Abstract
Methods for catalytically constructing of N–N axially chiral scaffolds have garnered significant attention since such compounds are widely present in natural products, bioactive molecules, and organic materials. Herein, we report a highly diastereoselective and enantioselective organocatalyzed [4 + 1] annulation method for synthesizing diverse valuable isoindolinones that possessing N–N axial and central chiralities. This methodology uses a chiral phosphoric acid as a bifunctional catalyst to promote a cascade sequence involving two nucleophilic additions, dehydration, and dearomatization processes. Control experiments and DFT calculations revealed a possible mechanism in which the stereoselectivity-determining step is likely to involve the irreversible formation of a hydroxy biaryl intermediate. Additionally, preliminary biological activity studies showed that some of these N–N axially chiral isoindolinones have potential in suppressing tumor-cell proliferation.
Subject terms: Synthetic chemistry methodology, Stereochemistry
Methods for catalytically constructing of N–N axially chiral scaffolds have garnered significant attention. Here, the authors report a highly diastereoselective and enantioselective organocatalyzed [4 + 1] annulation method for synthesizing diverse valuable isoindolinones that possessing N–N axial and central chiralities.
Introduction
Catalytic enantioselective synthesis of atropisomers has gained substantial attention over the past few years owing to its great potential in drug discovery, catalyst/ligand design and functional material development1–7, with significant achievements reported for the syntheses of C–C and C–N axially chiral compounds7–26. In sharp contrast, studies into N–N atropisomers and their syntheses remain largely underdeveloped and challenging, presumably due to the relatively low rotational barriers associated with N–N bonds. Although the N–N chiral axis is widely present in natural products, bioactive molecules, and organic materials27–33, the Lu group first reported the construction of N–N atropisomers in 202134. Since then, several strategies have been established for the construction of N–N atroposelective scaffolds (Fig. 1a)35–48. Organocatalyzed N–H alkylation and acylation reactions have been developed to access 1-aminopyrroles and 3-aminoquinazolinones36–39, while, Liu realized the synthesis of N–N bispyrroles through the use of Cu-catalyzed desymmetric Friedel–Crafts alkylation chemistry40. In addition, asymmetric annulation chemistry involving the de novo formation of aza-arenes41–45, and the C–H functionalization of pro-chiral N–N biaryls also provide facile access to N–N chiral bisindoles and N-pyrrolylindoles46,48. More recently, Li and Niu independently developed C–H activation/annulation for the atroposelective synthesis of N-aminoisoquinolinone49,50. Despite these advances, the development of methods for accessing novel N–N axially chiral scaffolds in a facile manner remains highly demanding yet challenging.
Fig. 1. Facile synthesis of unprecedented N − N atropisomers and our design.
a Status of catalytic construction of N − N atropisomers. b Concept of our design for catalytic synthesis of N − N atropisomeric isoindolinones bearing central chirality. c This work: synthesis of isoindolinones possessing N − N axial and central chirality. CPA chiral phosphoric acid, E electrophile.
In recent years, the catalytic enantioselective construction of centrally chiral atropisomers has become an emerging field because it offers a significant opportunity to expand potential applications by integrating new properties into atropisomers51–53. In sharp contrast to the widely explored multichiral C–C and C–N atropisomers, constructing N–N atropisomers with central chirality remains largely undeveloped (Fig. 1a)54,55. The generation of more than one chiral element in the catalytic process presents formidable challenges for both diastereo- and enantio-control. Bencivenni recently reported chemistry for the indirect synthesis of atropoisomeric hydrazides via a one-pot sequence involving two organocatalytic cycles54. However, direct approaches that provide highly stereoselective access to atropisomers bearing N–N axial and central chiralities appear to be unprecedented, despite being highly attractive.
On the other hand, isoindolinone motifs form the core structures of a variety of natural products and pharmacologically relevant molecules56–58. We recently became aware of a report detailing a facile access to isoindolinones by N-capping primary amines with 2-acylbenzaldehydes59–61; this transformation was assumed to involve an acid-promoted condensation-tautomerization cascade. In light of this stimulating work and recent advances in stereocontrol catalysed by chiral phosphoric acids (CPAs)11–14,62–65, we envisaged the possibility of synthesizing N–N atropisomers by harnessing asymmetric [4 + 1] annulation chemistry involving bulky hydrazine and an ortho-formyl-benzophenone in which contiguous axial and central chiralities are established through a CPA-catalyzed aromatization and dearomatization cascade process (Fig. 1b). In this strategy, 2-acylbenzaldehydes and hydrazines are used as 1,4-dielectrophiles and 1,1-dinucleophiles, respectively. A CPA is a suitable bifunctional catalyst that can promote sequential double nucleophilic additions and dehydration to generate reactive hydroxyisoindoline intermediate III, which is prone to asymmetric tautomerization (Path I). Another possible scenario involves the use of electrophilic reagents or species that can potentially react with III in an asymmetric dearomatization manner to furnish a N–N atropoisomer with a quaternary stereocenter (Path II). Herein, we present efficient chemistry for the synthesis of N–N atropoisomeric isoindolinones involving a highly diastereo- and enantioselective [4 + 1] annulation reaction (Fig. 1c). This transformation not only represents the first highly stereoselective and catalytic method for constructing centrally chiral N–N atropisomers, but also provides access to a new N–N atropoisomer family members that are potentially biologically active.
