Abstract
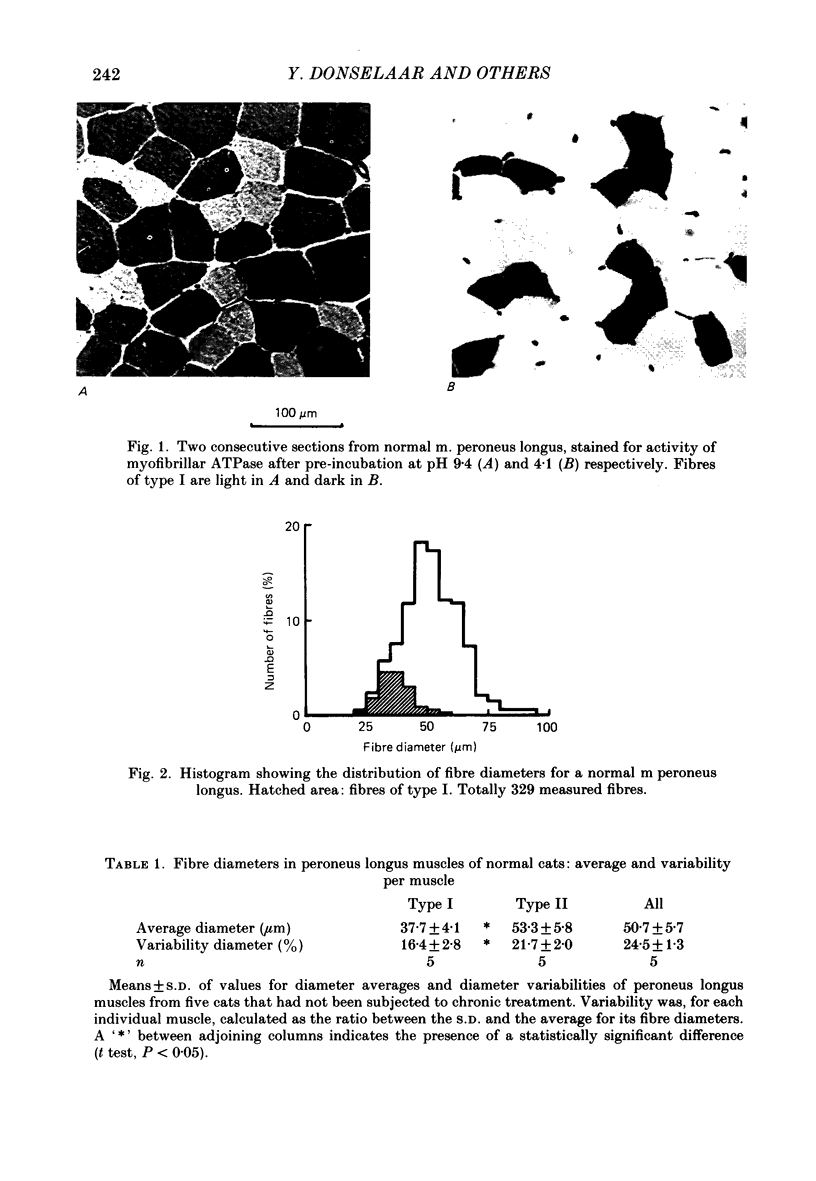
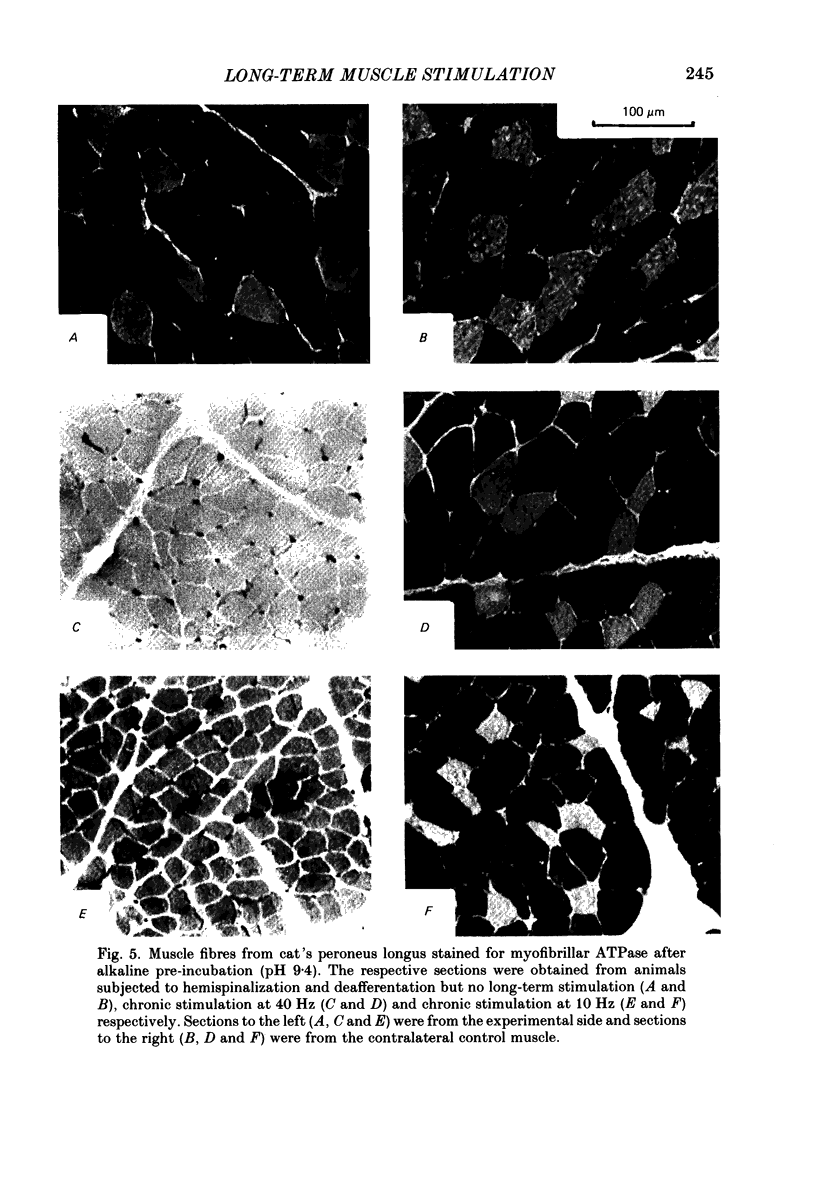
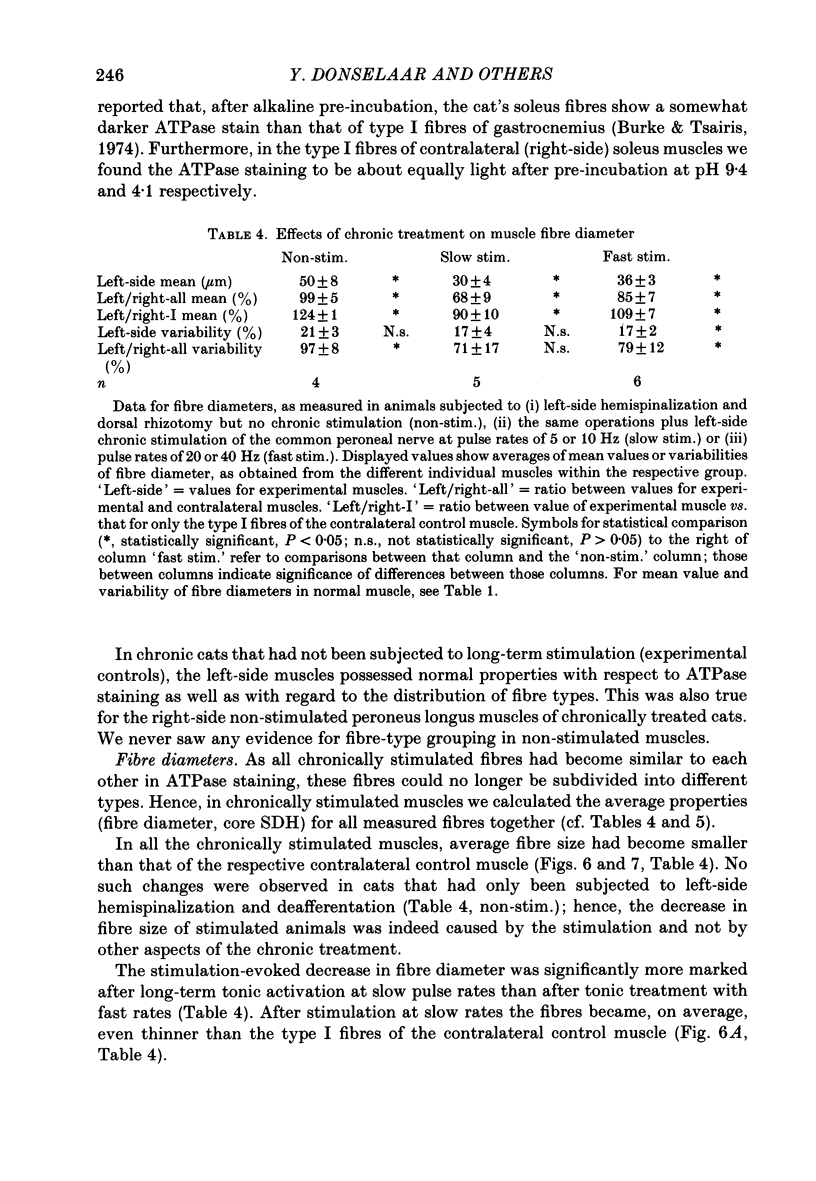
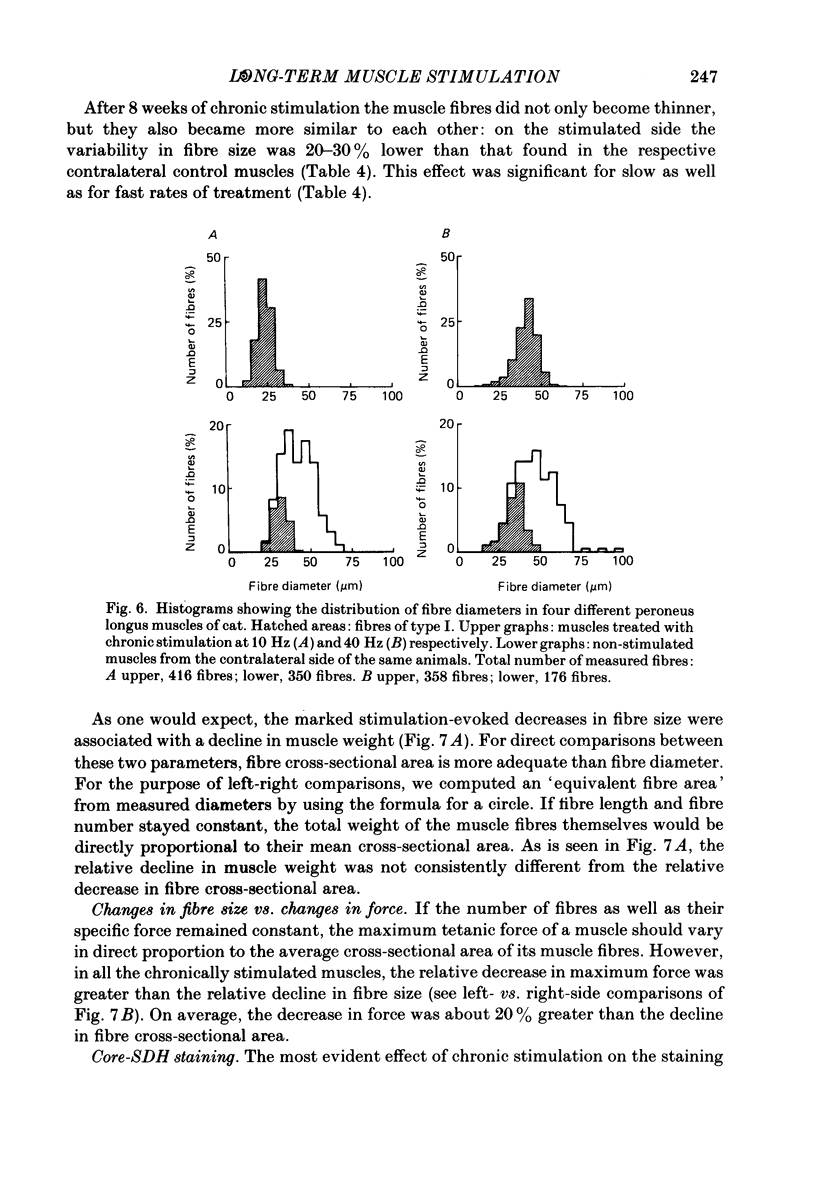
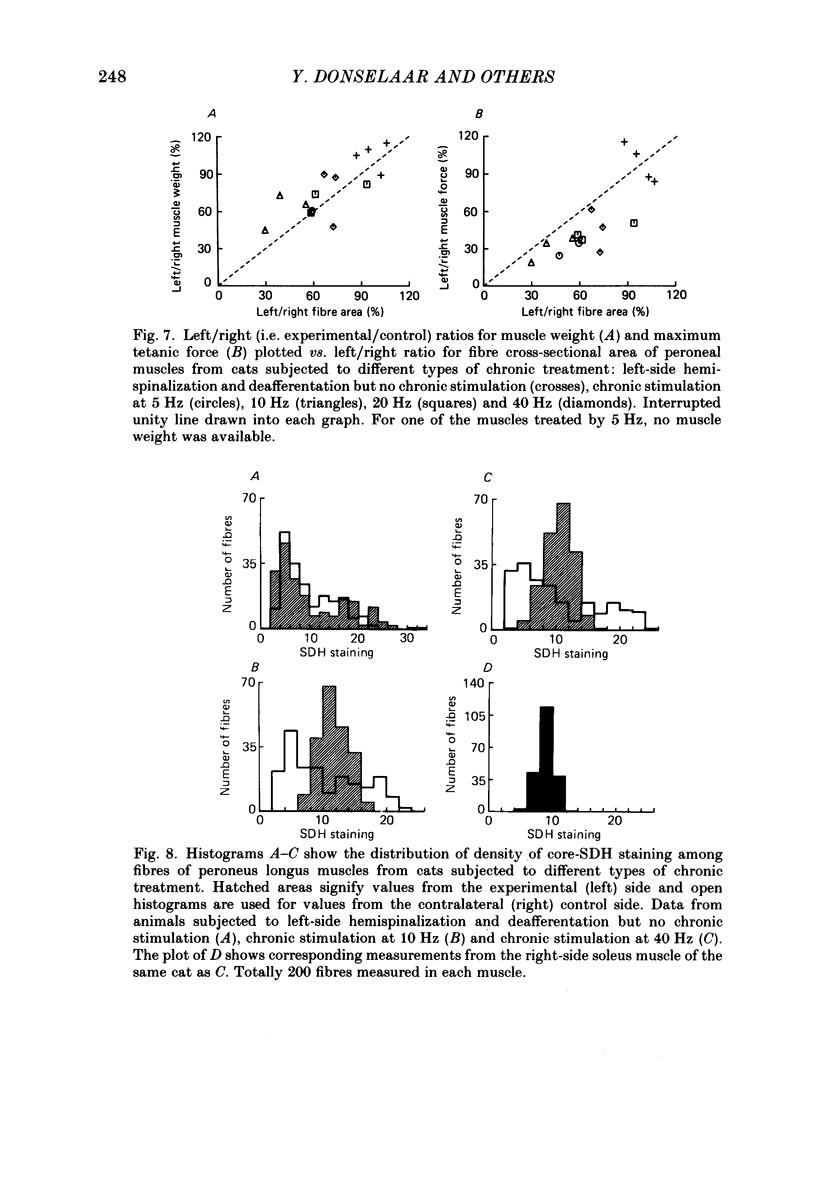
1. Normal and chronically stimulated peroneus longus muscles of the cat's hind limb were studied with respect to fibre size and staining properties for myofibrillar (myosin) adenosine triphosphatase (ATPase) and succinate dehydrogenase (SDH) activity. The intensity of staining for SDH activity was measured by microphotometry from the central portions of the muscle fibres ('core-SDH staining'). For comparison, histochemical properties were also studied in non-stimulated soleus muscles. 2. On account of the pH sensitivity of their myofibrillar ATPase, about 18% of the fibres in normal peroneus longus muscles were classified as type I, and about half of the remainder as II A and II B respectively. 3. In the normal peroneus longus muscles, the mean diameter of single muscle fibres generally varied between about 25 and 75 micron, whereby the average size of type I less than type II. 4. In the normal peroneus longus muscles the staining intensity for core SDH varied over a wide range. The average heaviness of staining was clearly ranked in the order type I greater than type II A greater than type II B. 5. Chronic