Abstract
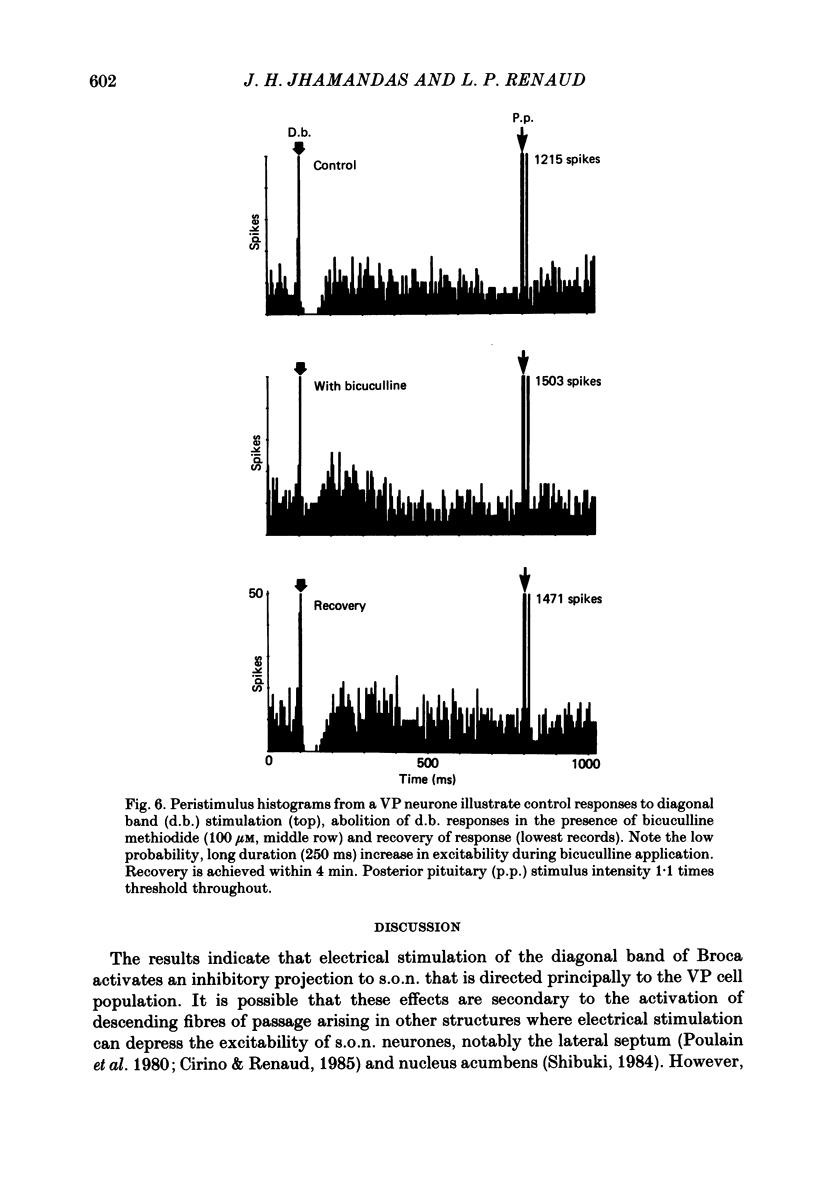
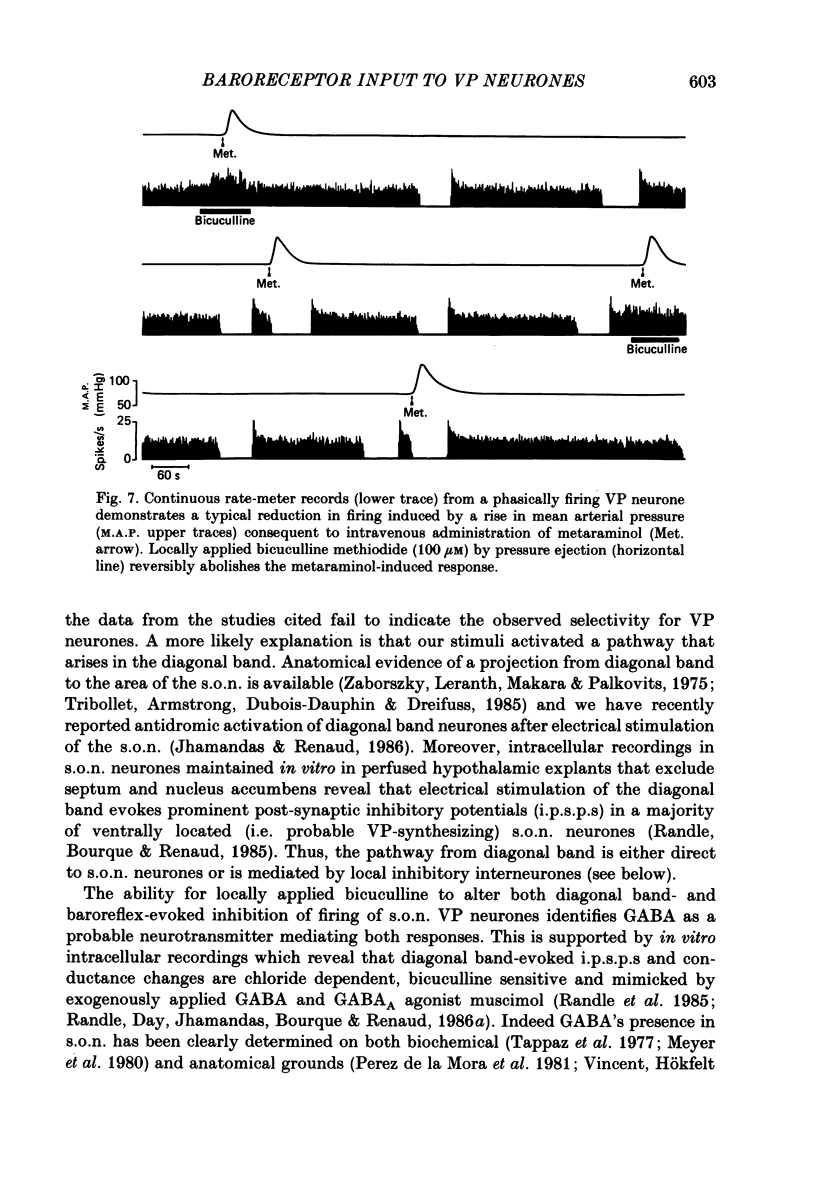
1. Extracellular recordings in pentobarbitone anaesthetized male Long-Evans rats examined the influence of electrical stimulation in the diagonal band of Broca on the excitability of 113 putative vasopressin-secreting and 22 putative oxytocin-secreting neurosecretory neurones in the hypothalamic supraoptic nucleus. 2. Single pulse or repetitive (5-20 Hz) stimulation in the ventral part of the diagonal band evoked a prominent reduction in the excitability of 83% of vasopressin-secreting neurones with no effect on the remainder. Amongst oxytocin-secreting neurones, 59% were unresponsive, 27% responded with an increase in activity while only 14% revealed an inhibitory pattern similar to vasopressin-secreting neurones. 3. Diagonal band stimulation-evoked inhibitions were reversibly abolished by local pressure applications of bicuculline methiodide (100 microM) to twenty out of twenty vasopressin secreting cells tested, whereas strychnine sulphate (100 microM) was without effect on four out of four cells tested. 4. In five out of five vasopressin-secreting cells tested, bicuculline applications reversibly abolished the reduction in their activity that follows peripheral baro-receptor activation. Failure to alter baroreflex-evoked depressions in firing during similar trials with prazosin hydrochloride (10 microM, six cells tested), timolol maleate (20 microM, six cells tested) or strychnine sulphate (100 microM, three cells tested) indicated the specificity of bicuculline's action. 5. These findings suggest that a GABAergic pathway from the diagonal band of Broca preferentially innervates vasopressin-secreting neurosecretory supraoptic nucleus (s.o.n.) neurones, and support the view that the baroreflex-induced depression in firing of s.o.n. vasopressin-secreting neurones is mediated in large part through this input.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong W. E., Gallagher M. J., Sladek C. D. Noradrenergic stimulation of supraoptic neuronal activity and vasopressin release in vitro: mediation by an alpha 1-receptor. Brain Res. 1986 Feb 12;365(1):192–197. doi: 10.1016/0006-8993(86)90739-0. [DOI] [PubMed] [Google Scholar]
- Armstrong W. E., Sladek C. D., Sladek J. R., Jr Characterization of noradrenergic control of vasopressin release by the organ-cultured rat hypothalamo-neurohypophyseal system. Endocrinology. 1982 Jul;111(1):273–279. doi: 10.1210/endo-111-1-273. [DOI] [PubMed] [Google Scholar]
- Arnauld E., Cirino M., Layton B. S., Renaud L. P. Contrasting actions of amino acids, acetylcholine, noradrenaline and leucine enkephalin on the excitability of supraoptic vasopressin-secreting neurons. A microiontophoretic study in the rat. Neuroendocrinology. 1983;36(3):187–196. doi: 10.1159/000123455. [DOI] [PubMed] [Google Scholar]
