Abstract
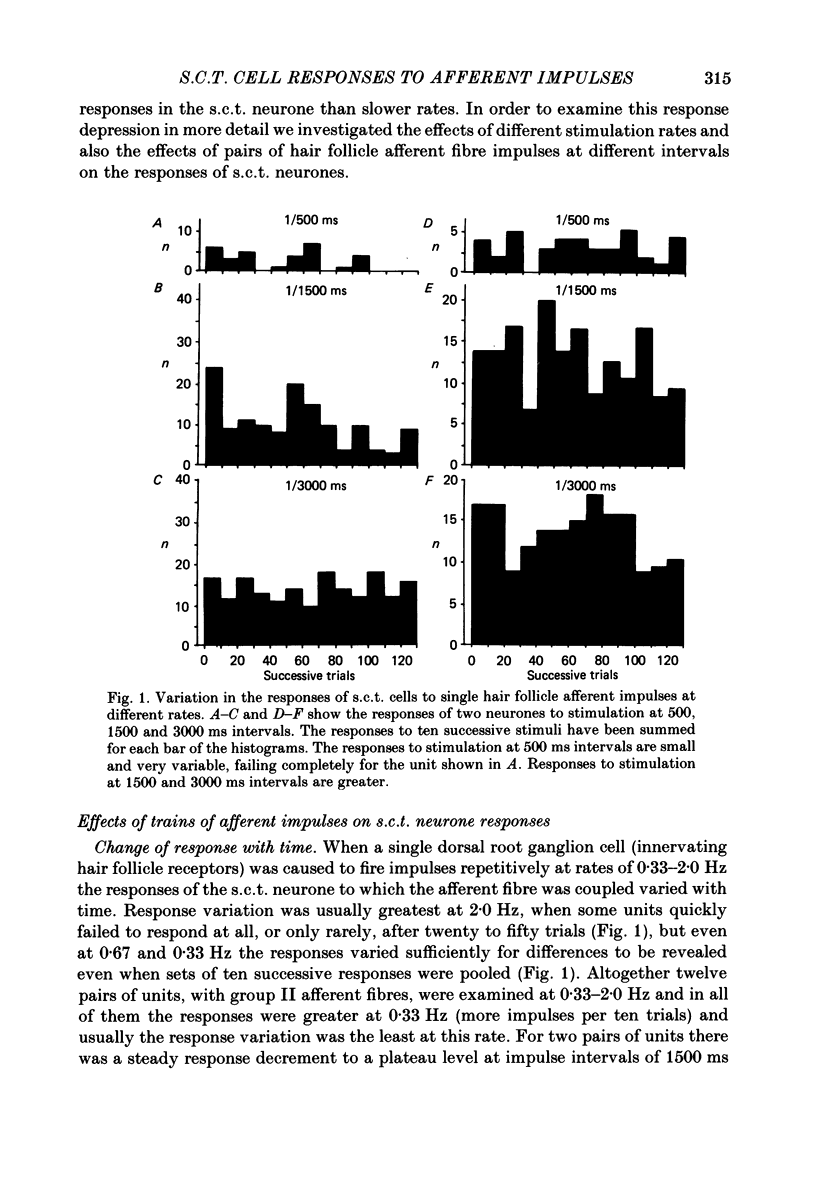
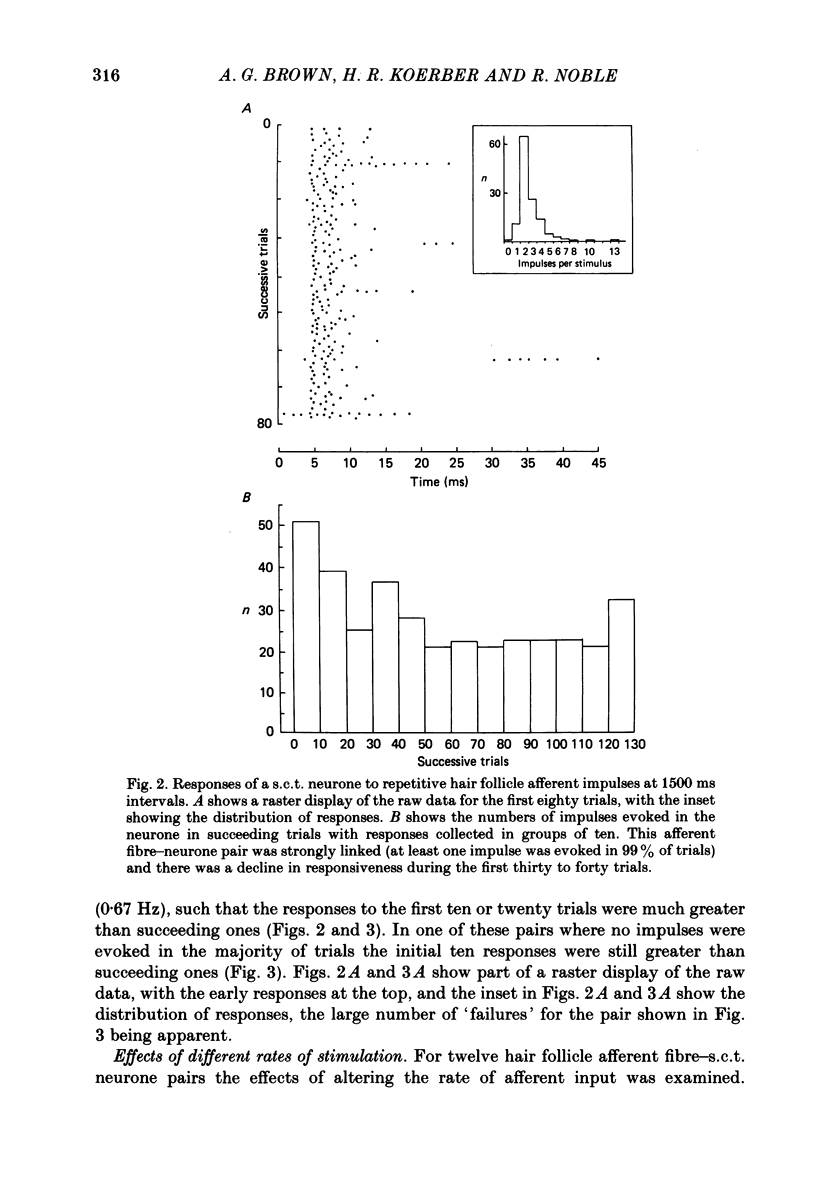
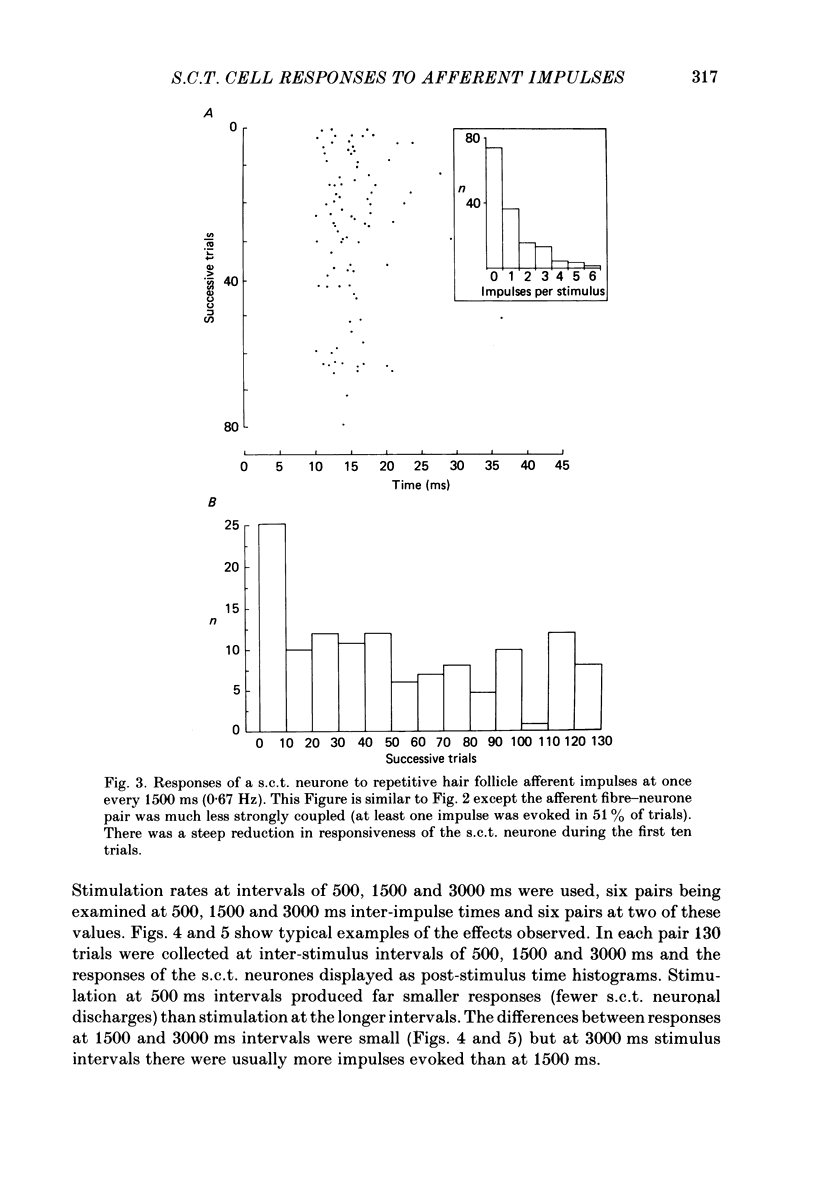
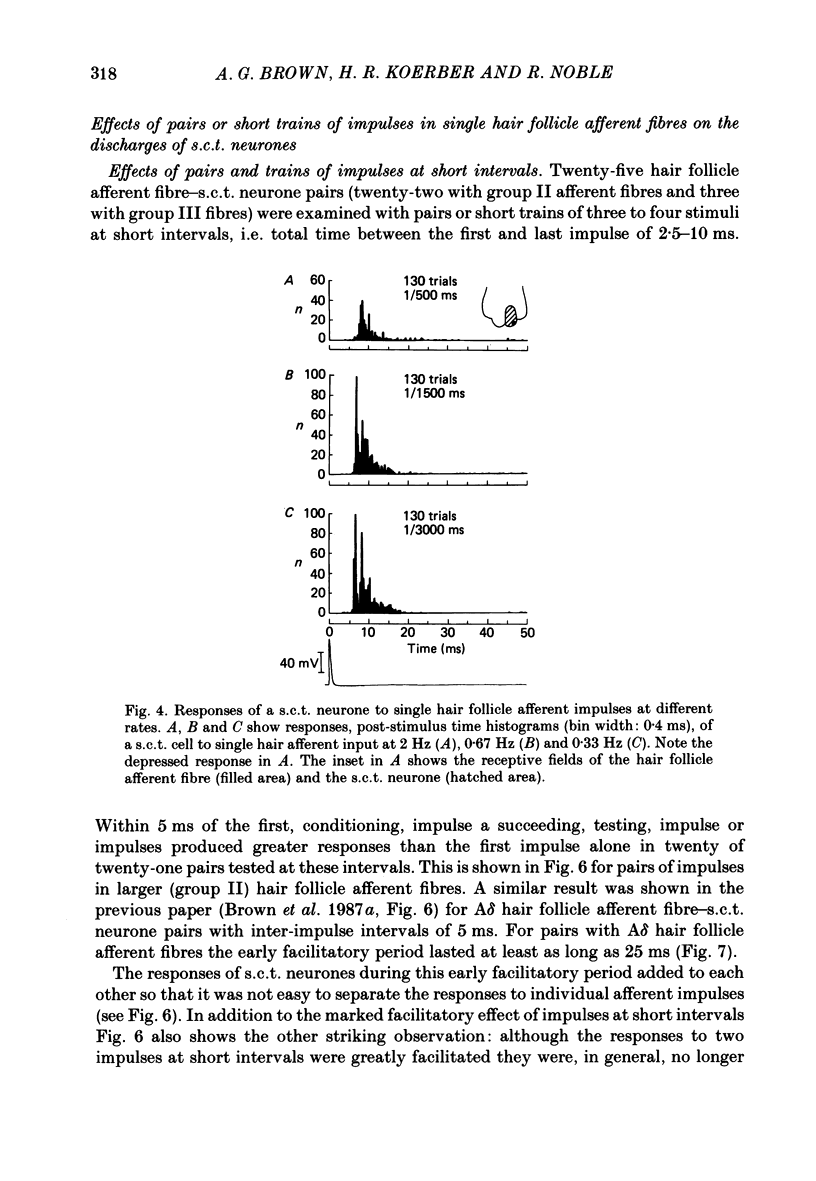
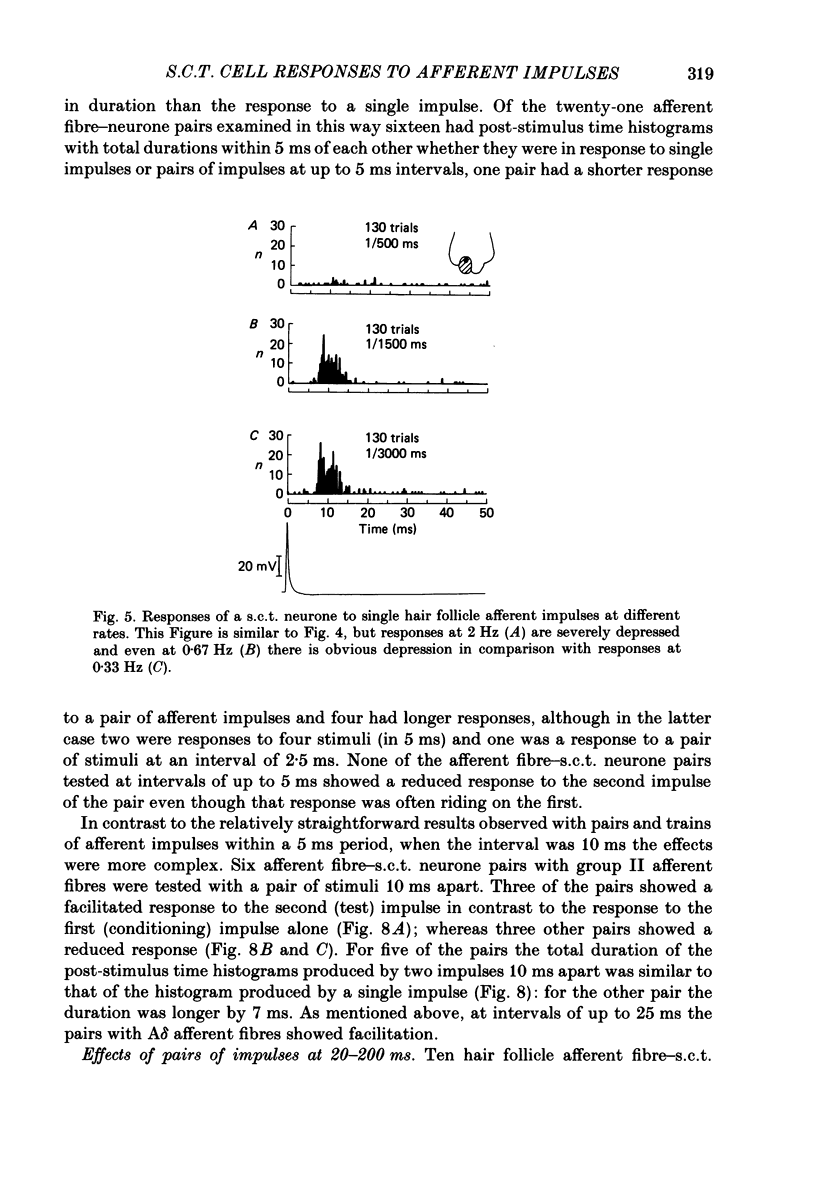
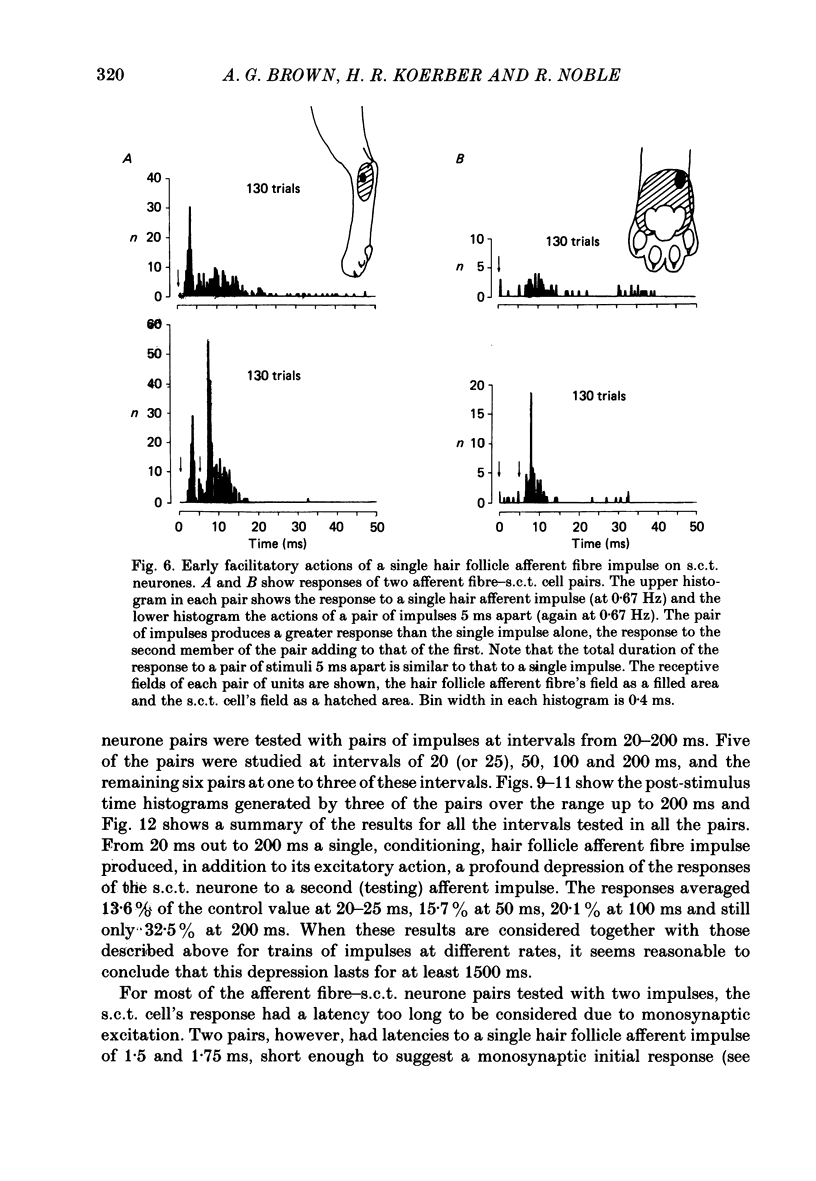
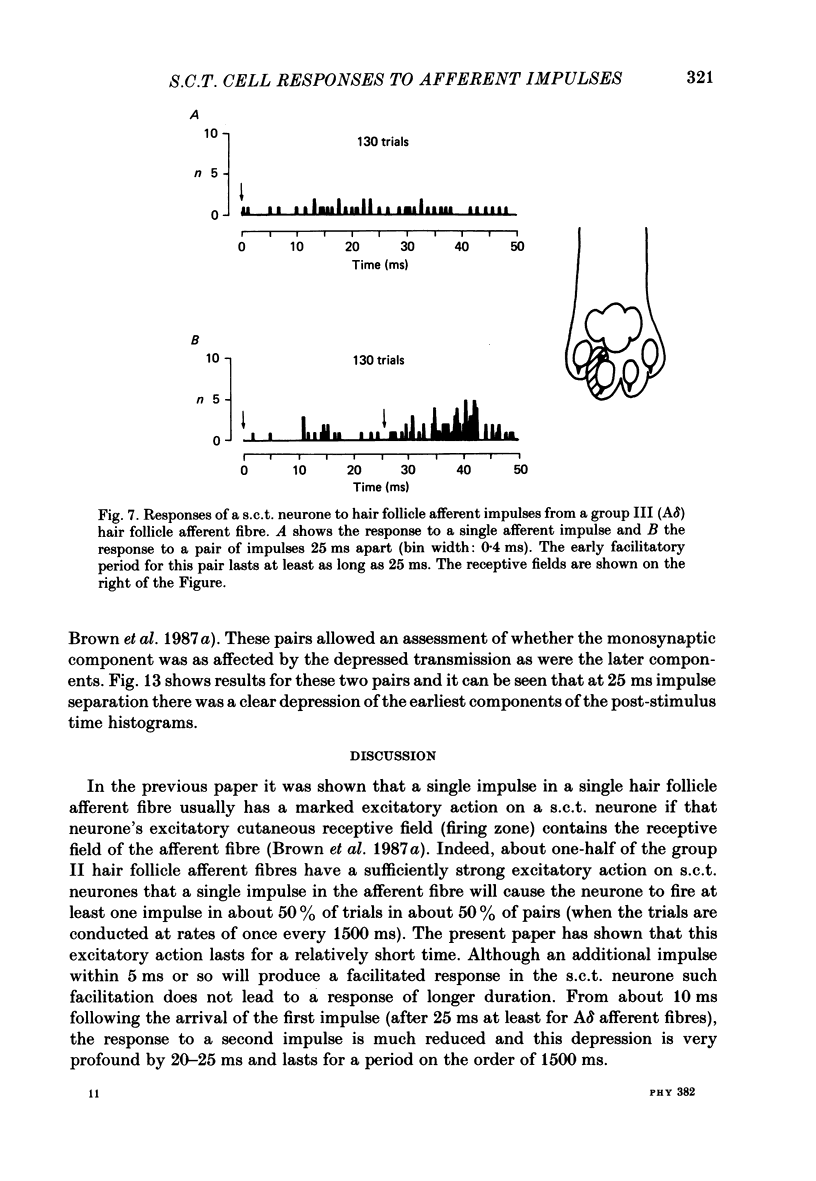
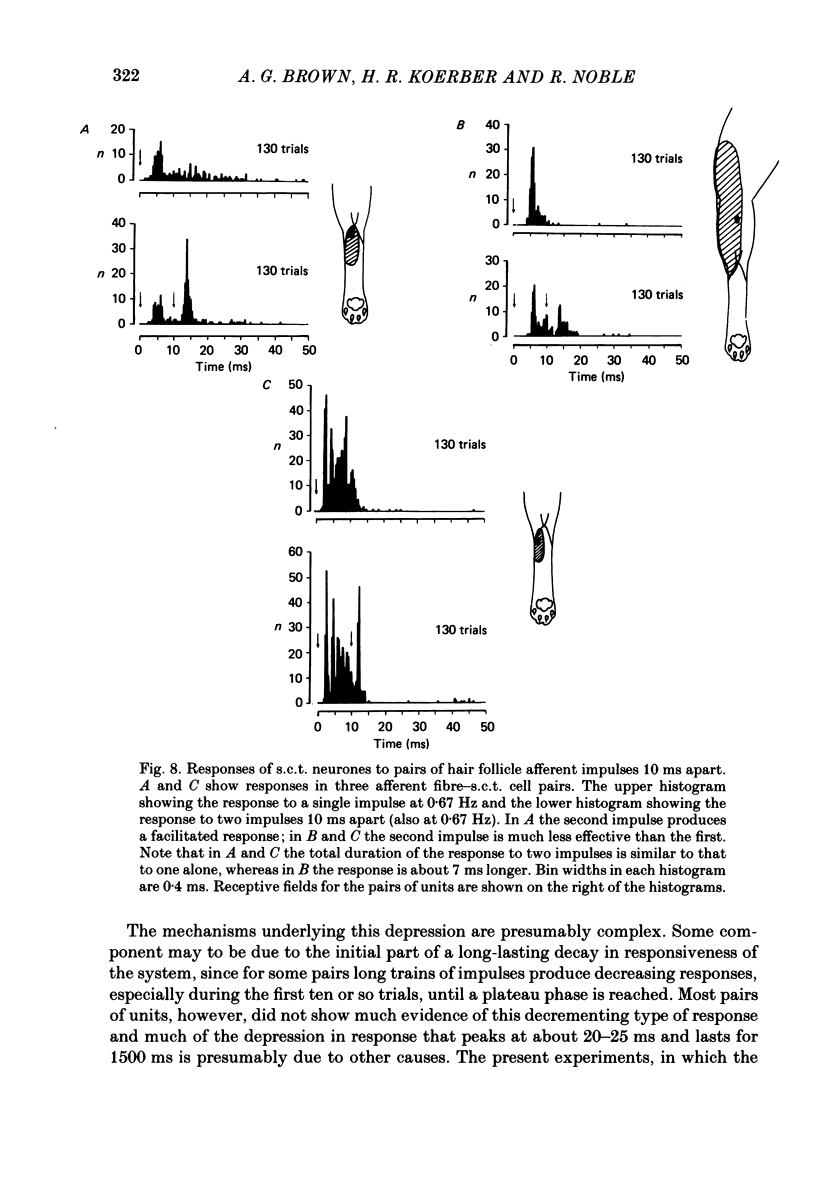
1. In cats under chloralose anaesthesia single lumbosacral dorsal root ganglion cells of hair