stimulation was given to the deafferented common peroneal nerve by aid of a portable and remotely controlled mini-stimulator. The stimulation was delivered in 'tonic' patterns (greater than or equal to 50% of total time taken up by activity) of 'fast' (20 or 40 Hz) or 'slow' (5 or 10 Hz) rates. 6. Prior to the period of long-term stimulation, the cats had been subjected to a dorsal rhizotomy and hemispinalization on the ipsilateral (left) side. In the absence of chronic stimulation, these operations had no evident effects on the sizes or staining properties of peroneus longus fibres. 7. After 8 weeks of treatment with tonic patterns of stimulation, the fibres of peroneus longus muscles clearly became more similar to each other with respect to their diameter as well as their staining for ATPase and SDH activity. With respect to ATPase staining, however, the chronically stimulated peroneus longus fibres had become more similar to non-stimulated soleus fibres than to non-stimulated type I fibres of peroneus longus. With respect to the staining for core SDH, the chronically stimulated fibres all became similar to normal II A fibres of peroneus longus. The 'fast' and 'slow' patterns of chronic stimulation had the same effects on the staining properties. 8. Chronically stimulated peroneus longus muscles showed a decrease in fibre diameter which corresponded, roughly, to the concomitant decrease in muscle weight.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF




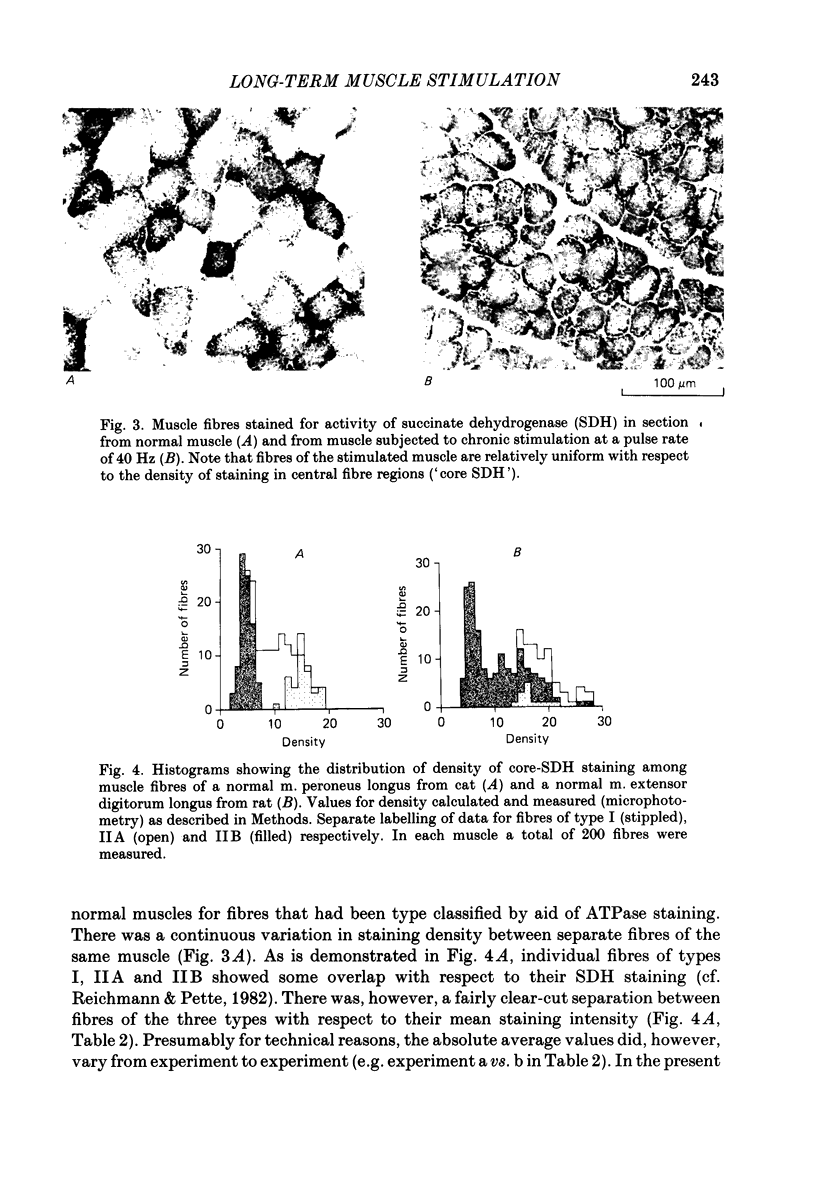
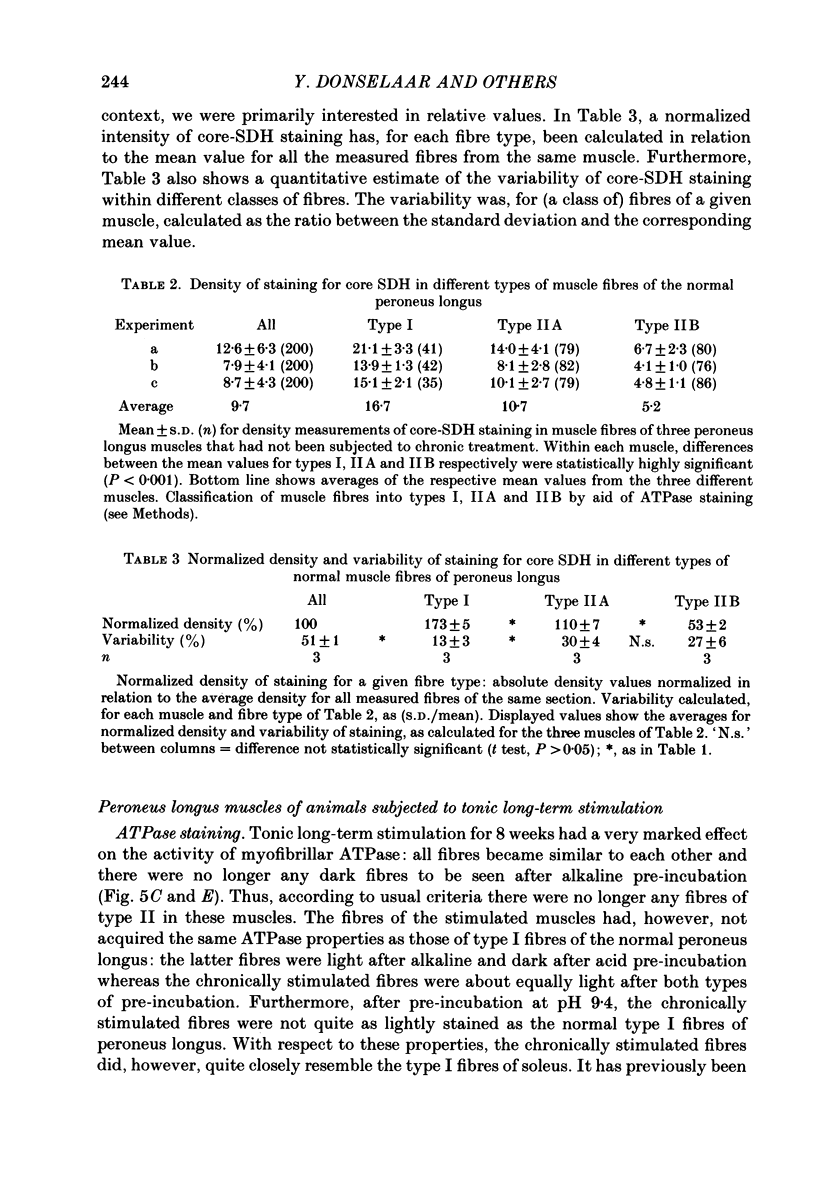






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ariano M. A., Armstrong R. B., Edgerton V. R. Hindlimb muscle fiber populations of five mammals. J Histochem Cytochem. 1973 Jan;21(1):51–55. doi: 10.1177/21.1.51. [DOI] [PubMed] [Google Scholar]
- Brooke M. H., Kaiser K. K. Muscle fiber types: how many and what kind? Arch Neurol. 1970 Oct;23(4):369–379. doi: 10.1001/archneur.1970.00480280083010. [DOI] [PubMed] [Google Scholar]
- Brown M. D., Cotter M. A., Hudlická O., Vrbová G. The effects of different patterns of muscle activity on capillary density, mechanical properties and structure of slow and fast rabbit muscles. Pflugers Arch. 1976 Feb 24;361(3):241–250. doi: 10.1007/BF00587288. [DOI] [PubMed] [Google Scholar]
- Buchegger A., Nemeth P. M., Pette D., Reichmann H. Effects of chronic stimulation on the metabolic heterogeneity of the fibre population in rabbit tibialis anterior muscle. J Physiol. 