Abstract
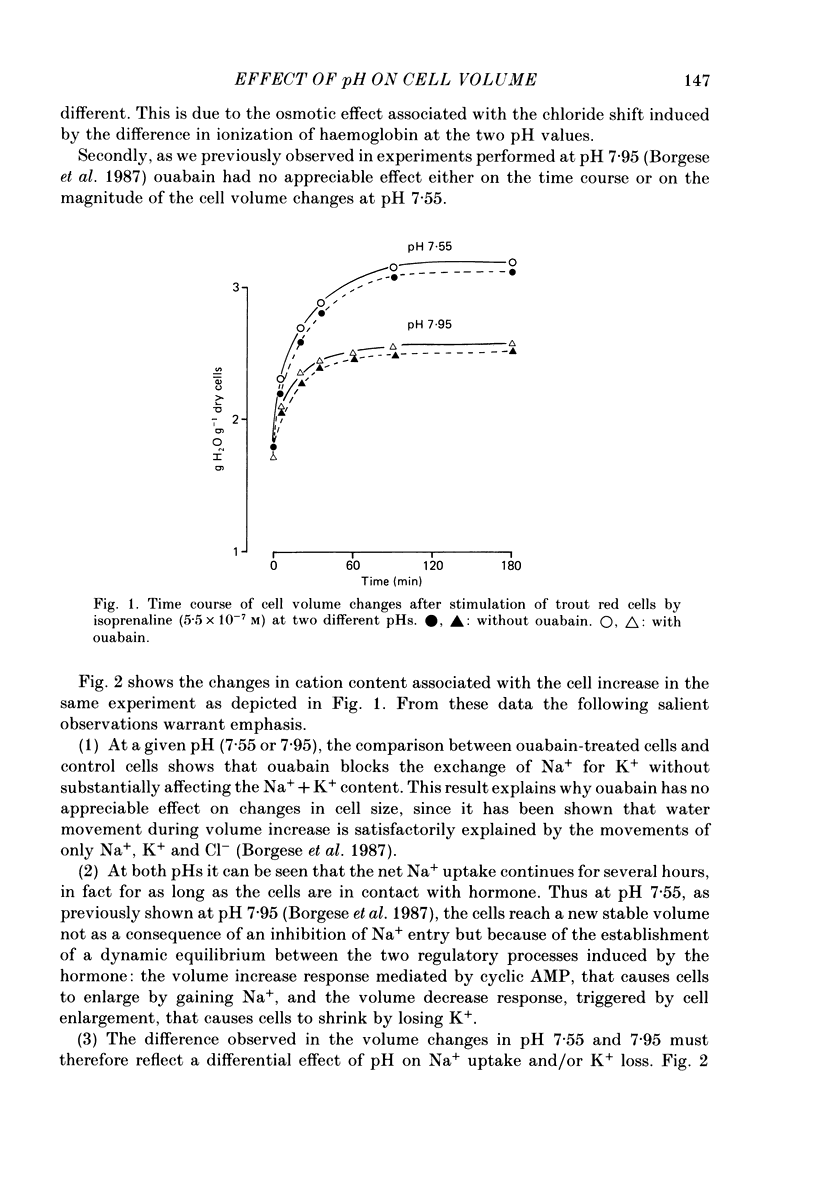
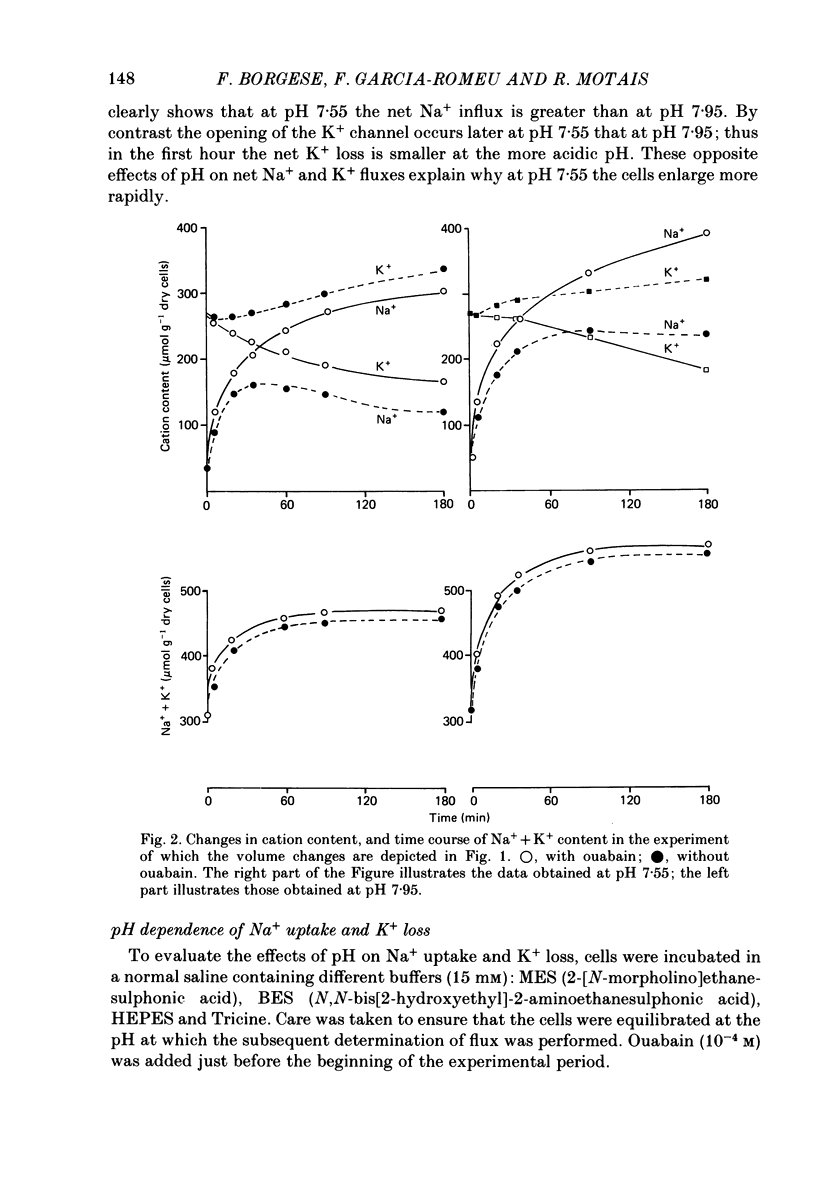
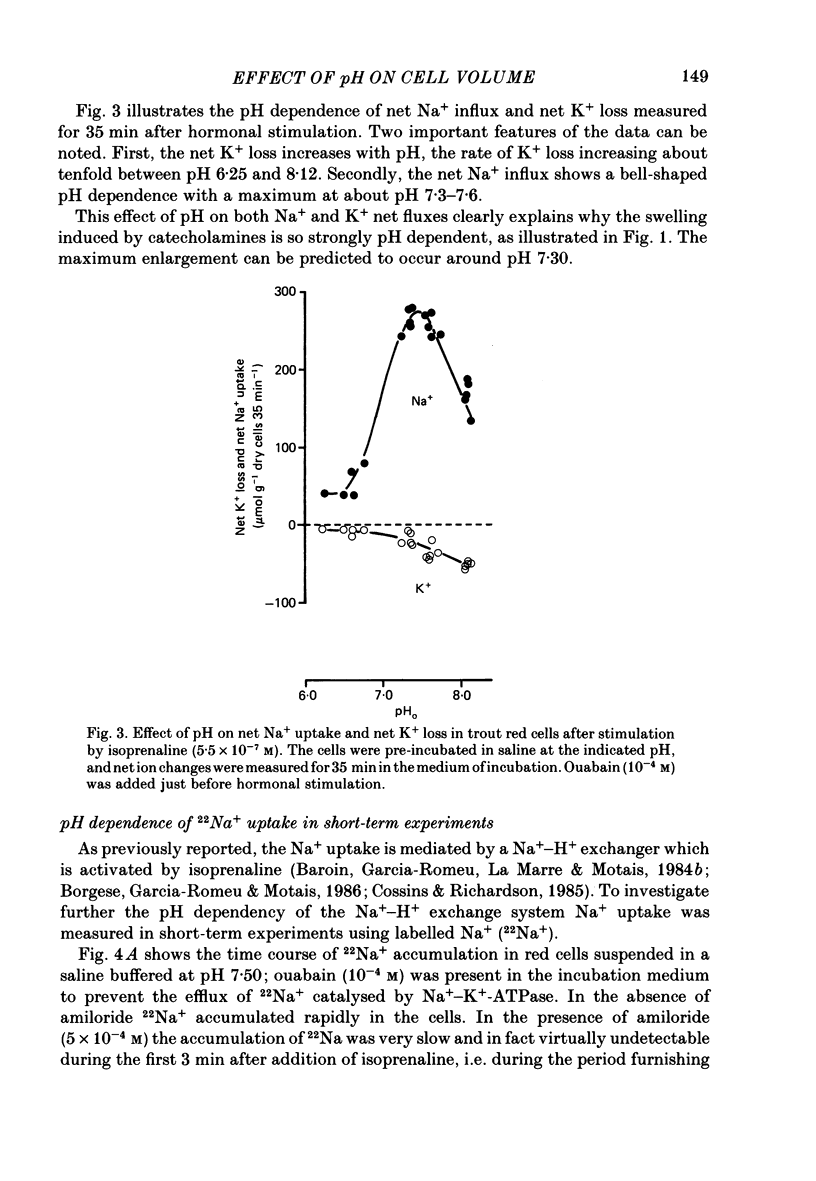
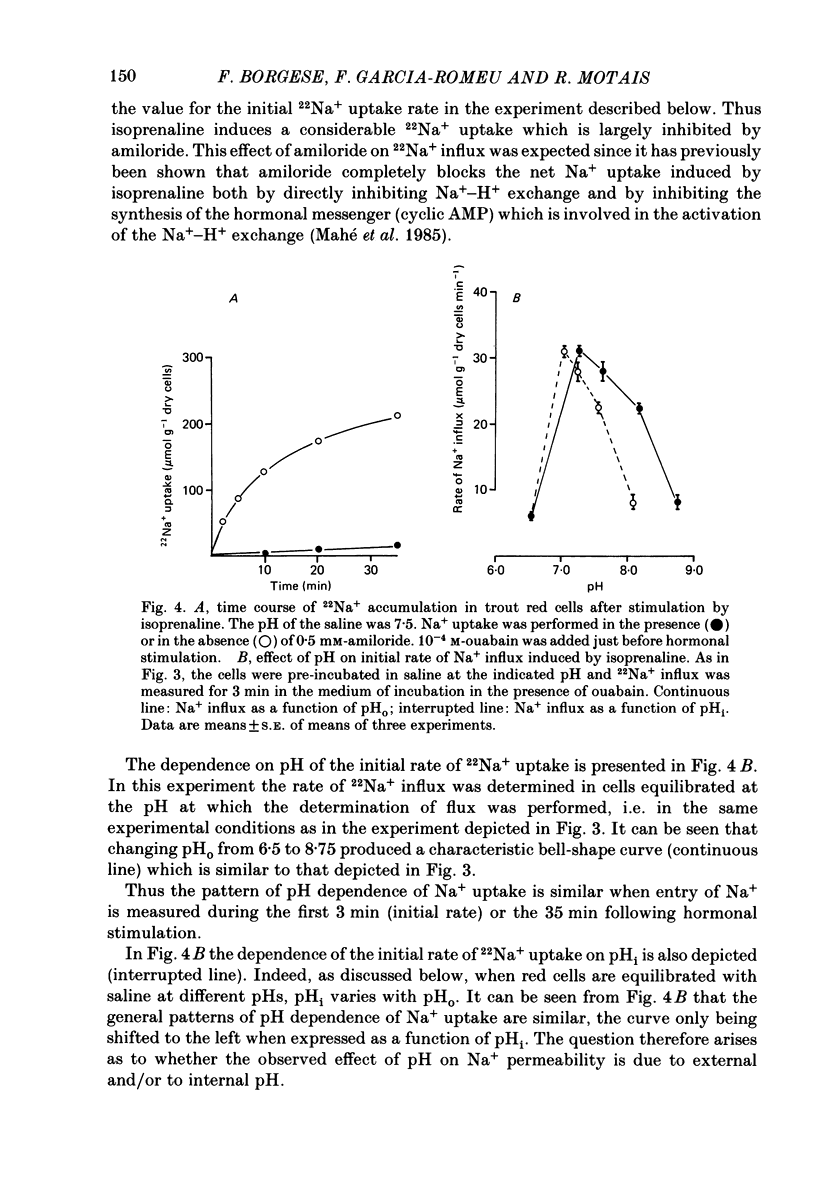
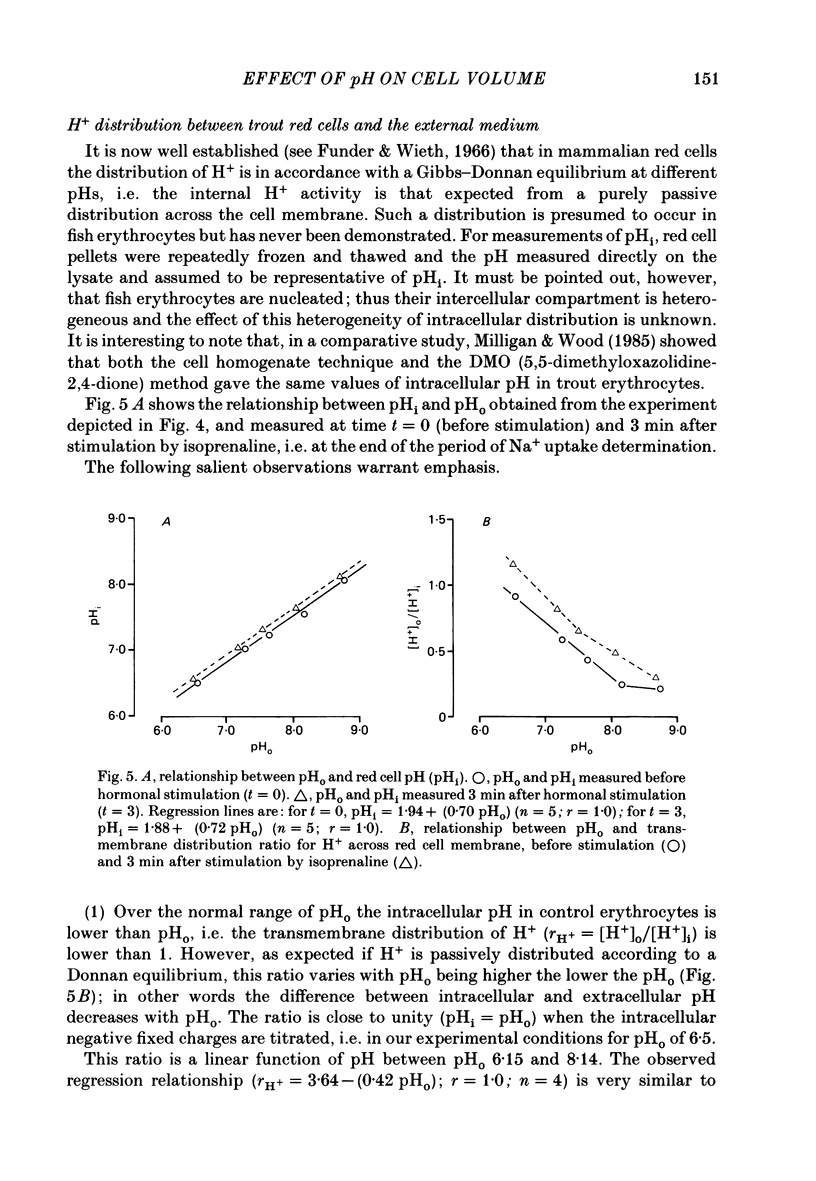
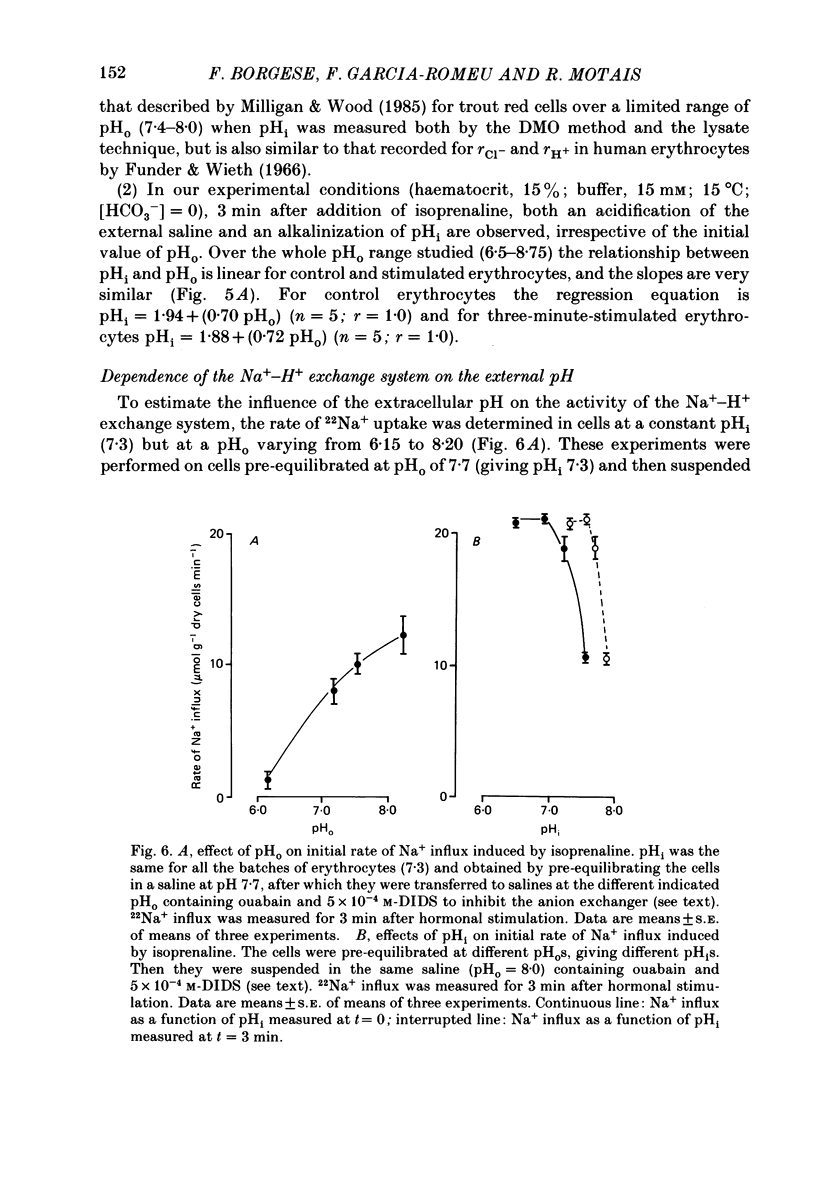
1. Trout red cells suspended in an isotonic medium containing catecholamines or adenosine 3',5'-phosphate (cyclic AMP) enlarge rapidly to reach a new steady-state volume which is maintained as long as hormone is present. 2. The present investigation demonstrates that the maximum swelling reached by the cells is strongly pH dependent. At pH 7.55 the cells enlarge more rapidly than at pH 7.95 and they reach a maximal volume which is much greater. It is explained by a differential effect of pH on two pathways controlling the movements of cations: K+ loss decreases as pH becomes more acidic in a roughly linear manner. On the contrary Na+ uptake increases as pH becomes more acidic with a maximum around pH 7.30 and then decreases. From this pH dependence it can be expected that the maximum enlargement occurs at about pH 7.30. 3. The complex relationship describing the change in the activity of the Na+-H+ exchanger as a function of pH (bell-shaped curve) is explained by the predominant influence of internal H+ on the antiporter in the alkaline range of pH and by the predominant influence of external H+ on the transporter in the acidic range.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson P. S. Mechanisms of active H+ secretion in the proximal tubule. Am J Physiol. 1983 Dec;245(6):F647–F659. doi: 10.1152/ajprenal.1983.245.6.F647. [DOI] [PubMed] [Google Scholar]
- Aronson P. S., Nee J., Suhm M. A. Modifier role of internal H+ in activating the Na+-H+ exchanger in renal microvillus membrane vesicles. Nature. 1982 Sep 9;299(5879):161–163. doi: 10.1038/299161a0. [DOI] [PubMed] [Google Scholar]
- Aronson P. S., Suhm M. A., Nee J. Interaction of external H+ with the Na+-H+ exchanger in renal microvillus membrane vesicles. J Biol Chem. 1983 Jun 10;258(11):6767–6771. [PubMed] [Google Scholar]
- Baroin A., Garcia-Romeu F., Lamarre T., Motais R. A transient sodium-hydrogen exchange system induced by catecholamines in erythrocytes of rainbow trout, Salmo gairdneri. J Physiol. 1984 Nov;356:21–31. doi: 10.1113/jphysiol.1984.sp015450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baroin A., Garcia-Romeu F., Lamarre T., Motais R. Hormone-induced co-transport with specific pharmacological properties in erythrocytes of rainbow trout, Salmo gairdneri. J Physiol. 1984 May;350:137–157. doi: 10.1113/jphysiol.1984.sp015193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgese F., Garcia-Romeu F., Motais R. Catecholamine-induced transport systems in trout erythrocyte. Na+/H+ countertransport or NaCl cotransport? J Gen Physiol. 