Results
Reaction development
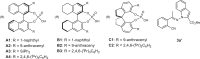
To test our hypothesis, we chose 2-acyl-benzaldehyde 2a as the 1,4-dielectrophile, N-aminoindole 1a as the dinucleophile in view of its good nucleophilicity as well as the wide existence of axially chiral indole scaffolds in bioactive compounds and natural products (Table 1). To our delight, BINOL-derived CPA (R)-A1 successfully catalyzed the expected asymmetric aromatization and tautomerization processes, to afford the N–N axially chiral isoindolinone 3a in 33% yield, 25% ee, and 10:1 dr (entry 1). We next examined various BINOL- and SPINOL-derived CPA catalysts bearing aryl substituents with varying electronic properties and steric effects (entries 2–9). Among these, (S)-A4 was identified as the optimal one, affording 3a with 53% yield, 91% ee, and 13:1 dr (entry 4). Solvent screening (see the Supplementary Information for details) showed that o-xylene and PhCl performed competitively, whereas DCE led to a sharp decrease in ee, albeit with a higher yield (entries 10–12). Toluene was determined to be the best solvent. Moreover, an enhanced yield and a slightly higher ee were obtained by decreasing the temperature to –20 °C (entry 13). Finally, reducing the catalyst loading to 5 mol% improved the yield to 73%, along with 93% ee and >20:1 dr (entry 14). It is worth mentioning that by-product 3a’ was observed in yields of less than 10% in these optimization studies (see the Supplementary Information for details)66.
Table 1.
Optimization of the reaction conditionsa
| Entry | Catalyst | Solvent | Yield (%)b | ee (%)c | drd |
|---|---|---|---|---|---|
| 1 | (R)-A1 | Toluene | 33 | −25 | 10:1 |
| 2 | (R)-A2 | Toluene | 23 | −7 | 17:1 |
| 3 | (R)-A3 | Toluene | 54 | −26 | 12:1 |
| 4 | (S)-A4 | Toluene | 53 | 91 | 13:1 |
| 5 | (R)-B1 | Toluene | 56 | −22 | 12:1 |
| 6 | (R)-B2 | Toluene | 60 | −9 | 10:1 |
| 7 | (R)-B3 | Toluene | 42 | −40 | 15:1 |
| 8 | (R)-C1 | Toluene | trace | ND | ND |
| 9 | (R)-C2 | Toluene | trace | ND | ND |
| 10 | (S)-A4 | DCE | 63 | 28 | 8:1 |
| 11 | (S)-A4 | o-Xylene | 50 | 89 | 10:1 |
| 12 | (S)-A4 | PhCl | 51 | 89 | 10:1 |
| 13e | (S)-A4 | Toluene | 65 | 92 | >20:1 |
| 14e, f | (S)-A4 | Toluene | 73 | 93 | >20:1 |
| 15e, g | (S)-A4 | Toluene | 61 | 91 | >20:1 |
 | |||||
aReaction conditions: 1a (0.1 mmol), 2a (0.05 mmol), catalyst (10 mol%), toluene (0.5 mL).
bIsolated yields are provided.
cThe ee values were determined by chiral HPLC.
dDiastereomeric ratios (dr) were determined from 1H NMR of the isolated product.
eReaction performed at −20 °C.
f5 mol% of (S)-A4 was used.
g2.5 mol% of (S)-A4 was used. DCE, 1,2-dichloroethane; PhCl, chlorobenzene.
Substrates scope exploration
Having identified the optimal conditions (entry 14, Table 1), we next examined the scope of the asymmetric [4 + 1] annulation reaction (Fig. 2). A wide range of N-aminoindoles 1 bearing substituents with different electronic properties at the C5, C4, and C3 positions exhibited good reactivities in this reaction, with products 3a–3k formed in yields of 54–77% with excellent enantioselectivities and diastereoselectivities (89–98% ee, >20:1 dr). Other ester and even amide groups at the C2 position of the indole ring were also tolerated, to afford 3 l and 3 m in moderate-to-good yields and high stereoselectivities. To further broaden the substrate scope, we also examined N-aminopyrroles. To ensure that a sufficiently rotationally restricted N–N axis was generated, we initially examined the use of 2,4-disubstituted N-aminopyrrole 1n. Gratifyingly, the desired product 3n was efficiently formed (64% yield, >99% ee, >20:1 dr) using (R)-C2 as the catalyst. Moreover, multi-substituted N-aminopyrroles also performed well to afford 3o and 3p. In addition, various ester units were well tolerated in this system, with 3q–3t produced in good yields and excellent stereoselectivities (56–72% yield, 94–96% ee, >20:1 dr). The absolute configurations of 3i and 3n were assigned to be (RN-N, SC) and (RN-N, RC), respectively, by X-ray crystallography.
Fig. 2. Scope of the asymmetric [4 + 1] annulation for N–N axially chiral isoindolinones.
Reaction conditions: 1 (0.4 mmol), 2 (0.2 mmol), (S)-A4 (5 mol%), toluene (2 mL), −20 °C, 36 h. Isolated yields. a(R)-C2 (5 mol%) was used as catalyst, 25 °C, 24 h. Tol., toluene.
We next studied the feasibility of asymmetric [4 + 1] annulation chemistry involving different 2-acylbenzaldehydes. A variety of 2-acetylbenzaldehydes bearing substituents with different electronic properties at the C4–C6 positions reacted smoothly with 1a to highly efficiently and stereoselectively afford N–N axially chiral isoindolinones 3u–3aa (57–74% yield, 88–99% ee, >20:1 dr). Changing of the methyl group to n-propyl did not significantly affect the efficiency or stereoselectivity of the reaction.