follicle afferent fibres were stimulated intracellularly to produce trains or pairs of impulses. At the same time, single spinocervical tract (s.c.t.) neurones were recorded extracellularly, from their axons in the upper lumbar spinal cord. Afferent fibre-neurone pairs were chosen in which the receptive field of the fibre was contained within the excitatory receptive field (firing zone) of the neurone. 2. Trains of impulses of 2.0 Hz were less effective in increasing the probability of s.c.t. cell firing than trains at 0.67 Hz, and this latter rate was usually less effective than trains at 0.33 Hz. 3. Successive responses to individual members of a train of hair follicle afferent impulses were variable. In some pairs of units succeeding responses declined until a fairly consistent plateau was reached. In others there was no decline and the responses remained irregular. 4. Pairs or short trains of impulses revealed two phenomena: over the first 5 ms or so following an impulse in a group II hair follicle afferent fibre, a second or small group of impulses produced a greater response from the s.c.t. neurone but at intervals of 25-200 ms there was a profound depression of the responses evoked by the second member of a pair of impulses. For A delta afferent fibres the early facilitation lasted for at least 25 ms. 5. It is concluded that a single impulse in a single hair follicle afferent fibre from within the excitatory receptive field of a s.c.t. neurone has complex actions on transmission through that neurone. An initial excitatory influence is followed by a long-lasting depression that influences transmission through the system for at least 1500 ms. Possible mechanisms underlying this depression are discussed.