1984 May;350:109–119. doi: 10.1113/jphysiol.1984.sp015191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke R. E., Tsairis P. Trophic functions of the neuron. II. Denervation and regulation of muscle. The correlation of physiological properties with histochemical characteristics in single muscle units. Ann N Y Acad Sci. 1974 Mar 22;228(0):145–159. doi: 10.1111/j.1749-6632.1974.tb20507.x. [DOI] [PubMed] [Google Scholar]
- Caccia M. R., Meola G., Brignoli G., Andreussi L., Scarlato G. Physiological and histochemical changes of the extensor digitorum longus and soleus muscles after lateral cordotomy in the albino rat. Exp Neurol. 1978 Dec;62(3):647–657. doi: 10.1016/0014-4886(78)90275-3. [DOI] [PubMed] [Google Scholar]
- Close R. I. Dynamic properties of mammalian skeletal muscles. Physiol Rev. 1972 Jan;52(1):129–197. doi: 10.1152/physrev.1972.52.1.129. [DOI] [PubMed] [Google Scholar]
- Dum R. P., Burke R. E., O'Donovan M. J., Toop J., Hodgson J. A. Motor-unit organization in flexor digitorum longus muscle of the cat. J Neurophysiol. 1982 Jun;47(6):1108–1125. doi: 10.1152/jn.1982.47.6.1108. [DOI] [PubMed] [Google Scholar]
- Edjtehadi G. D., Lewis D. M. Histochemical reactions of fibres in a fast twitch muscle of the cat. J Physiol. 1979 Feb;287:439–453. doi: 10.1113/jphysiol.1979.sp012669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eerbeek O., Kernell D., Verhey B. A. Effects of fast and slow patterns of tonic long-term stimulation on contractile properties of fast muscle in the cat. J Physiol. 