In addition, we also attempted to construct a contiguous N–N axis and quaternary stereocenter by harnessing an appropriate electrophile to trap the in-situ generated hydroxyisoindoline intermediate III in presence of a CPA; however, our efforts were unsuccessful. The enol species significantly favored tautomerization over nucleophilic addition in the acidic system. Interestingly, N-aminoindole 1 bearing a bulky CO2iPr or CO2tBu ester moiety at the C2 position, underwent an unexpected condensation and oxidative asymmetric dearomatization (Fig. 3). This transformation predominantly delivered N–N axially chiral isoindolinonyl hydroperoxide 4a or 4b bearing a quaternary stereocenter with excellent stereoselectivities (92–97% ee, >20:1 dr). The structure and absolute configuration of 4b were confirmed by X-ray crystallography. We subsequently expanded the scope of this chiral isoindolinonyl hydroperoxide-forming chemistry; 2-acylbenzaldehydes bearing either electron-donating or electron-withdrawing groups performed well in this transformation, to highly efficiently yield 4c–4 f with excellent stereoselectivities. A n-propyl or aryl substituent was well tolerated, rendering products 4g–4k with high efficiency (56–64% yields, 85–96% ee and >20:1 dr), irrespective of the electronic nature or position of the substituent on the phenyl ring. The protocol also tolerated 5-methyl- and 5-bromo-substituted N-aminoindoles, furnishing 4l–4o in moderate-to-good yields with excellent stereocontrol (94–98% ee, >20:1). Also, 1 y, which is derived from (–)-borneol, was a suitable substrate for preparing 4p, albeit in notably lower yield.
Fig. 3. Scope of N–N axially chiral isoindolinones bearing a quaternary stereocenter.
Reaction conditions: 1 (0.4 mmol), 2 (0.2 mmol), (S)-A4 (5 mol%), toluene (2 mL), −20 °C, 36 h. Isolated yields. a0 °C, 72 h. bp-Xylene, 25 °C, 24 h.
Moreover, the chiral products 3p, 3 u, 4 f and 4j were stirred in toluene at 110 °C for 12 h. Such four compounds could be recovered in high yields, with high enantioselectivities and diastereoselectivities retained, which indicated that the N–N axially chiral isoindolinones have high chemical stability and configurational stability (see the Supplementary Information for details).
Synthetic applications and mechanistic studies
To further highlight the synthetic practicality and utility of this catalytic method, we performed scaled-up experiments and further transformed the chiral products (Fig. 4). 1e was reacted with 2a, while 1o was reacted with 2a, each on a 1 mmol scale, with products 3e and 3n obtained without obvious erosions in yield or stereoselectivity (Fig. 4a, b). In addition, selective C3-bromination of the pyrrole ring of 3n proceeded well to form 3p, which underwent further Suzuki-coupling with minimal erosion of enantiopurity (Fig. 4c). The ester substituent in 3n was readily converted into a carboxyl group, with the high ee of the starting material maintained.
Fig. 4. Scale-up experiments and synthetic application.
a, b Mmol-scale synthesis. c Transformations of enantioenriched products. NBS N-Bromosuccinimide, DMF N,N-Dimethylformamide, THF tetrahydrofuran.
A series of control experiments were carried out to gain some insights into the reaction mechanism (Fig. 5). First, phthalaldehyde was reacted with 1e to form the desired product 7, whereas the 1,2-diacetylbenzene reacted with 1e to afford a complex mixture with no cyclized compound detected (Fig. 5, eq 1 and 2), which indicates that dehydration is essential for the [4 + 1] annulation reaction. Moreover, treating side product 3a’ with H2O (1 equiv.) under typical conditions did not afford 3a (Fig. 5, eq 3), which suggests that 3a’ is not a reaction intermediate. Interestingly, replacing the CPA catalyst with achiral diphenylphosphoric acid provided racemic products 3i and 3n with excellent diastereoselectivities (Fig. 5, eq 4 and 5). In addition, we treated 4 d’ under the standard conditions to shed light on the formation of hydroperoxide; 4 d was not formed under these conditions, thereby excluding the involvement of a direct oxidation pathway (Fig. 5, eq 6). Some deuterium experiments were conducted (see the Supplementary Information for details). We introduced RPA1-D to the reaction of 1a and 2a, providing product 3a in 53% yield with 24% deuterium incorporation. The result indicates that CPA works as an acid and proton source in the reaction for 3a.
Fig. 5. Control experiments.
Control experiments for the [4+1] annulation or the oxidation process.
Density functional theory studies
We used density functional theory (DFT) calculations to model the formation of isoindolinone 3a by the reaction of hydrazine 1a with 2-acylbenzaldehyde 2a to better understand the mechanism and key steps responsible for the observed stereoselectivity. As shown in Fig. 6a, the first addition of the amino group of 1a to the aldehyde reversibly generates racemic intermediate I, which is 1.4 kcal/mol higher in energy than the reactants. The second addition to the ketone moiety forms intermediate II, which contains two chiral centers and one chiral axis. Consequently, eight stereoisomers are possible. The relative energies of the four diastereomers range from −3.5 to −0.6 kcal/mol (Fig. 6b). The subsequent dehydration and tautomerization, which generates intermediate III and product 3a, respectively, are highly exergonic and irreversible. The calculated rotational barriers for intermediates II, III, and 3a are shown in Fig. 6c. The irreversible formation of intermediate III is highly likely to be the stereodetermining step owing to the calculated high barriers, leading to the formation of only one major enantiomer of III. The exact process by which the chiral phosphoric acid catalyst facilitates this step and controls the stereoselectivity is currently being explored in our laboratories.
