Abstract
1. Synaptic transmission and neuronal morphology were studied in the nucleus tractus solitarius and in the dorsal vagal motor nucleus (solitary complex), in coronal brain-stem slices of rat or cat, superfused in vitro. 2. Electrical stimulation of afferent fibres of the solitary tract evoked two different types of post-synaptic response recorded intracellularly in different solitary complex neurones. Labelling with horseradish peroxidase showed that these two sorts of orthodromically evoked responses were correlated with different post-synaptic neuronal morphologies. 3. The majority of recorded neurones (n = 93) showed a prolonged reduction in excitability following the initial solitary-tract-evoked excitatory post-synaptic potential (e.p.s.p.). A smaller number of neurones (n = 53) showed a prolonged increase in excitability following solitary tract stimulation. In no case did the solitary tract stimulation induce a burst of action potentials at high frequency. 4. The time-to-peak and the half-width of the initial solitary-tract-evoked e.p.s.p. were shorter in neurones with prolonged increased excitability than in those with prolonged reduced excitability. In neurones with prolonged reduced excitability, this e.p.s.p. was followed by a hyperpolarization lasting 60-100 ms. The latency of this inhibitory post-synaptic potential (i.p.s.p.) was 3-5 ms longer than that of the initial e.p.s.p. and its reversal potential was 10 mV more negative than the reversal potential of the response measured following application of gamma-aminobutyric acid or