Full text
PDF

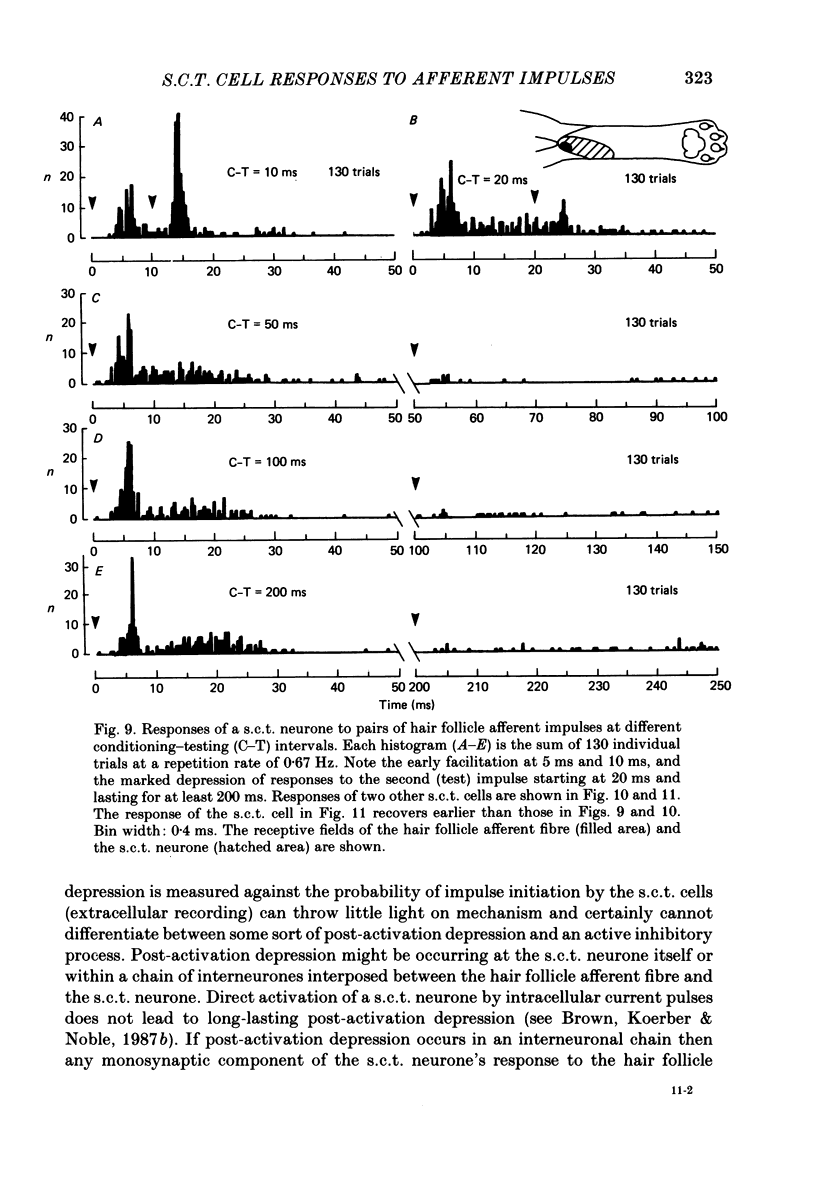
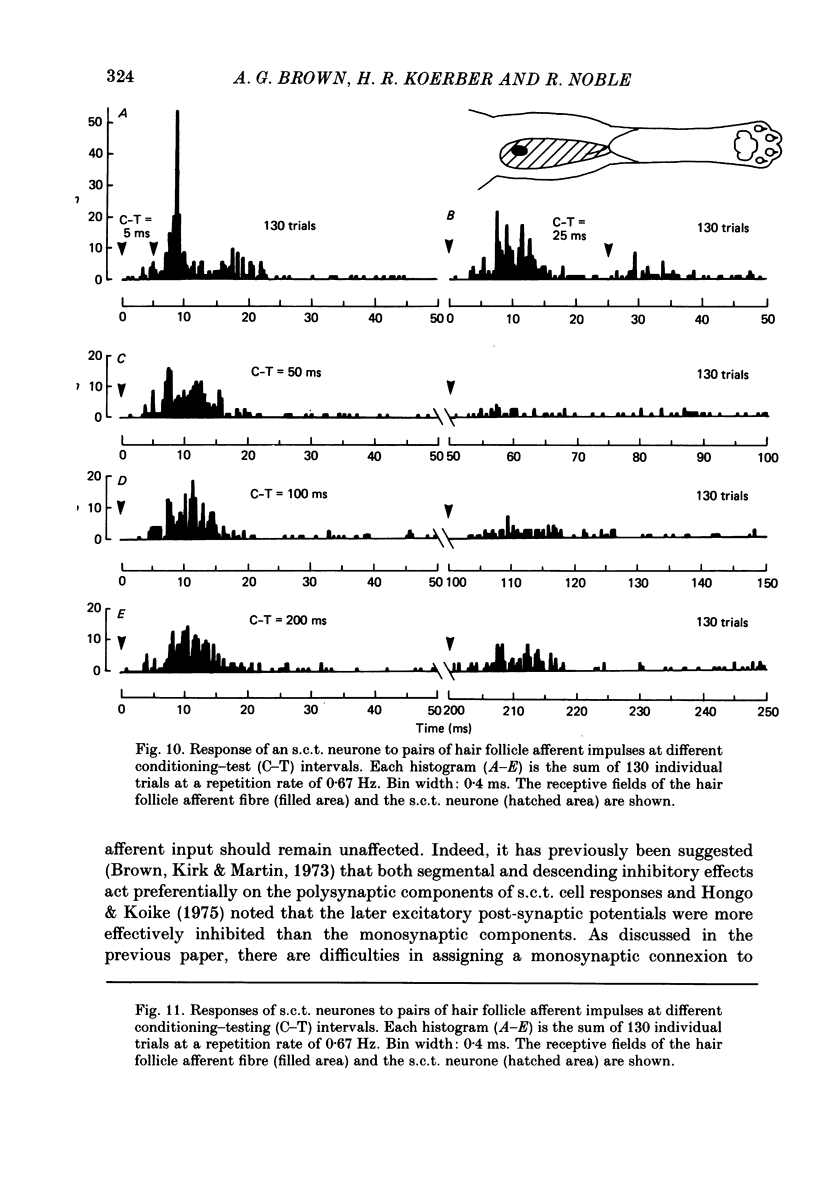
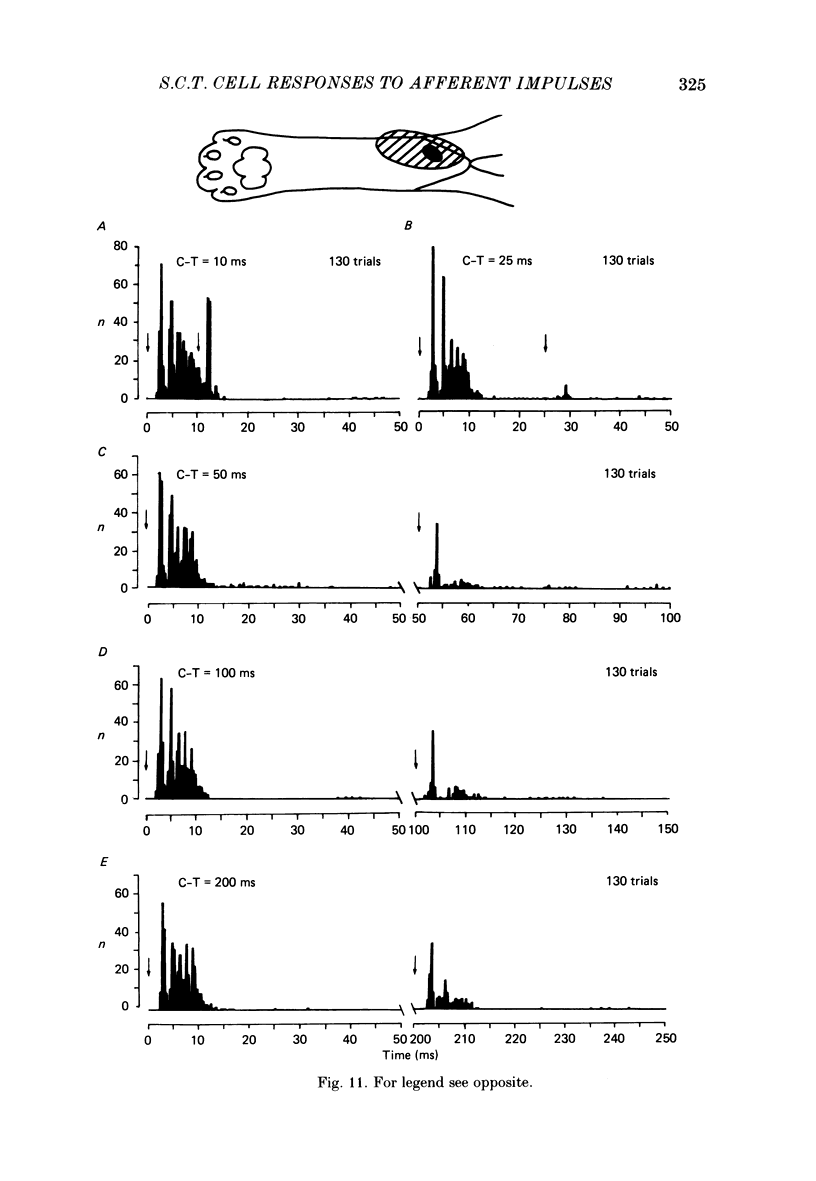
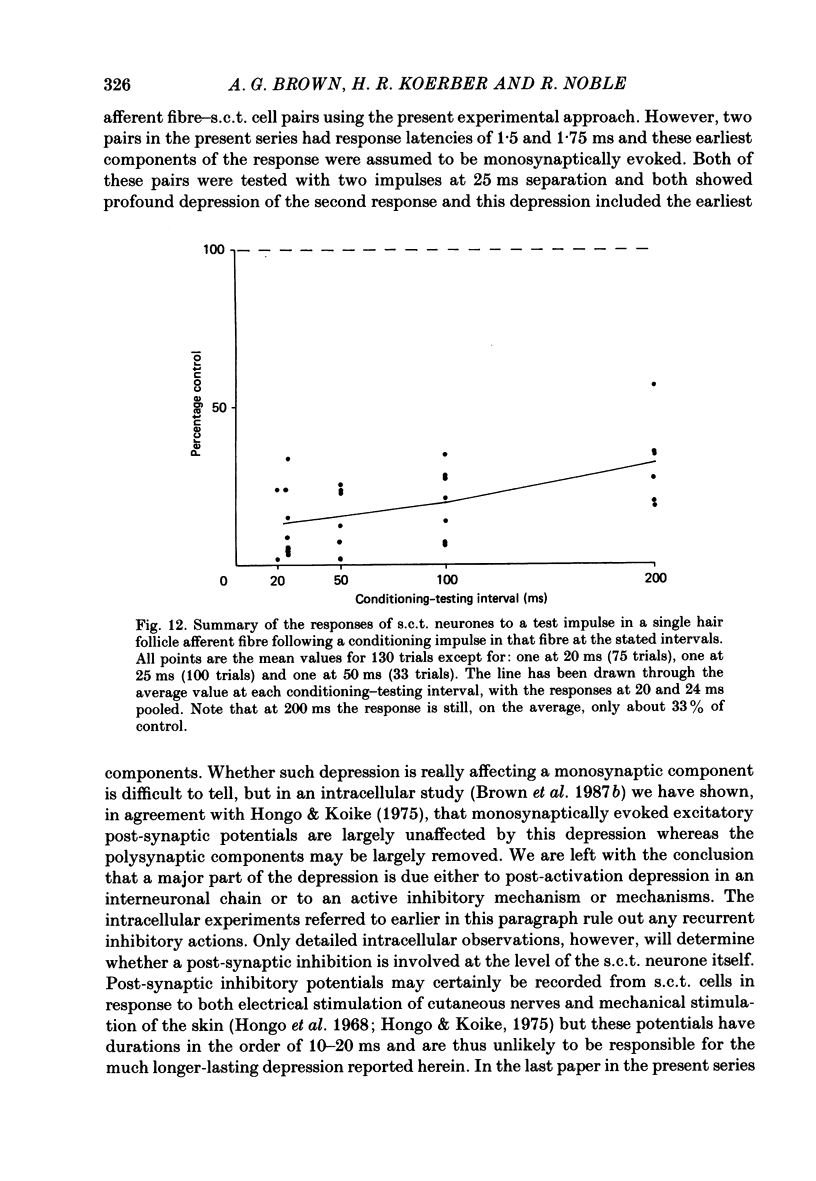


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown A. G., Brown P. B., Fyffe R. E., Pubols L. M. Receptive field organization and response properties of spinal neurones with axons ascending the dorsal columns in the cat. J Physiol. 1983 Apr;337:575–588. doi: 10.1113/jphysiol.1983.sp014643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., Fyffe R. E. Form and function of dorsal horn neurones with axons ascending the dorsal columns in cat. J Physiol. 1981 Dec;321:31–47. doi: 10.1113/jphysiol.1981.sp013970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., Kirk E. J., Martin H. F., 3rd Descending and segmental inhibition of transmission through the spinocervical tract. J Physiol. 1973 May;230(3):689–705. doi: 10.1113/jphysiol.1973.sp010212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., Koerber H. R., Noble R. An intracellular study of spinocervical tract cell responses to natural stimuli and single hair afferent fibres in cats. J Physiol. 