Fig. 6. DFT calculations for the reaction free energies of model reaction between 1a and 2a to form product 3a.
a The energy profile of the [4 + 1] annulation of 1a and 2a. b The relative energies of the four different diastereomers II-1 − II-4. c The computed rotational barriers of intermediates II, III, and product 3a.
Additionally, we propose a plausible mechanism for the formation of products 3 and 4 based on our experimental observations and the DFT calculations discussed above, as well as literature precedent (Fig. 7)40–54,67,68. The process begins with acid-promoted addition and dehydration to form intermediate A, which tautomerizes by protonation at the Re face of the hydroxypyrrole-ring plane to give product 3. Alternatively, it is proposed that the oxidative dearomatizaion is initiated by the removal of a proton and the triplet O2 is more likely to oxidize the generated anion A’ to a radical species B through a single-electron transfer (SET) process67,68. Subsequently, the residual superoxide radical anion attacks the C4 position of the pyrrole ring of intermediate B from the Si face to form C, which is finally protonated to afford product 4. Preliminary results from DFT-calculated energetics suggest that this mechanism is feasible. The theoretical result indicated that the deprotonated anion 3b-4 is much easier to be oxidized by triplet oxygen through a SET process to generate radical 3b-2, which is endergonic by 11.0 kcal/mol. The subsequent addition of the superoxide radical anion to radical 3b-2 is highly exergonic (-31.0 kcal/mol), which provides the driving force for the whole process. Moreover, we have performed additional DFT calculations to investigate the thermodynamic preferences for the two types of products (see the Supplementary Information for details). The preliminary results indicate that the formation of 3a (Re face) is thermodynamically preferred. In contrast, the observed product (4b) for the peroxidation (Si face) is less stable than the minor diastereomer 4b-1, which suggests that the CPA catalyst is likely involved in the peroxidation step.
Fig. 7. Proposed mechanism for the generation of products 3 and 4.
Plausible mechanism.
Anticancer performance evaluation
Finally, we were intrigued by the potential biological activities of the synthesized enantioenriched N − N atropisomers. Isoindolinones exhibited various biological activities, including anticancer, antibacterial, antiviral activities, and have been extensively studied as key core scaffolds for diverse drug candidates69–73. Therefore, it is of crucial significance to assess the antitumor performance of our synthesized N − N axially chiral isoindolinones, as shown in Figs. 2, 3. Cell counting kit-8 (CCK-8) assay was used to preliminarily screen the viability of 4T1 cell after treatment with a series of products 3 and 4. The results of CCK-8 illustrated that compounds 3a, 3n, and 4a partially inhibited tumor-cell growth, with the cell viabilities of 66.5%, 56.8%, and 53.1% at the concentration of 50 μM, respectively (Fig. 8a–c). These preliminary results shown that our synthesized N − N axially chiral isoindolinones have potential in inhibiting tumor-cell growth.
Fig. 8. Anticancer performance assessment.
a–c Cell viability of compounds 3a, 3n, and 4a (n = 5 biologically independent samples). d–f Confocal images of 4T1 cells stained with DCFH-DA and calcein-AM/PI after treatment with various doses of compounds 3a, 3n, and 4a. g–i Flow cytometry analysis of apoptotic cells after treatment with different concentrations of compounds 3a, 3n, and 4a.
Generally, overproduction of reactive oxygen species (ROS) is a key marker in the early stage of apoptotic cells. 2’,7’-dichlorodihydrofluorescein diacetate (DCFH-DA) was utilized as the ROS fluorescent probe to assess the intracellular ROS levels after diverse treatments. As displayed in Fig. 8d–f, the confocal images illustrated that the green fluorescence signals of ROS increased with elevating doses of compounds 3a, 3n, and 4a, indicating the effective apoptosis induction. Furthermore, 4T1 cells were treated with increasing concentrations of these compounds, and then labeled with calcein acetoxymethyl ester (calcein-AM) and propidium iodide (PI) to visualize live and dead cells. After treatment with 50 µM of 3a, 3n, and 4a, a distinct red fluorescence signal of PI was observed in 4T1 cells, suggesting their high inhibitory activity against cancer cells. Moreover, flow cytometry analysis was conducted using annexin V-fluorescein isothiocyanate/PI (annexin V-FITC/PI) staining assay to confirm the apoptosis induction by these three compounds (Fig. 8g–i). The results revealed that compounds 3a, 3n, and 4a induced apoptosis of 4T1 cells in a dose-dependent manner, with apoptosis rates of 50.2%, 55% and 41.5% at 50 µM, respectively, which verified that the anticancer activity of compounds 3a, 3n, and 4a may be related to the induction of apoptosis.
Discussion
In summary, we established an unprecedented, highly diastereoselective, and atroposelective protocol for the synthesis of N–N axially chiral isoindolinones that proceeds via a Brønsted acid-catalyzed asymmetric [4 + 1] annulation, which represents the first example of the direct construction of N–N atropisomers with central chirality and excellent stereocontrol. This methodology is promoted by a chiral phosphoric acid that bifunctionally catalyzes a sequence of two nucleophilic additions, dehydration, and dearomatization. Control experiments and DFT calculations revealed that the mechanism possibly involves the irreversible formation of the hydroxy-biaryl intermediate as likely the key step responsible for the observed stereoselectivity. In addition, preliminary biological activity studies revealed that some of these N–N axially chiral isoindolinones exhibited potential tumor-cell inhibitory activity.