glycine. In neurones with prolonged increased excitability, at a membrane potential of -40 to -50 mV, the initial solitary tract e.p.s.p. was followed by a prolonged depolarization lasting 100-400 ms. 5. Background synaptic activity was high in neurones with prolonged increased excitability, consisting of unitary e.p.s.p.s with an amplitude of more than 0.8 mV. This activity was increased for a period of 300-800 ms following solitary tract stimulation. Spontaneous excitatory potentials of more than 0.5 mV were not seen in neurones with prolonged reduced excitability. In these neurones, after intracellular injection of choride ions, reversed unitary i.p.s.p.s formed a background activity which was increased following stimulation of the solitary tract. 6. Neurones with prolonged reduced excitability were found in the medial, ventral and ventrolateral part of the nucleus tractus solitarius and in the dorsal vagal motor nucleus where they were identified by their antidromic response to stimulation ventral and lateral to the tractus solitarius.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF




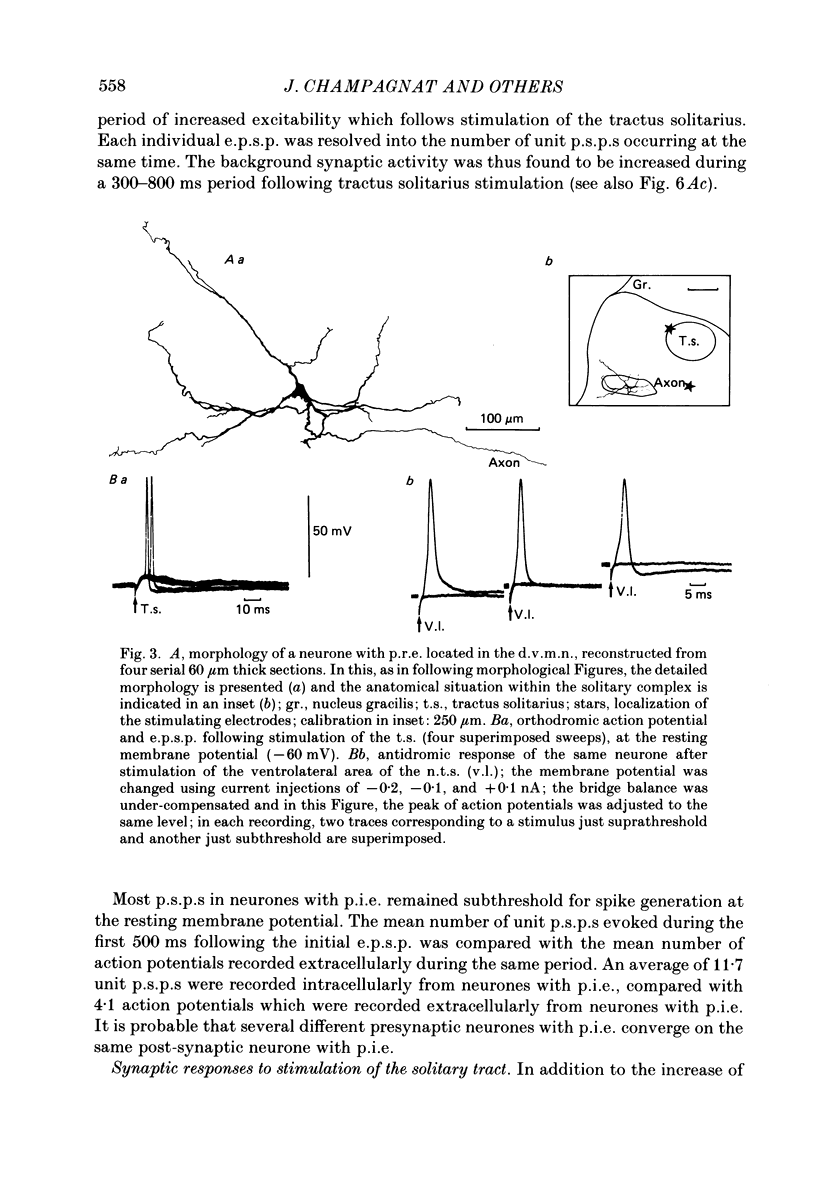
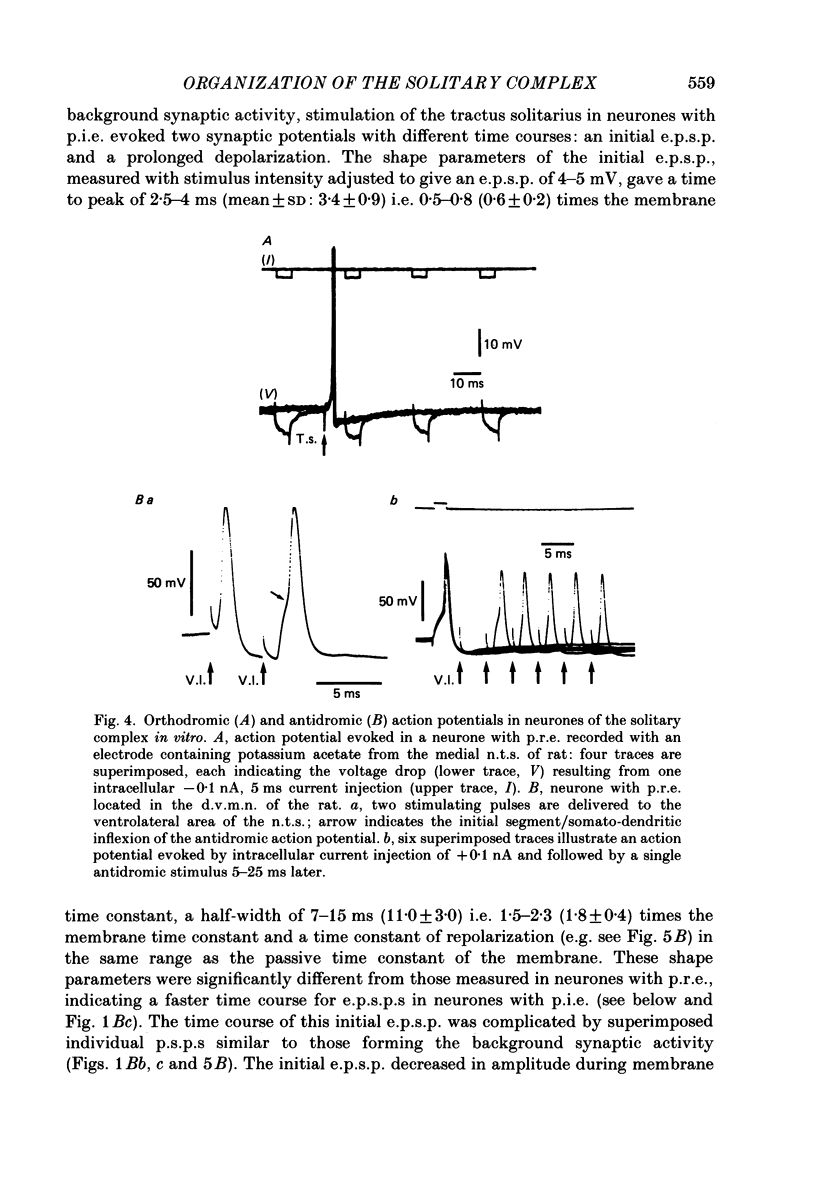
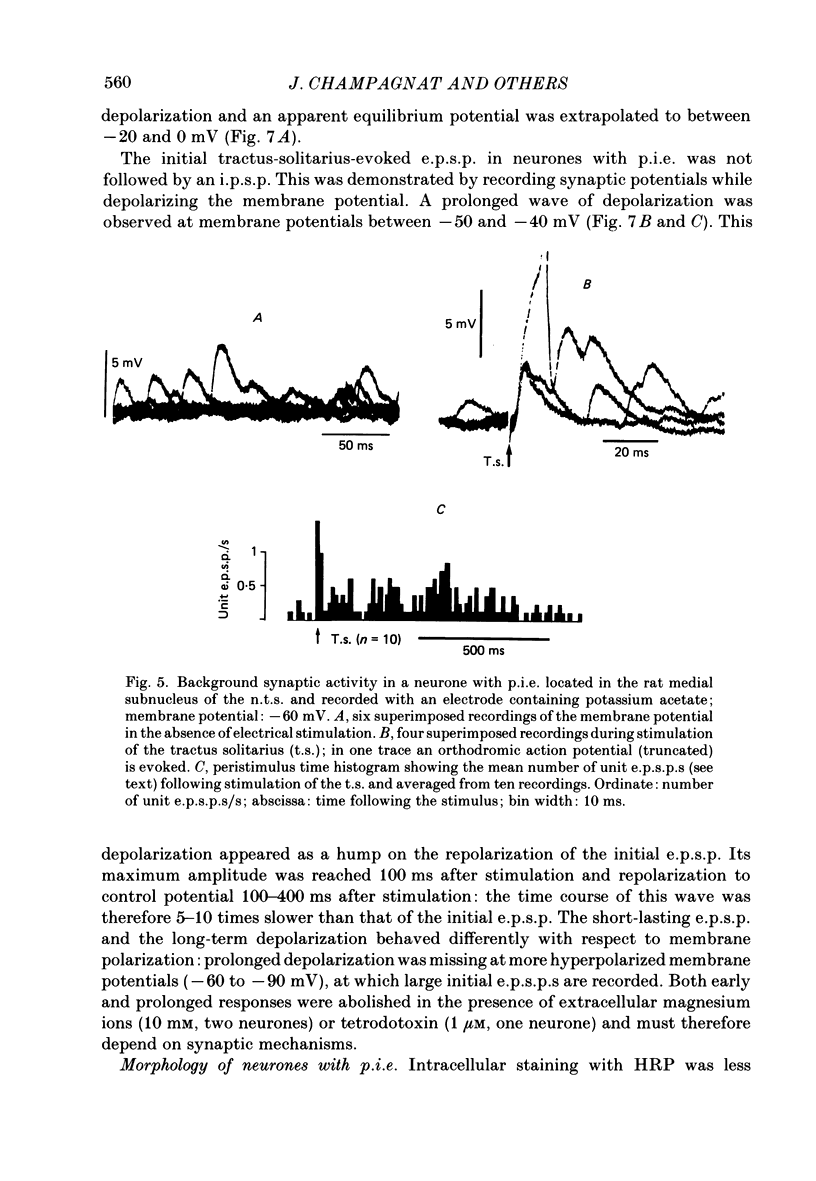
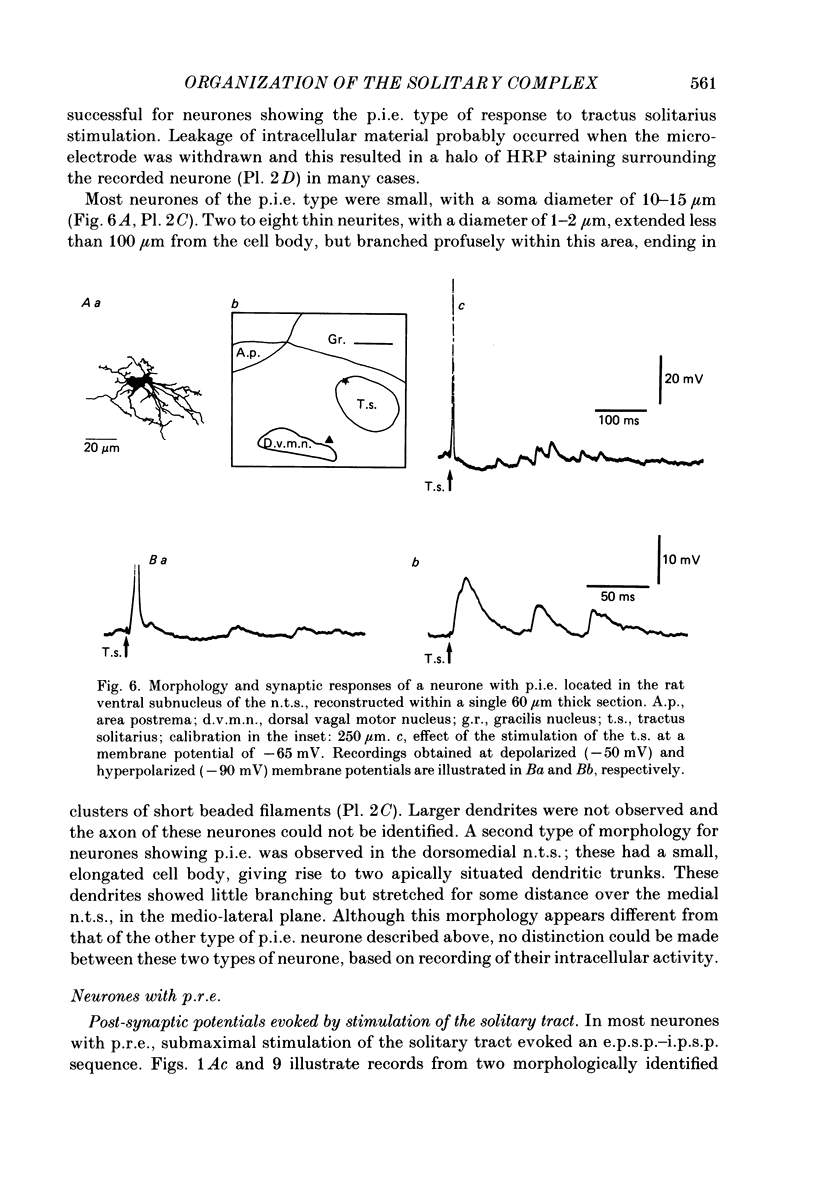
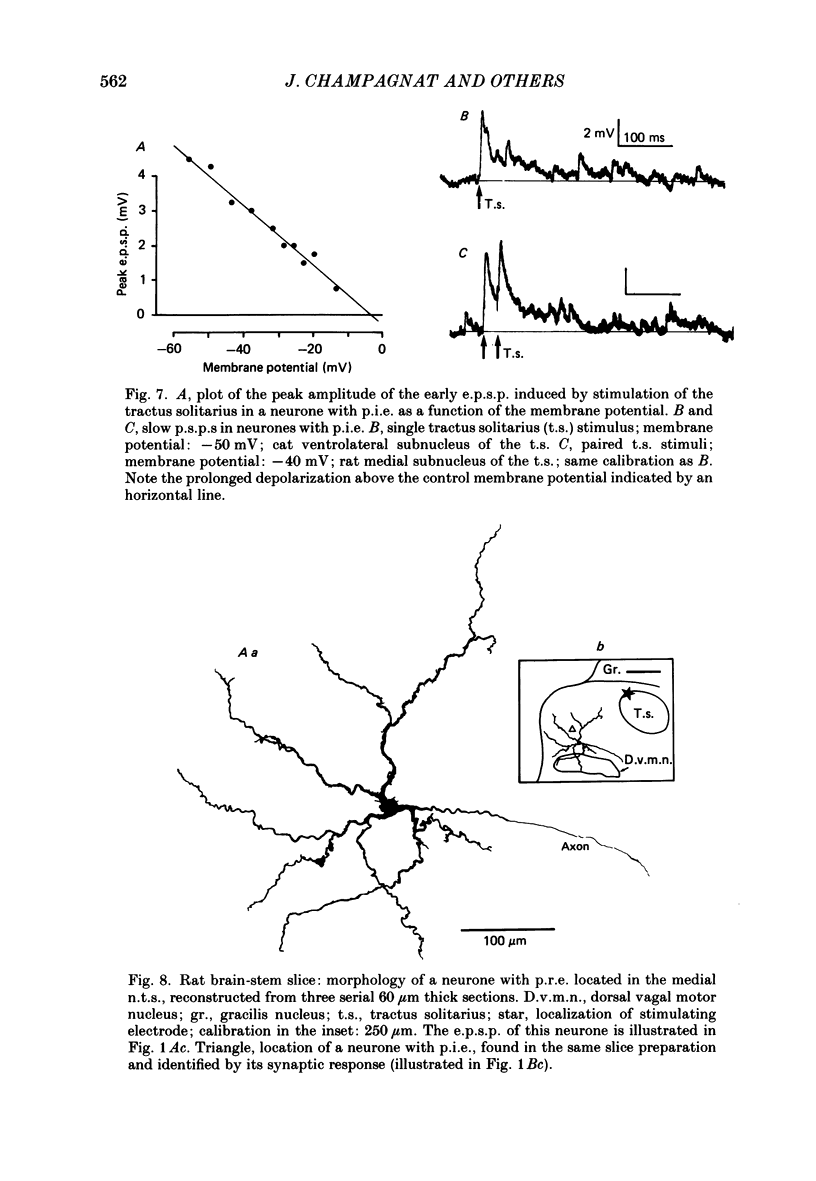
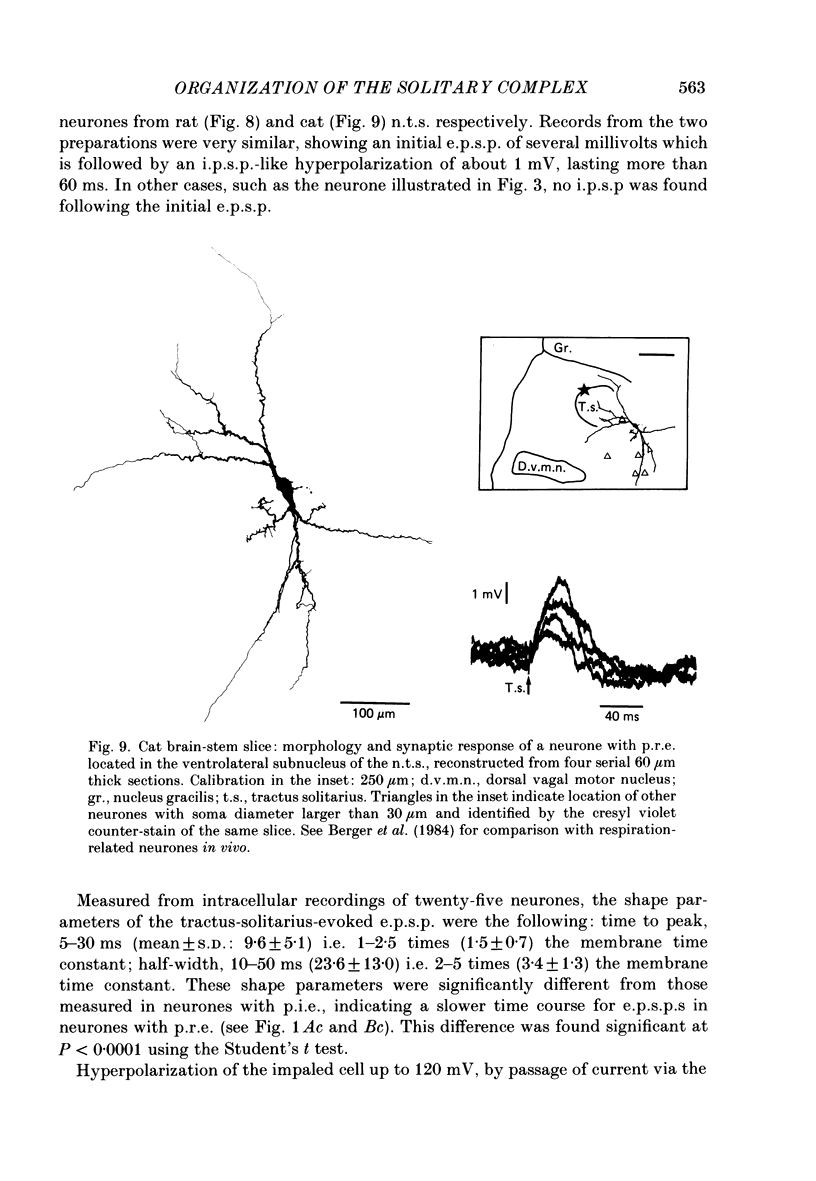
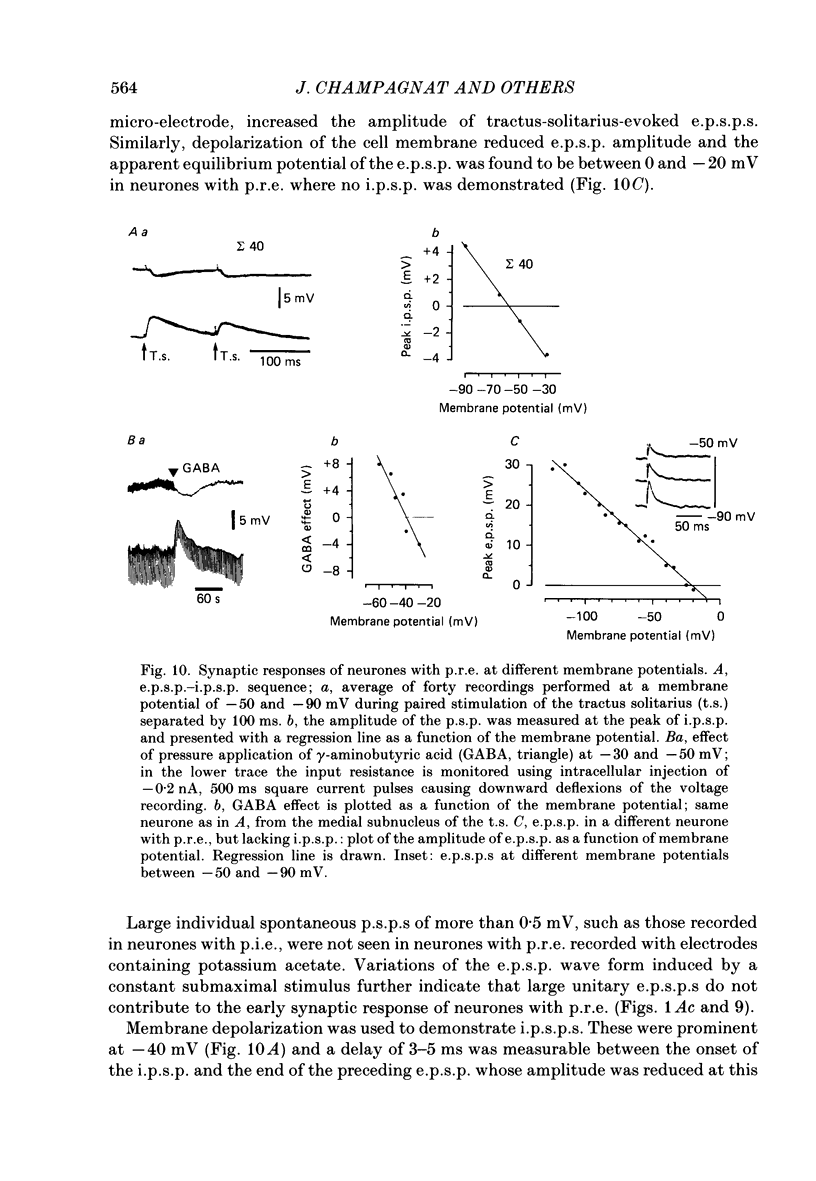

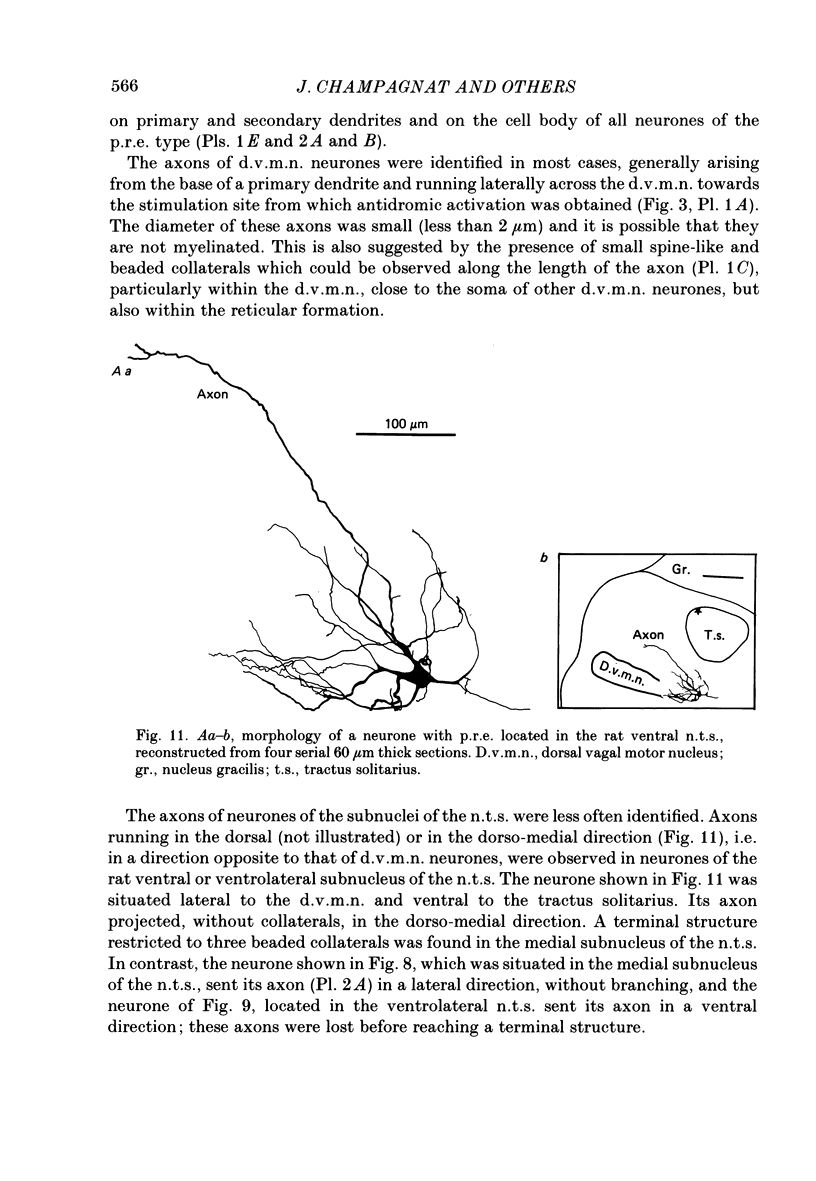






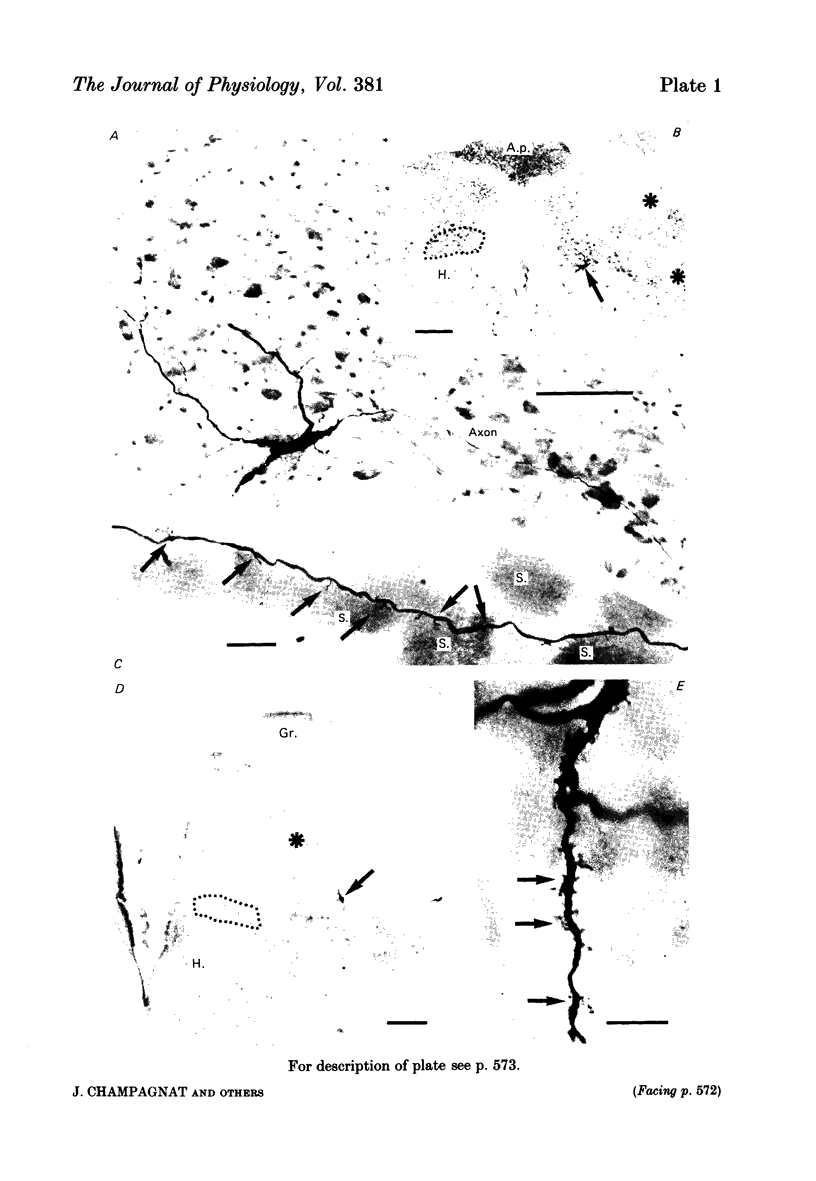
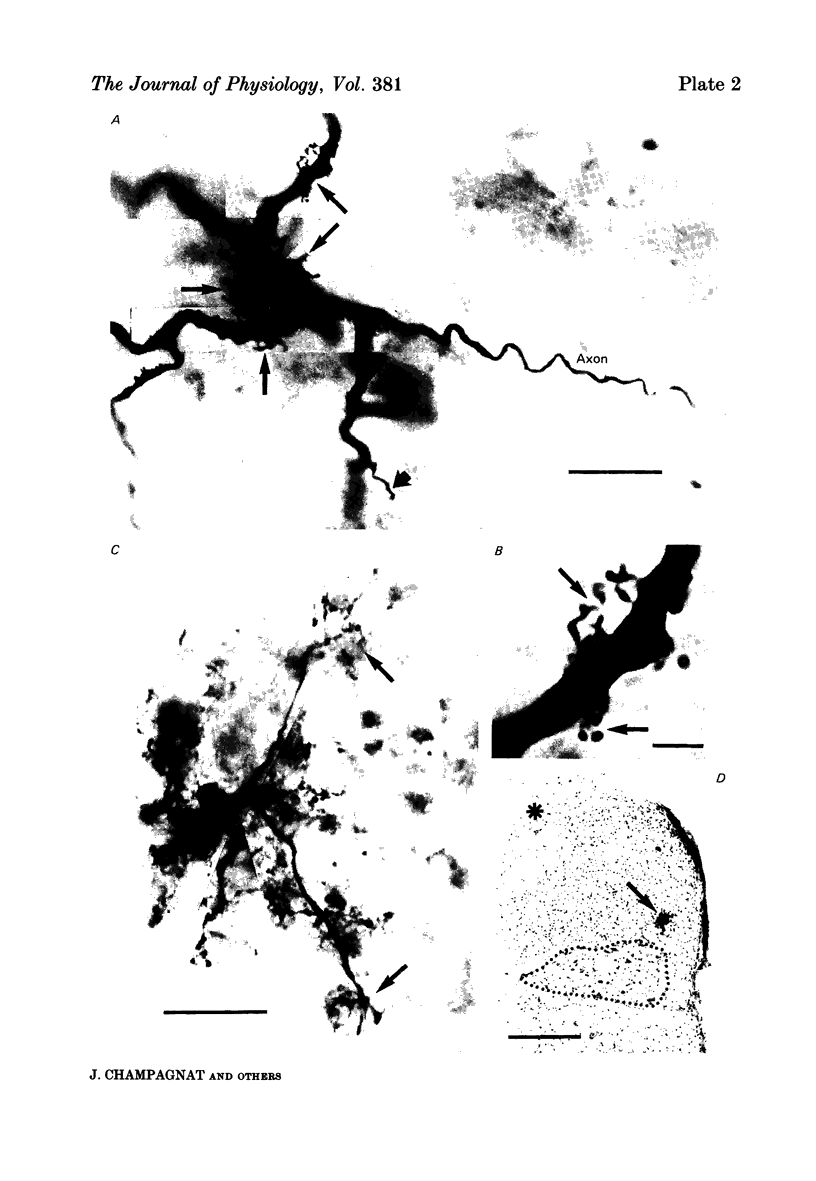