- Baertschi A. J., Vallet P. G. Osmosensitivity of the hepatic portal vein area and vasopressin release in rats. J Physiol. 1981 Jun;315:217–230. doi: 10.1113/jphysiol.1981.sp013743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks D., Harris M. C. Lesions of the locus coeruleus abolish baroreceptor-induced depression of supraoptic neurones in the rat. J Physiol. 1984 Oct;355:383–398. doi: 10.1113/jphysiol.1984.sp015425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker J. L., Crayton J. W., Nicoll R. A. Noradrenaline and acetylcholine responses of supraoptic neurosecretory cells. J Physiol. 1971 Oct;218(1):19–32. doi: 10.1113/jphysiol.1971.sp009602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bioulac B., Gaffori O., Harris M., Vincent J. D. Effects of acetylcholine, sodium glutamate and GABA on the discharge of supraoptic neurons in the rat. Brain Res. 1978 Oct 6;154(1):159–162. doi: 10.1016/0006-8993(78)91064-8. [DOI] [PubMed] [Google Scholar]
- Blessing W. W., Sved A. F., Reis D. J. Destruction of noradrenergic neurons in rabbit brainstem elevates plasma vasopressin, causing hypertension. Science. 1982 Aug 13;217(4560):661–663. doi: 10.1126/science.6124043. [DOI] [PubMed] [Google Scholar]
- Cirino M., Renaud L. P. Influence of lateral septum and amygdala stimulation on the excitability of hypothalamic supraoptic neurons. An electrophysiological study in the rat. Brain Res. 1985 Feb 11;326(2):357–361. doi: 10.1016/0006-8993(85)90045-9. [DOI] [PubMed] [Google Scholar]
- Davidoff R. A., Aprison M. H., Werman R. The effects of strychnine on the inhibition of interneurons by glycine and gamma-aminobutyric acid. Int J Neuropharmacol. 1969 Mar;8(2):191–194. doi: 10.1016/0028-3908(69)90013-6. [DOI] [PubMed] [Google Scholar]
- Day T. A., Ferguson A. V., Renaud L. P. Facilitatory influence of noradrenergic afferents on the excitability of rat paraventricular nucleus neurosecretory cells. J Physiol. 1984 Oct;355:237–249. doi: 10.1113/jphysiol.1984.sp015416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day T. A., Randle J. C., Renaud L. P. Opposing alpha- and beta-adrenergic mechanisms mediate dose-dependent actions of noradrenaline on supraoptic vasopressin neurones in vivo. Brain Res. 1985 Dec 9;358(1-2):171–179. doi: 10.1016/0006-8993(85)90961-8. [DOI] [PubMed] [Google Scholar]
- Day T. A., Renaud L. P. Electrophysiological evidence that noradrenergic afferents selectively facilitate the activity of supraoptic vasopressin neurons. Brain Res. 1984 Jun 15;303(2):233–240. doi: 10.1016/0006-8993(84)91209-5. [DOI] [PubMed] [Google Scholar]