Methods
General procedure for the synthesis of enantioenriched 3 and 4
General Procedure
At –20 oC or 25 oC, to an oven-dried 10-mL vial charged with a solution of the 1H-indol-1-amine or 2-methyl-1H-pyrrol-1-amine 1 (0.4 mmol) and the substituted 2-acetylbenzaldehyde 2a (0.2 mmol) in toluene (1.5 mL) was added a solution of the catalyst (S)-A4 (7.5 mg, 0.01 mmol, 5 mol%) or (R)-C2 (6.7 mg, 0.01 mmol, 5 mol%) in toluene (0.5 mL). The reaction mixture was stirred at the same temperature for 24 h or 36 h. The mixture was concentrated under reduced pressure and then purified by silica gel (deactivated by triethylamine) flash chromatography to afford the desired product 3 or 4.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Source data
Acknowledgements
This work was supported by the National Natural Science Foundation of China (22201073 to X.L., 21925201 to P.-N.L., 22161160319 to P.-N.L., T2322018 to H.X., 22171130 to P.Y., 32171391 to H.X.) and the Fundamental Research Funds for the Central Universities. We thank the Research Center of Analysis and Test of East China University of Science and Technology for help in the characterization. P.Y. acknowledges Shenzhen Science and Technology Program (KQTD20210811090112004) for financial support. Computational work was supported by the Center for Computational Science and Engineering and the CHEM High-Performance Supercomputer Cluster (CHEM-HPC) of the Department of Chemistry, Southern University of Science and Technology.
Author contributions
X.L. conceived, directed the project and wrote the paper. X.-Z.W. and Q.-Y.C. performed and analyzed the experiments. P.Y. and B.S. performed the DFT studies. H.X. designed and performed the biological experiments. P.-N.L. directed the project. All the authors discussed the results and commented on the manuscript.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Data availability
All data generated and analyzed during this study are included in this Article and its Supplementary Information. The X-ray crystallographic coordinate for structures 3i, 3n, and 4b have been deposited at the Cambridge Crystallographic Data Centre under deposition numbers CCDC 2323929 (for 3i), 2323930 (for 3n), and 2323931 (for 4b), respectively and can be obtained free of charge from the CCDC via http://www.ccdc.cam.ac.uk/data_request/cif. All data are available from the corresponding author upon request. Source data are present with this paper. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Xingguang Li, Xin-Ze Wang, Boming Shen.
Contributor Information
Xingguang Li, Email: lixingguang@ecust.edu.cn.
Peiyuan Yu, Email: yupy@sustech.edu.cn.
Pei-Nian Liu, Email: liupn@ecust.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-025-56838-2.
References
- 1.Bringmann, G., Gulder, T., Gulder, T. A. M. & Breuning, M. Atroposelective total synthesis of axially chiral biaryl natural products. Chem. Rev.111, 563–639 (2011). [DOI] [PubMed] [Google Scholar]
- 2.Toenjes, S. T. & Gustafson, J. L. Atropisomerism in medicinal chemistry: challenges and opportunities. Future Med. Chem.10, 409–422 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perreault, S., Chandrasekhar, J. & Patel, L. Atropisomerism in drug discovery: A medicinal chemistry perspective inspired by atropisomeric class I PI3K inhibitors. Acc. Chem. Res.55, 2581–2593 (2022). [DOI] [PubMed] [Google Scholar]
- 4.Zhou, Q.-L. Privileged Chiral Ligands and Catalysts (Wiley, 2011).
- 5.Da, B.-C. & Tan, B. Application for axially chiral ligands. In axially chiral compounds: Asymmetric synthesis and applications part II (Wiley, 2021).