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Backman S. B., Anders C., Ballantyne D., Röhrig N., Camerer H., Mifflin S., Jordan D., Dickhaus H., Spyer K. M., Richter D. W. Evidence for a monosynaptic connection between slowly adapting pulmonary stretch receptor afferents and inspiratory beta neurones. Pflugers Arch. 1984 Oct;402(2):129–136. doi: 10.1007/BF00583324. [DOI] [PubMed] [Google Scholar]
- Bell C. C., Finger T. E., Russell C. J. Central connections of the posterior lateral line lobe in mormyrid fish. Exp Brain Res. 1981;42(1):9–22. doi: 10.1007/BF00235724. [DOI] [PubMed] [Google Scholar]
- Berger A. J., Averill D. B., Cameron W. E. Morphology of inspiratory neurons located in the ventrolateral nucleus of the tractus solitarius of the cat. J Comp Neurol. 1984 Mar 20;224(1):60–70. doi: 10.1002/cne.902240106. [DOI] [PubMed] [Google Scholar]
- Berger A. J., Averill D. B. Projection of single pulmonary stretch receptors to solitary tract region. J Neurophysiol. 1983 Mar;49(3):819–830. doi: 10.1152/jn.1983.49.3.819. [DOI] [PubMed] [Google Scholar]
- Champagnat J., Denavit-Saubie M., Siggins G. R. Rhythmic neuronal activities in the nucleus of the tractus solitarius isolated in vitro. Brain Res. 1983 Nov 28;280(1):155–159. doi: 10.1016/0006-8993(83)91184-8. [DOI] [PubMed] [Google Scholar]
- Champagnat J., Jacquin T., Richter D. W. Voltage-dependent currents in neurones of the nuclei of the solitary tract of rat brainstem slices. Pflugers Arch. 1986 Apr;406(4):372–379. doi: 10.1007/BF00590939. [DOI] [PubMed] [Google Scholar]