1986 Apr;87(4):551–566. doi: 10.1085/jgp.87.4.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgese F., Garcia-Romeu F., Motais R. Control of cell volume and ion transport by beta-adrenergic catecholamines in erythrocytes of rainbow trout, Salmo gairdneri. J Physiol. 1987 Jan;382:123–144. doi: 10.1113/jphysiol.1987.sp016359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne P. K., Cossins A. R. On the instability of K+ influx in erythrocytes of the rainbow trout, Salmo gairdneri, and the role of catecholamine hormones in maintaining in vivo influx activity. J Exp Biol. 1982 Dec;101:93–104. doi: 10.1242/jeb.101.1.93. [DOI] [PubMed] [Google Scholar]
- Grinstein S., Cohen S., Rothstein A. Cytoplasmic pH regulation in thymic lymphocytes by an amiloride-sensitive Na+/H+ antiport. J Gen Physiol. 1984 Mar;83(3):341–369. doi: 10.1085/jgp.83.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinstein S., Goetz J. D., Rothstein A. 22Na+ fluxes in thymic lymphocytes. II. Amiloride-sensitive Na+/H+ exchange pathway; reversibility of transport and asymmetry of the modifier site. J Gen Physiol. 1984 Oct;84(4):585–600. doi: 10.1085/jgp.84.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean T., Frelin C., Vigne P., Barbry P., Lazdunski M. Biochemical properties of the Na+/H+ exchange system in rat brain synaptosomes. Interdependence of internal and external pH control of the exchange activity. J Biol Chem. 1985 Aug 15;260(17):9678–9684. [PubMed] [Google Scholar]
- Mahé Y., Garcia-Romeu F., Motais R. Inhibition by amiloride of both adenylate cyclase activity and the Na+/H+ antiporter in fish erythrocytes. Eur J Pharmacol. 1985 Oct 22;116(3):199–206. doi: 10.1016/0014-2999(85)90154-2. [DOI] [PubMed] [Google Scholar]
- Milligan C. L., Wood C. M. Intracellular pH transients in rainbow trout tissues measured by dimethadione distribution. Am J Physiol. 1985 Jun;248(6 Pt 2):R668–R673. doi: 10.1152/ajpregu.1985.248.6.R668. [DOI] [PubMed] [Google Scholar]
- Paris S., Pouysségur J. Biochemical characterization of the amiloride-sensitive Na+/H+ antiport in Chinese hamster lung fibroblasts. J Biol Chem. 1983 Mar 25;258(6):3503–3508. [PubMed] [Google Scholar]
- Rindler M. J., Saier M. H., Jr Evidence for Na+/H+ antiport in cultured dog kidney cells (MDCK). J Biol Chem. 1981 Nov 10;256(21):10820–10825. [PubMed] [Google Scholar]
- Romano L., Passow H. Characterization of anion transport system in trout red blood cell. Am J Physiol. 1984 Mar;246(3 Pt 1):C330–C338. doi: 10.1152/ajpcell.1984.246.3.C330. [DOI] [PubMed] [Google Scholar]