- 6.Erbas-Cakmak, S., Leigh, D. A., McTernan, C. T. & Nussbaumer, A. L. Artificial molecular machines. Chem. Rev.115, 10081–10206 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumarasamy, E., Raghunathan, R., Sibi, M. P. & Sivaguru, J. Nonbiaryl and heterobiaryl atropisomers: molecular templates with promise for atropselective chemical transformations. Chem. Rev.115, 11239–11300 (2015). [DOI] [PubMed] [Google Scholar]
- 8.Wencel-Delord, J., Panossian, A., Leroux, F. R. & Colobert, F. Recent advances and new concepts for the synthesis of axially stereoenriched biaryls. Chem. Soc. Rev.44, 3418–3430 (2015). [DOI] [PubMed] [Google Scholar]
- 9.Link, A. & Sparr, C. Stereoselective arene formation. Chem. Soc. Rev.47, 3804–3815 (2018). [DOI] [PubMed] [Google Scholar]
- 10.Metrano, A. J. & Miller, S. J. Peptide-Based catalysts reach the outer sphere through remote desymmetrization and atroposelectivity. Acc. Chem. Res.52, 199–215 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang, Y.-C., Jiang, F. & Shi, F. Organocatalytic asymmetric synthesis of indole-based chiral heterocycles: strategies, reactions, and outreach. Acc. Chem. Res.53, 425–446 (2020). [DOI] [PubMed] [Google Scholar]
- 12.Shao, Y.-D. & Cheng, D.-J. Chiral phosphoric acid: A powerful organocatalyst for the asymmetric synthesis of heterocycles with chiral atropisomerism. ChemCatChem13, 1271–1289 (2021). [Google Scholar]
- 13.Cheng, J. K., Xiang, S.-H., Li, S., Ye, L. & Tan, B. Recent advances in catalytic asymmetric construction of atropisomers. Chem. Rev.121, 4805–4902 (2021). [DOI] [PubMed] [Google Scholar]
- 14.Cheng, J. K., Xiang, S.-H. & Tan, B. Organocatalytic enantioselective synthesis of axially chiral molecules: Development of strategies and skeletons. Acc. Chem. Res.55, 2920–2937 (2022). [DOI] [PubMed] [Google Scholar]
- 15.Sheng, F.-T., Yang, S., Wu, S.-F., Zhang, Y.-C. & Shi, F. Catalytic asymmetric synthesis of axially chiral 3,3’-bisindoles by direct coupling of indole rings. Chin. J. Chem.40, 2151–2160 (2022). [Google Scholar]
- 16.Hang, Q.-Q. et al. Design and catalytic atroposelective synthesis of axially chiral isochromenone-indoles. Sci. China Chem.65, 1929–1937 (2022). [Google Scholar]
- 17.Liu, Y.-W., Chen, Y.-H., Cheng, J. K., Xiang, S.-H. & Tan, B. Enantioselective synthesis of 3-arylindole atropisomers via organocatalytic indolization of iminoquinones. Chem. Synth.3, 1–8 (2023). [Google Scholar]
- 18.Zhang, X., Zhao, K. & Gu, Z. Transition metal-catalyzed biaryl atropisomer synthesis via a torsional strain promoted ring-opening reaction. Acc. Chem. Res.55, 1620–1633 (2022). [DOI] [PubMed] [Google Scholar]
- 19.Portolani, C., Centonze, G., Righi, P. & Bencivenni, G. Role of cinchona alkaloids in the enantio- and diastereoselective synthesis of axially chiral compounds. Acc. Chem. Res.55, 3551–3571 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li, T.-Z., Liu, S.-J., Tan, W. & Shi, F. Catalytic asymmetric construction of axially chiral indole-based frameworks: an emerging area. Chem. Eur. J.26, 15779–15792 (2020). [DOI] [PubMed] [Google Scholar]
- 21.Kitagawa, O. Chiral Pd-catalyzed enantioselective syntheses of various N–C axially chiral compounds and their synthetic applications. Acc. Chem. Res.54, 719–730 (2021). [DOI] [PubMed] [Google Scholar]
- 22.Colobert, F. & Shi, B.-F. C–N atropopure compounds: new directions. Chem. Catal.1, 483–485 (2021). [Google Scholar]
- 23.Zhang, H.-H. & Shi, F. Organocatalytic atroposelective synthesis of indole derivatives bearing axial chirality: strategies and applications. Acc. Chem. Res.55, 2562–2580 (2022). [DOI] [PubMed] [Google Scholar]
- 24.Wu, Y.-J., Liao, G. & Shi, B.-F. Stereoselective construction of atropisomers featuring a C–N chiral axis. Green Synth. Catal.3, 117–136 (2022). [Google Scholar]
- 25.Rodríguez-Salamanca, P., Fernández, R., Hornillos, V. & Lassaletta, J. M. Asymmetric synthesis of axially chiral C–N atropisomers. Chem. Eur. J.28, e202104442 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang, X. K., Liu, Y.-Z., Shao, H. W. & Ma, X. F. Advances in atroposelectively de novo synthesis of axially chiral heterobiaryl scaffolds. Molecules27, 8517 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang, Q. et al. N–N-Coupled indolo-sesquiterpene atropo-diastereomers from a marine-derived actinomycete. Eur. J. Org. Chem.2012, 5256–5262 (2012). [Google Scholar]
- 28.Xu, Z., Baunach, M., Ding, L. & Hertweck, C. Bacterial synthesis of diverse indole terpene alkaloids by an unparalleled cyclization sequence. Angew. Chem. Int. Ed.51, 10293–10297 (2012). [DOI] [PubMed] [Google Scholar]
- 29.Blair, L. M. & Sperry, J. Natural products containing a nitrogen–nitrogen bond. J. Nat. Prod.76, 794–812 (2013). [DOI] [PubMed] [Google Scholar]