1984 Jul;352:73–90. doi: 10.1113/jphysiol.1984.sp015278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallego R., Kuno M., Núez R., Snider W. D. Dependence of motoneurone properties on the length of immobilized muscle. J Physiol. 1979 Jun;291:179–189. doi: 10.1113/jphysiol.1979.sp012806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksson J., Galbo H., Blomstrand E. Role of the motor nerve in activity-induced enzymatic adaptation in skeletal muscle. Am J Physiol. 1982 May;242(5):C272–C277. doi: 10.1152/ajpcell.1982.242.5.C272. [DOI] [PubMed] [Google Scholar]
- Hudlicka O., Aitman T., Heilig A., Leberer E., Tyler K. R., Pette D. Effects of different patterns of long-term stimulation on blood flow, fuel uptake and enzyme activities in rabbit fast skeletal muscles. Pflugers Arch. 1984 Nov;402(3):306–311. doi: 10.1007/BF00585514. [DOI] [PubMed] [Google Scholar]
- Hudlická O., Tyler K. R., Srihari T., Heilig A., Pette D. The effect of different patterns of long-term stimulation on contractile properties and myosin light chains in rabbit fast muscles. Pflugers Arch. 1982 Apr;393(2):164–170. doi: 10.1007/BF00582940. [DOI] [PubMed] [Google Scholar]
- Kernell D., Eerbeek O., Verhey B. A. Motor unit categorization on basis of contractile properties: an experimental analysis of the composition of the cat's m. peroneus longus. Exp Brain Res. 1983;50(2-3):211–219. doi: 10.1007/BF00239185. [DOI] [PubMed] [Google Scholar]