- Champagnat J., Siggins G. R., Koda L. Y., Denavit-Saubié M. Synaptic responses of neurons of the nucleus tractus solitarius in vitro. Brain Res. 1985 Jan 28;325(1-2):49–56. doi: 10.1016/0006-8993(85)90301-4. [DOI] [PubMed] [Google Scholar]
- Chiba T., Kato M. Synaptic structures and quantification of catecholaminergic axons in the nucleus tractus solitarius of the rat: possible modulatory roles of catecholamines in baroreceptor reflexes. Brain Res. 1978 Aug 4;151(2):323–338. doi: 10.1016/0006-8993(78)90888-0. [DOI] [PubMed] [Google Scholar]
- Cohen M. I. Neurogenesis of respiratory rhythm in the mammal. Physiol Rev. 1979 Oct;59(4):1105–1173. doi: 10.1152/physrev.1979.59.4.1105. [DOI] [PubMed] [Google Scholar]
- Connors B. W., Gutnick M. J., Prince D. A. Electrophysiological properties of neocortical neurons in vitro. J Neurophysiol. 1982 Dec;48(6):1302–1320. doi: 10.1152/jn.1982.48.6.1302. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Hösli L., Johnston G. A., Johnston I. H. The hyperpolarization of spinal motoneurones by glycine and related amino acids. Exp Brain Res. 1968;5(3):235–258. doi: 10.1007/BF00238666. [DOI] [PubMed] [Google Scholar]
- Davies J. G., Kirkwood P. A., Sears T. A. The detection of monosynaptic connexions from inspiratory bulbospinal neurones to inspiratory motoneurones in the cat. J Physiol. 1985 Nov;368:33–62. doi: 10.1113/jphysiol.1985.sp015845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denavit-Saubié M., Champagnat J., Zieglgänsberger W. Effects of opiates and methionine-enkephalin on pontine and bulbar respiratory neurones of the cat. Brain Res. 1978 Oct 20;155(1):55–67. doi: 10.1016/0006-8993(78)90305-0. [DOI] [PubMed] [Google Scholar]