- 30.Klein, J. T. et al. Synthesis and structure-activity relationships of N-propyl-N-(4-pyridinyl)-1H-indol-1-amine (besipirdine) and related analogs as potential therapeutic agents for alzheimer′s disease. J. Med. Chem.39, 570–581 (1996). [DOI] [PubMed] [Google Scholar]
- 31.Shoeb, M. et al. Isolation, structure elucidation and bioactivity of schischkiniin, a unique indole alkaloid from the seeds of centaurea schischkinii. Tetrahedron61, 9001–9006 (2005). [Google Scholar]
- 32.Suzuki, K., Nomura, I., Ninomiya, M., Tanaka, K. & Koketsu, M. Synthesis and antimicrobial activity of β-carboline derivatives with N2-alkyl modifications. Bioorg. Med. Chem. Lett.28, 2976–2978 (2018). [DOI] [PubMed] [Google Scholar]
- 33.Liu, X.-Y., Zhang, Y.-L., Fei, X., Liao, L.-S. & Fan, J. 9,9′-Bicarbazole: New molecular skeleton for organic light emitting diodes. Chem. Eur. J.25, 4501–4508 (2019). [DOI] [PubMed] [Google Scholar]
- 34.Mei, G.-J. et al. Rational design and atroposelective synthesis of N–N axially chiral compounds. Chem. 7, 2743–2757 (2021). [Google Scholar]
- 35.Centonze, G., Portolani, C., Righi, P. & Bencivenni, G. Enantioselective strategies for the synthesis of N–N atropisomers. Angew. Chem. Int. Ed. 62, e202303966 (2023). [DOI] [PubMed] [Google Scholar]
- 36.Lin, W. et al. Asymmetric synthesis of N–N axially chiral compounds via organocatalytic atroposelective N-acylation. Chem. Sci.13, 141–148 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Balanna, K. et al. N-heterocyclic carbene-catalyzed atroposelective synthesis of N–N axially chiral 3-amino quinazolinones. ACS Catal. 13, 8752–8759 (2023). [Google Scholar]
- 38.Song, C. Y. et al. Catalytic N-acylation for access to N − N atropisomeric N-aminoindoles: choice of acylation reagents and mechanistic insights. ACS Catal.14, 6926–6935 (2024). [Google Scholar]
- 39.Ranganathappa, S. S. et al. Atroposelective synthesis of N − N axially chiral indoles and pyrroles via NHC-catalyzed diastereoselective (3 + 3) annulation strategy. ACS Catal. 14, 6965–6972 (2024). [Google Scholar]
- 40.Wang, X.-M. et al. Enantioselective synthesis of nitrogen−nitrogen biaryl atropisomers via copper-catalyzed Friedel-Crafts alkylation reaction. J. Am. Chem. Soc.143, 15005–15010 (2021). [DOI] [PubMed] [Google Scholar]
- 41.Chen, K.-W. et al. Organocatalytic atroposelective synthesis of N − N axially chiral indoles and pyrroles by de novo ring formation. Angew. Chem. Int. Ed.61, e202116829 (2022). [DOI] [PubMed] [Google Scholar]
- 42.Chen, Z.-H. et al. Organocatalytic enantioselective synthesis of axially chiral N,N’-bisindoles. Angew. Chem. Int. Ed.62, e202300419 (2023). [DOI] [PubMed] [Google Scholar]
- 43.Gao, Y. et al. Atroposelective synthesis of 1,1′-bipyrroles bearing a chiral N − N axis: chiral phosphoric acid catalysis with lewis acid induced enantiodivergence. Angew. Chem. Int. Ed. 61, e202200371 (2022). [DOI] [PubMed] [Google Scholar]
- 44.Yang, G. M., He, Y., Wang, T. Y., Li, Z. P. & Wang, J. Atroposelective synthesis of planar-chiral indoles via carbene catalyzed macrocyclization. Angew. Chem. Int. Ed. 63, e202316739 (2024). [DOI] [PubMed] [Google Scholar]
- 45.Hutskalova, V. & Sparr, C. Control over stereogenic N–N axes by Pd-Catalyzed 5-endo-hydroaminocyclizations. Synthesis55, 1770–1782 (2023). [Google Scholar]
- 46.Zhang, P. et al. Enantioselective synthesis of N − N bisindole atropisomers. Angew. Chem. Int. Ed.61, e202212101 (2022). [DOI] [PubMed] [Google Scholar]
- 47.Yin, S.-Y., Zhou, Q., Liu, C.-X., Gu, Q. & You, S.-L. Enantioselective synthesis of N–N biaryl atropisomers through iridium(I)-catalyzed C–H alkylation with acrylates. Angew. Chem. Int. Ed. 62, e202305067 (2023). [DOI] [PubMed] [Google Scholar]
- 48.Yao, W. et al. Enantioselective synthesis of N − N atropisomers by palladium-catalyzed C − H functionalization of pyrroles. Angew. Chem. Int. Ed.62, e202218871 (2023). [DOI] [PubMed] [Google Scholar]
- 49.Wang, Y.-S. et al. Rhodium-catalyzed enantioselective and diastereodivergent access to diaxially chiral heterocycles. Nat. Commun.14, 4661 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li, T. et al. Cobalt-catalyzed atroposelective C − H activation/annulation to access N − N axially chiral frameworks. Nat. Commun.14, 5271 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bai, X.-F., Cui, Y.-M., Cao, J. & Xu, L.-W. Atropisomers with axial and point chirality: Synthesis and applications. Acc. Chem. Res.55, 2545–2561 (2022). [DOI] [PubMed] [Google Scholar]
- 52.Luc, A. & Wencel-Delord, J. One reaction – double stereoinduction: C–H activation as a privileged route towards complex atropisomeric molecules. Chem. Commun.59, 8159–8167 (2023). [DOI] [PubMed] [Google Scholar]
- 53.Zhang, H.-H., Zhen, T., Liu, S.-J. & Shi, F. Catalytic asymmetric synthesis of atropisomers bearing multiple chiral elements: An emerging field. Angew. Chem. Int. Ed.63, e202311053 (2024). [DOI] [PubMed] [Google Scholar]