- Kugelberg E., Lindegren B. Transmission and contraction fatigue of rat motor units in relation to succinate dehydrogenase activity of motor unit fibres. J Physiol. 1979 Mar;288:285–300. [PMC free article] [PubMed] [Google Scholar]
- Mayer R. F., Burke R. E., Toop J., Walmsley B., Hodgson J. A. The effect of spinal cord transection on motor units in cat medial gastrocnemius muscles. Muscle Nerve. 1984 Jan;7(1):23–31. doi: 10.1002/mus.880070105. [DOI] [PubMed] [Google Scholar]
- McDonagh J. C., Binder M. D., Reinking R. M., Stuart D. G. A commentary on muscle unit properties in cat hindlimb muscles. J Morphol. 1980 Nov;166(2):217–230. doi: 10.1002/jmor.1051660208. [DOI] [PubMed] [Google Scholar]
- Nemeth P., Pette D. Succinate dehydrogenase activity in fibres classified by myosin ATPase in three hind limb muscles of rat. J Physiol. 1981 Nov;320:73–80. doi: 10.1113/jphysiol.1981.sp013935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peckham P. H., Mortimer J. T., Van Der Meulen J. P. Physiologic and metabolic changes in white muscle of cat following induced exercise. Brain Res. 1973 Feb 28;50(2):424–429. doi: 10.1016/0006-8993(73)90745-2. [DOI] [PubMed] [Google Scholar]