- Donoghue S., Felder R. B., Gilbey M. P., Jordan D., Spyer K. M. Post-synaptic activity evoked in the nucleus tractus solitarius by carotid sinus and aortic nerve afferents in the cat. J Physiol. 1985 Mar;360:261–273. doi: 10.1113/jphysiol.1985.sp015616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge F. L., Gill-Kumar P., Millhorn D. E. Input-output relationships of central neural circuits involved in respiration in cats. J Physiol. 1981 Feb;311:81–95. doi: 10.1113/jphysiol.1981.sp013574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel M., Seller H. Interaction of baroreceptor afferents from carotid sinus and aorta at the nucleus tractus solitarii. Pflugers Arch. 1970;318(1):7–20. doi: 10.1007/BF00588539. [DOI] [PubMed] [Google Scholar]
- Higgins G. A., Hoffman G. E., Wray S., Schwaber J. S. Distribution of neurotensin-immunoreactivity within baroreceptive portions of the nucleus of the tractus solitarius and the dorsal vagal nucleus of the rat. J Comp Neurol. 1984 Jun 20;226(2):155–164. doi: 10.1002/cne.902260202. [DOI] [PubMed] [Google Scholar]
- Jack J. J., Redman S. J., Wong K. The components of synaptic potentials evoked in cat spinal motoneurones by impulses in single group Ia afferents. J Physiol. 1981 Dec;321:65–96. doi: 10.1113/jphysiol.1981.sp013972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson O., Hökfelt T., Elde R. P. Immunohistochemical distribution of somatostatin-like immunoreactivity in the central nervous system of the adult rat. Neuroscience. 1984 Oct;13(2):265–339. doi: 10.1016/0306-4522(84)90233-1. [DOI] [PubMed] [Google Scholar]