1987 Jan;382:331–354. doi: 10.1113/jphysiol.1987.sp016370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., Koerber H. R., Noble R. Excitatory actions of single impulses in single hair follicle afferent fibres on spinocervical tract neurones in the cat. J Physiol. 1987 Jan;382:291–312. doi: 10.1113/jphysiol.1987.sp016368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., Noble R., Rowe M. J. Receptive field profiles and integrative properties of spinocervical tract cells in the cat. J Physiol. 1986 May;374:335–348. doi: 10.1113/jphysiol.1986.sp016082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G. The spinocervical tract. Prog Neurobiol. 1981;17(1-2):59–96. doi: 10.1016/0301-0082(81)90004-6. [DOI] [PubMed] [Google Scholar]
- Hongo T., Jankowska E., Lundberg A. Post-synaptic excitation and inhibition from primary afferents in neurones of the spinocervical tract. J Physiol. 1968 Dec;199(3):569–592. doi: 10.1113/jphysiol.1968.sp008669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell D. J., Bannatyne B. A., Fyffe R. E., Brown A. G. Ultrastructure of hair follicle afferent fibre terminations in the spinal cord of the cat. J Neurocytol. 1982 Aug;11(4):571–582. doi: 10.1007/BF01262425. [DOI] [PubMed] [Google Scholar]