- Harris M. C. Effects of chemoreceptor and baroreceptor stimulation on the discharge of hypothalamic supraoptic neurones in rats. J Endocrinol. 1979 Jul;82(1):115–125. doi: 10.1677/joe.0.0820115. [DOI] [PubMed] [Google Scholar]
- Jhamandas J. H., Renaud L. P. Diagonal band neurons may mediate arterial baroreceptor input to hypothalamic vasopressin-secreting neurons. Neurosci Lett. 1986 Apr 11;65(2):214–218. doi: 10.1016/0304-3940(86)90307-1. [DOI] [PubMed] [Google Scholar]
- Meyer D. K., Oertel W. H., Brownstein M. J. Deafferentation studies on the glutamic acid decarboxylase content of the supraoptic nucleus of the rat. Brain Res. 1980 Oct 27;200(1):165–168. doi: 10.1016/0006-8993(80)91102-6. [DOI] [PubMed] [Google Scholar]
- Nagai T., McGeer P. L., McGeer E. G. Distribution of GABA-T-intensive neurons in the rat forebrain and midbrain. J Comp Neurol. 1983 Aug 1;218(2):220–238. doi: 10.1002/cne.902180209. [DOI] [PubMed] [Google Scholar]
- Panula P., Revuelta A. V., Cheney D. L., Wu J. Y., Costa E. An immunohistochemical study on the location of GABAergic neurons in rat septum. J Comp Neurol. 1984 Jan 1;222(1):69–80. doi: 10.1002/cne.902220107. [DOI] [PubMed] [Google Scholar]
- Poulain D. A., Ellendorff F., Vincent J. D. Septal connections with identified oxytocin and vasopressin neurones in the supraoptic nucleus of the rat. An electrophysiological investigation. Neuroscience. 1980;5(2):379–387. doi: 10.1016/0306-4522(80)90113-x. [DOI] [PubMed] [Google Scholar]
- Poulain D. A., Wakerley J. B. Electrophysiology of hypothalamic magnocellular neurones secreting oxytocin and vasopressin. Neuroscience. 1982 Apr;7(4):773–808. doi: 10.1016/0306-4522(82)90044-6. [DOI] [PubMed] [Google Scholar]
- Pérez de la Mora M., Possani L. D., Tapia R., Teran L., Palacios R., Fuxe K., Hökfelt T., Ljungdahl A. Demonstration of central gamma-aminobutyrate-containing nerve terminals by means of antibodies against glutamate decarboxylase. Neuroscience. 1981;6(5):875–895. doi: 10.1016/0306-4522(81)90169-x. [DOI] [PubMed] [Google Scholar]
- Randle J. C., Bourque C. W., Renaud L. P. Alpha-adrenergic activation of rat hypothalamic supraoptic neurons maintained in vitro. Brain Res. 1984 Jul 30;307(1-2):374–378. doi: 10.1016/0006-8993(84)90499-2. [DOI] [PubMed] [Google Scholar]
- Randle J. C., Mazurek M., Kneifel D., Dufresne J., Renaud L. P. Alpha 1-adrenergic receptor activation releases vasopressin and oxytocin from perfused rat hypothalamic explants. Neurosci Lett. 1986 Apr 11;65(2):219–223. doi: 10.1016/0304-3940(86)90308-3. [DOI] [PubMed] [Google Scholar]