- 54.Portolani, C. et al. Synthesis of atropisomeric hydrazides by one-pot sequential enantioselective and diastereoselective catalysis. Angew. Chem. Int. Ed.61, e202209895 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang, S.-J. et al. Organocatalytic diastereo- and atroposelective construction of N–N axially chiral pyrroles and indoles. Nat. Commun.15, 518 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Speck, K. & Magauer, T. The chemistry of isoindole natural products. Beilstein. J. Org. Chem.9, 2048–2078 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thong, W. L., Shin-Ya, K., Nishiyama, M. & Kuzuyama, T. Discovery of an antibacterial isoindolinone-containing tetracyclic polyketide by cryptic gene activation and characterization of its biosynthetic gene cluster. ACS Chem. Biol.13, 2615–2622 (2018). [DOI] [PubMed] [Google Scholar]
- 58.Chessari, G. & Hardcastle, I. R. Structure-Based design of potent and orally active isoindolinone inhibitors of MDM2-p53 protein−protein interaction. J. Med. Chem. 64, 4071–4088 (2021). [DOI] [PubMed] [Google Scholar]
- 59.Augner, D., Gerbino, D. C., Slavov, N., Neudçrfl, J.-M. & Schmalz, H.-G. N-Capping of primary amines with 2-acylbenzaldehydes to give isoindolinones. Org. Lett.13, 5374–5377 (2011). [DOI] [PubMed] [Google Scholar]
- 60.Zhang, Y. et al. Chiral phosphoric acid catalyzed asymmetric Ugi reaction by dynamic kinetic resolution of the primary multicomponent adduct. Angew. Chem. Int. Ed.55, 5282–5285 (2016). [DOI] [PubMed] [Google Scholar]
- 61.Min, C., Lin, Y. & Seidel, D. Catalytic enantioselective synthesis of Mariline A and related isoindolinones through a biomimetic approach. Angew. Chem. Int. Ed.56, 15353–15357 (2017). [DOI] [PubMed] [Google Scholar]
- 62.You, S.-L., Cai, Q. & Zeng, M. Chiral Brønsted acid catalyzed Friedel–Crafts alkylation reactions. Chem. Soc. Rev.38, 2190–2201 (2009). [DOI] [PubMed] [Google Scholar]
- 63.Parmar, D., Sugiono, E., Raja, S. & Rueping, M. Complete field guide to asymmetric BINOL-phosphate derived Brønsted acid and metal catalysis: History and classification by mode of activation; Brønsted acidity, hydrogen bonding, ion pairing, and metal phosphates. Chem. Rev.114, 9047–9153 (2014). [DOI] [PubMed] [Google Scholar]
- 64.Parmar, D., Sugiono, E., Raja, S. & Rueping, M. Addition and correction to complete field guide to asymmetric BINOL-phosphate derived Brønsted acid and metal catalysis: History and classification by mode of activation; Brønsted acidity, hydrogen bonding, ion pairing, and metal phosphates. Chem. Rev.117, 10608–10620 (2017). [DOI] [PubMed] [Google Scholar]
- 65.Da, B.-C., Xiang, S.-H., Li, S. & Tan, B. Chiral phosphoric acid catalyzed asymmetric synthesis of axially chiral compounds. Chin. J. Chem.39, 1787–1796 (2021). [Google Scholar]
- 66.Fazullina, A. A., Ryapisova, L. V., Kashevarova, L. B. & Fridland, S. V. Reactions of benzaldehyde dimethylhydrazone with phosphorous acids. Russ. J. Gen. Chem.83, 2119–2121 (2013). [Google Scholar]
- 67.Tian, H. et al. Pyrrole-assisted and easy oxidation of cyclic α-amino acid-derived diketopiperazines under mild conditions. Adv. Synth. Catal. 353, 1525–1533 (2011). [Google Scholar]
- 68.Petsi, M. & Zografos, A. L. 2,5-Diketopiperazine catalysts as activators of dioxygen in oxidative processes. ACS Catal. 10, 7093–7099 (2020). [Google Scholar]
- 69.Breytenbach, J. C. et al. Synthesis and antimicrobial activity of some isoindolin-1-ones derivatives. Bioorg. Med. Chem. Lett.10, 1629–1631 (2000). [DOI] [PubMed] [Google Scholar]
- 70.Maugeri, C. et al. New anti-viral drugs for the treatment of the common cold. Bioorg. Med. Chem.16, 3091–3107 (2008). [DOI] [PubMed] [Google Scholar]
- 71.Zhao, X.-Z., Maddali, K., Marchand, C., Pommier, Y. & Burke, T. R. Diketoacid-genre HIV-1 integrase inhibitors containing enantiomeric arylamide functionality. Bioorg. Med. Chem.17, 5318–5324 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pereira, N. A. L. et al. Enantiopure indolizinoindolones with in vitro activity against blood- and liver-stage malaria parasites. ChemMedChem10, 2080–2089 (2015). [DOI] [PubMed] [Google Scholar]
- 73.Bhatia, R. K. Isoindole derivatives: Propitious anticancer structural motifs. Curr. Top. Med. Chem.17, 189–207 (2016). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated and analyzed during this study are included in this Article and its Supplementary Information. The X-ray crystallographic coordinate for structures 3i, 3n, and 4b have been deposited at the Cambridge Crystallographic Data Centre under deposition numbers CCDC 2323929 (for 3i), 2323930 (for 3n), and 2323931 (for 4b), respectively and can be obtained free of charge from the CCDC via http://www.ccdc.cam.ac.uk/data_request/cif. All data are available from the corresponding author upon request. Source data are present with this paper. Source data are provided with this paper.