- Pette D., Müller W., Leisner E., Vrbová G. Time dependent effects on contractile properties, fibre population, myosin light chains and enzymes of energy metabolism in intermittently and continuously stimulated fast twitch muscles of the rabbit. Pflugers Arch. 1976 Jul 30;364(2):103–112. doi: 10.1007/BF00585177. [DOI] [PubMed] [Google Scholar]
- Pette D., Ramirez B. U., Müller W., Simon R., Exner G. U., Hildebrand R. Influence of intermittent long-term stimulation on contractile, histochemical and metabolic properties of fibre populations in fast and slow rabbit muscles. Pflugers Arch. 1975 Dec 19;361(1):1–7. doi: 10.1007/BF00587333. [DOI] [PubMed] [Google Scholar]
- Pette D., Smith M. E., Staudte H. W., Vrbová G. Effects of long-term electrical stimulation on some contractile and metabolic characteristics of fast rabbit muscles. Pflugers Arch. 1973 Feb 6;338(3):257–272. doi: 10.1007/BF00587391. [DOI] [PubMed] [Google Scholar]
- Pette D., Tyler K. R. Response of succinate dehydrogenase activity in fibres of rabbit tibialis anterior muscle to chronic nerve stimulation. J Physiol. 1983 May;338:1–9. doi: 10.1113/jphysiol.1983.sp014655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pool C. W., Diegenbach P. C., Scholten G. Quantitative succinate-dehydrogenase histochemistry. I. A Methodological study on mammalian and fish muscle. Histochemistry. 1979;64(3):251–262. doi: 10.1007/BF00495025. [DOI] [PubMed] [Google Scholar]