- Jordan D., Khalid M. E., Schneiderman N., Spyer K. M. The location and properties of preganglionic vagal cardiomotor neurones in the rabbit. Pflugers Arch. 1982 Nov 11;395(3):244–250. doi: 10.1007/BF00584817. [DOI] [PubMed] [Google Scholar]
- Joseph S. A., Pilcher W. H., Bennett-Clarke C. Immunocytochemical localization of ACTH perikarya in nucleus tractus solitarius: evidence for a second opiocortin neuronal system. Neurosci Lett. 1983 Aug 8;38(3):221–225. doi: 10.1016/0304-3940(83)90372-5. [DOI] [PubMed] [Google Scholar]
- Kalia M., Mesulam M. M. Brain stem projections of sensory and motor components of the vagus complex in the cat: II. Laryngeal, tracheobronchial, pulmonary, cardiac, and gastrointestinal branches. J Comp Neurol. 1980 Sep 15;193(2):467–508. doi: 10.1002/cne.901930211. [DOI] [PubMed] [Google Scholar]
- Kirchheim H. R. Systemic arterial baroreceptor reflexes. Physiol Rev. 1976 Jan;56(1):100–177. doi: 10.1152/physrev.1976.56.1.100. [DOI] [PubMed] [Google Scholar]
- Krnjević K., Puil E., Werman R. GABA and glycine actions on spinal motoneurons. Can J Physiol Pharmacol. 1977 Jun;55(3):658–669. doi: 10.1139/y77-090. [DOI] [PubMed] [Google Scholar]
- McLean J. H., Hopkins D. A. Ultrastructural identification of labeled neurons in the dorsal motor nucleus of the vagus nerve following injections of horseradish peroxidase into the vagus nerve and brainstem. J Comp Neurol. 1982 Apr 10;206(3):243–252. doi: 10.1002/cne.902060304. [DOI] [PubMed] [Google Scholar]
