [Google Scholar]
- LANDGREN S., PHILLIPS C. G., PORTER R. Cortical fields of origin of the monosynaptic pyramidal pathways to some alpha motoneurones of the baboon's hand and forearm. J Physiol. 1962 Apr;161:112–125. doi: 10.1113/jphysiol.1962.sp006876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANDGREN S., PHILLIPS C. G., PORTER R. Minimal synaptic actions of pyramidal impulses on some alpha motoneurones of the baboon's hand and forearm. J Physiol. 1962 Apr;161:91–111. doi: 10.1113/jphysiol.1962.sp006875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence D. G., Kuypers H. G. The functional organization of the motor system in the monkey. I. The effects of bilateral pyramidal lesions. Brain. 1968 Mar;91(1):1–14. doi: 10.1093/brain/91.1.1. [DOI] [PubMed] [Google Scholar]
- Lawrence D. G., Porter R., Redman S. J. Corticomotoneuronal synapses in the monkey: light microscopic localization upon motoneurons of intrinsic muscles of the hand. J Comp Neurol. 1985 Feb 22;232(4):499–510. doi: 10.1002/cne.902320407. [DOI] [PubMed] [Google Scholar]
- Lemon R. N. Functional properties of monkey motor cortex neurones receiving afferent input from the hand and fingers. J Physiol. 1981 Feb;311:497–519. doi: 10.1113/jphysiol.1981.sp013601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir R. B., Lemon R. N. Corticospinal neurons with a special role in precision grip. Brain Res. 1983 Feb 21;261(2):312–316. doi: 10.1016/0006-8993(83)90635-2. [DOI] [PubMed] [Google Scholar]
- NAPIER J. R. The prehensile movements of the human hand. J Bone Joint Surg Br. 1956 Nov;38-B(4):902–913. doi: 10.1302/0301-620X.38B4.902. [DOI] [PubMed] [Google Scholar]
- PENFIELD W. Mechanisms of voluntary movement. Brain. 1954;77(1):1–17. doi: 10.1093/brain/77.1.1. [DOI] [PubMed] [Google Scholar]
- PHILLIPS C. G., PORTER R. THE PYRAMIDAL PROJECTION TO MOTONEURONES OF SOME MUSCLE GROUPS OF THE BABOON'S FORELIMB. Prog Brain Res. 1964;12:222–245. doi: 10.1016/s0079-6123(08)60625-1. [DOI] [PubMed] [Google Scholar]
- PRESTON J. B., WHITLOCK D. G. Intracellular potentials recorded from motoneurons following precentral gyrus stimulation in primate. J Neurophysiol. 1961 Jan;24:91–100. doi: 10.1152/jn.1961.24.1.91. [DOI] [PubMed] [Google Scholar]
- Passingham R. E., Perry V. H., Wilkinson F. The long-term effects of removal of sensorimotor cortex in infant and adult rhesus monkeys. Brain. 1983 Sep;106(Pt 3):675–705. doi: 10.1093/brain/106.3.675. [DOI] [PubMed] [Google Scholar]
- Passingham R., Perry H., Wilkinson F. Failure to develop a precision grip in monkeys with unilateral neocortical lesions made in infancy. Brain Res. 1978 Apr 28;145(2):410–414. doi: 10.1016/0006-8993(78)90878-8. [DOI] [PubMed] [Google Scholar]
- Porter R., Hore J. Time course of minimal corticomotoneuronal excitatory postsynaptic potentials in lumbar motoneurons of the monkey. J Neurophysiol. 1969 May;32(3):443–451. doi: 10.1152/jn.1969.32.3.443. [DOI] [PubMed] [Google Scholar]
- Sears T. A., Stagg D. Short-term synchronization of intercostal motoneurone activity. J Physiol. 1976 Dec;263(3):357–381. doi: 10.1113/jphysiol.1976.sp011635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapovalov A. I. Neuronal organization and synaptic mechanisms of supraspinal motor control in vertebrates. Rev Physiol Biochem Pharmacol. 1975;72:1–54. doi: 10.1007/BFb0031545. [DOI] [PubMed] [Google Scholar]
- Shinoda Y., Yokota J., Futami T. Divergent projection of individual corticospinal axons to motoneurons of multiple muscles in the monkey. Neurosci Lett. 1981 Apr 9;23(1):7–12. doi: 10.1016/0304-3940(81)90182-8. [DOI] [PubMed] [Google Scholar]
- Stephens J. A., Usherwood T. P. The mechanical properties of human motor units with special reference to their fatiguability and recruitment threshold. Brain Res. 1977 Apr 8;125(1):91–97. doi: 10.1016/0006-8993(77)90361-4. [DOI] [PubMed] [Google Scholar]
- Swadlow H. A., Waxman S. G., Rosene D. L. Latency variability and the identification of antidromically activated neurons in mammalian brain. Exp Brain Res. 1978 Jul 14;32(3):439–443. doi: 10.1007/BF00238715. [DOI] [PubMed] [Google Scholar]
- Woolsey C. N., Górska T., Wetzel A., Erickson T. C., Earls F. J., Allman J. M. Complete unilateral section of the pyramidal tract at the medullar level in Macaca mulatta. Brain Res. 1972 May 12;40(1):119–123. doi: 10.1016/0006-8993(72)90116-3. [DOI] [PubMed] [Google Scholar]
- Wray S. H. Innervation ratios for large and small limb muscles in the baboon. J Comp Neurol. 1969 Oct;137(2):227–250. doi: 10.1002/cne.901370207. [DOI] [PubMed] [Google Scholar]