- Reichmann H., Pette D. A comparative microphotometric study of succinate dehydrogenase activity levels in type I, IIA and IIB fibres of mammalian and human muscles. Histochemistry. 1982;74(1):27–41. doi: 10.1007/BF00495049. [DOI] [PubMed] [Google Scholar]
- Rubinstein N., Mabuchi K., Pepe F., Salmons S., Gergely J., Sreter F. Use of type-specific antimyosins to demonstrate the transformation of individual fibers in chronically stimulated rabbit fast muscles. J Cell Biol. 1978 Oct;79(1):252–261. doi: 10.1083/jcb.79.1.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmons S., Henriksson J. The adaptive response of skeletal muscle to increased use. Muscle Nerve. 1981 Mar-Apr;4(2):94–105. doi: 10.1002/mus.880040204. [DOI] [PubMed] [Google Scholar]
- Salmons S., Vrbová G. The influence of activity on some contractile characteristics of mammalian fast and slow muscles. J Physiol. 1969 May;201(3):535–549. doi: 10.1113/jphysiol.1969.sp008771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreter F. A., Pinter K., Jolesz F., Mabuchi K. Fast to slow transformation of fast muscles in response to long-term phasic stimulation. Exp Neurol. 1982 Jan;75(1):95–102. doi: 10.1016/0014-4886(82)90009-7. [DOI] [PubMed] [Google Scholar]