- Sawchenko P. E., Swanson L. W. The organization of noradrenergic pathways from the brainstem to the paraventricular and supraoptic nuclei in the rat. Brain Res. 1982 Nov;257(3):275–325. doi: 10.1016/0165-0173(82)90010-8. [DOI] [PubMed] [Google Scholar]
- Sgro S., Ferguson A. V., Renaud L. P. Subfornical organ--supraoptic nucleus connections: an electrophysiologic study in the rat. Brain Res. 1984 Jun 11;303(1):7–13. doi: 10.1016/0006-8993(84)90205-1. [DOI] [PubMed] [Google Scholar]
- Sherlock D. A., Field P. M., Raisman G. Retrograde transport of horseradish peroxidase in the magnocellular neurosecretory system of the rat. Brain Res. 1975 May 9;88(3):403–414. doi: 10.1016/0006-8993(75)90653-8. [DOI] [PubMed] [Google Scholar]
- Shibuki K. Supraoptic neurosecretory cells: synaptic inputs from the nucleus accumbens in the rat. Exp Brain Res. 1984;53(2):341–348. doi: 10.1007/BF00238164. [DOI] [PubMed] [Google Scholar]
- Tappaz M. L., Brownstein M. J., Kopin I. J. Glutamate decarboxylase (GAD) and gamma-aminobutyric acid (GABA) in discrete nuclei of hypothalamus and substantia nigra. Brain Res. 1977 Apr 8;125(1):109–121. doi: 10.1016/0006-8993(77)90363-8. [DOI] [PubMed] [Google Scholar]
- Tappaz M. L., Wassef M., Oertel W. H., Paut L., Pujol J. F. Light- and electron-microscopic immunocytochemistry of glutamic acid decarboxylase (GAD) in the basal hypothalamus: morphological evidence for neuroendocrine gamma-aminobutyrate (GABA). Neuroscience. 1983 Jun;9(2):271–287. doi: 10.1016/0306-4522(83)90293-2. [DOI] [PubMed] [Google Scholar]
- Tribollet E., Armstrong W. E., Dubois-Dauphin M., Dreifuss J. J. Extra-hypothalamic afferent inputs to the supraoptic nucleus area of the rat as determined by retrograde and anterograde tracing techniques. Neuroscience. 1985 May;15(1):135–148. doi: 10.1016/0306-4522(85)90128-9. [DOI] [PubMed] [Google Scholar]
- Vincent S. R., Hökfelt T., Wu J. Y. GABA neuron systems in hypothalamus and the pituitary gland. Immunohistochemical demonstration using antibodies against glutamate decarboxylase. Neuroendocrinology. 1982 Feb;34(2):117–125. doi: 10.1159/000123288. [DOI] [PubMed] [Google Scholar]
- Záborszky L., Léránth C., Makara G. B., Palkovits M. Quantitative studies on the supraoptic nucleus in the rat. II. Afferent fiber connections. Exp Brain Res. 1975 May 22;22(5):525–540. doi: 10.1007/BF00237352. [DOI] [PubMed] [Google Scholar]
- van den Pol A. N. Dual ultrastructural localization of two neurotransmitter-related antigens: colloidal gold-labeled neurophysin-immunoreactive supraoptic neurons receive peroxidase-labeled glutamate decarboxylase- or gold-labeled GABA-immunoreactive synapses. J Neurosci. 1985 Nov;5(11):2940–2954. doi: 10.1523/JNEUROSCI.05-11-02940.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]