- Mei N., Condamin M., Boyer A. The composition of the vagus nerve of the cat. Cell Tissue Res. 1980;209(3):423–431. doi: 10.1007/BF00234756. [DOI] [PubMed] [Google Scholar]
- Merrill E. G., Lipski J., Kubin L., Fedorko L. Origin of the expiratory inhibition of nucleus tractus solitarius inspiratory neurones. Brain Res. 1983 Mar 14;263(1):43–50. doi: 10.1016/0006-8993(83)91198-8. [DOI] [PubMed] [Google Scholar]
- Miller A. J. Deglutition. Physiol Rev. 1982 Jan;62(1):129–184. doi: 10.1152/physrev.1982.62.1.129. [DOI] [PubMed] [Google Scholar]
- Morin-Surun M. P., Champagnat J., Boudinot E., Denavit-Saubie M. Differentiation of two respiratory areas in the cat medulla using kainic acid. Respir Physiol. 1984 Dec;58(3):323–334. doi: 10.1016/0034-5687(84)90008-2. [DOI] [PubMed] [Google Scholar]
- Paintal A. S. Vagal sensory receptors and their reflex effects. Physiol Rev. 1973 Jan;53(1):159–227. doi: 10.1152/physrev.1973.53.1.159. [DOI] [PubMed] [Google Scholar]
- Rall W., Burke R. E., Smith T. G., Nelson P. G., Frank K. Dendritic location of synapses and possible mechanisms for the monosynaptic EPSP in motoneurons. J Neurophysiol. 1967 Sep;30(5):1169–1193. doi: 10.1152/jn.1967.30.5.1169. [DOI] [PubMed] [Google Scholar]
- Richter D. W. Generation and maintenance of the respiratory rhythm. J Exp Biol. 1982 Oct;100:93–107. doi: 10.1242/jeb.100.1.93. [DOI] [PubMed] [Google Scholar]
- Ritchie T. C., Westlund K. N., Bowker R. M., Coulter J. D., Leonard R. B. The relationship of the medullary catecholamine containing neurones to the vagal motor nuclei. Neuroscience. 1982 Jun;7(6):1471–1482. doi: 10.1016/0306-4522(82)90258-5. [DOI] [PubMed] [Google Scholar]