- Maxwell D. J., Fyffe R. E., Brown A. G. Fine structure of normal and degenerating primary afferent boutons associated with characterized spinocervical tract neurons in the cat. Neuroscience. 1984 May;12(1):151–163. doi: 10.1016/0306-4522(84)90144-1. [DOI] [PubMed] [Google Scholar]
- Maxwell D. J., Fyffe R. E., Brown A. G. Fine structure of spinocervical tract neurones and the synaptic boutons in contact with them. Brain Res. 1982 Feb 11;233(2):394–399. doi: 10.1016/0006-8993(82)91212-4. [DOI] [PubMed] [Google Scholar]
- Tapper D. N., Wiesenfeld Z. A dorsal spinal neural network in cat. I. Responses to single impulses in single type I cutaneous input fibers. J Neurophysiol. 1980 Dec;44(6):1190–1213. doi: 10.1152/jn.1980.44.6.1190. [DOI] [PubMed] [Google Scholar]
- Tapper D. N., Wiesenfeld Z., Craig A. D., Jr A dorsal spinal neural network in cat. II. Changes in responsiveness initiated by single conditioning impulses in single type 1 cutaneous input fibers. J Neurophysiol. 1983 Feb;49(2):534–547. doi: 10.1152/jn.1983.49.2.534. [DOI] [PubMed] [Google Scholar]