- Shapiro R. E., Miselis R. R. The central organization of the vagus nerve innervating the stomach of the rat. J Comp Neurol. 1985 Aug 22;238(4):473–488. doi: 10.1002/cne.902380411. [DOI] [PubMed] [Google Scholar]
- Spyer K. M. Central nervous integration of cardiovascular control. J Exp Biol. 1982 Oct;100:109–128. doi: 10.1242/jeb.100.1.109. [DOI] [PubMed] [Google Scholar]
- Takagi H., Kubota Y., Mori S., Tateishi K., Hamaoka T., Tohyama M. Fine structural studies of cholecystokinin-8-like immunoreactive neurons and axon terminals in the nucleus of tractus solitarius of the rat. J Comp Neurol. 1984 Aug 10;227(3):369–379. doi: 10.1002/cne.902270307. [DOI] [PubMed] [Google Scholar]
- Urbán L., Randić M. Slow excitatory transmission in rat dorsal horn: possible mediation by peptides. Brain Res. 1984 Jan 9;290(2):336–341. doi: 10.1016/0006-8993(84)90952-1. [DOI] [PubMed] [Google Scholar]
- van der Kooy D., Koda L. Y., McGinty J. F., Gerfen C. R., Bloom F. E. The organization of projections from the cortex, amygdala, and hypothalamus to the nucleus of the solitary tract in rat. J Comp Neurol. 1984 Mar 20;224(1):1–24. doi: 10.1002/cne.902240102. [DOI] [PubMed] [Google Scholar]
- von Euler C., Hayward J. N., Marttila I., Wyman R. J. Respiratory neurones of the ventrolateral nucleus of the solitary tract of cat: vagal input, spinal connections and morphological identification. Brain Res. 1973 Oct 26;61:1–22. doi: 10.1016/0006-8993(73)90512-x. [DOI] [PubMed] [Google Scholar]