Abstract
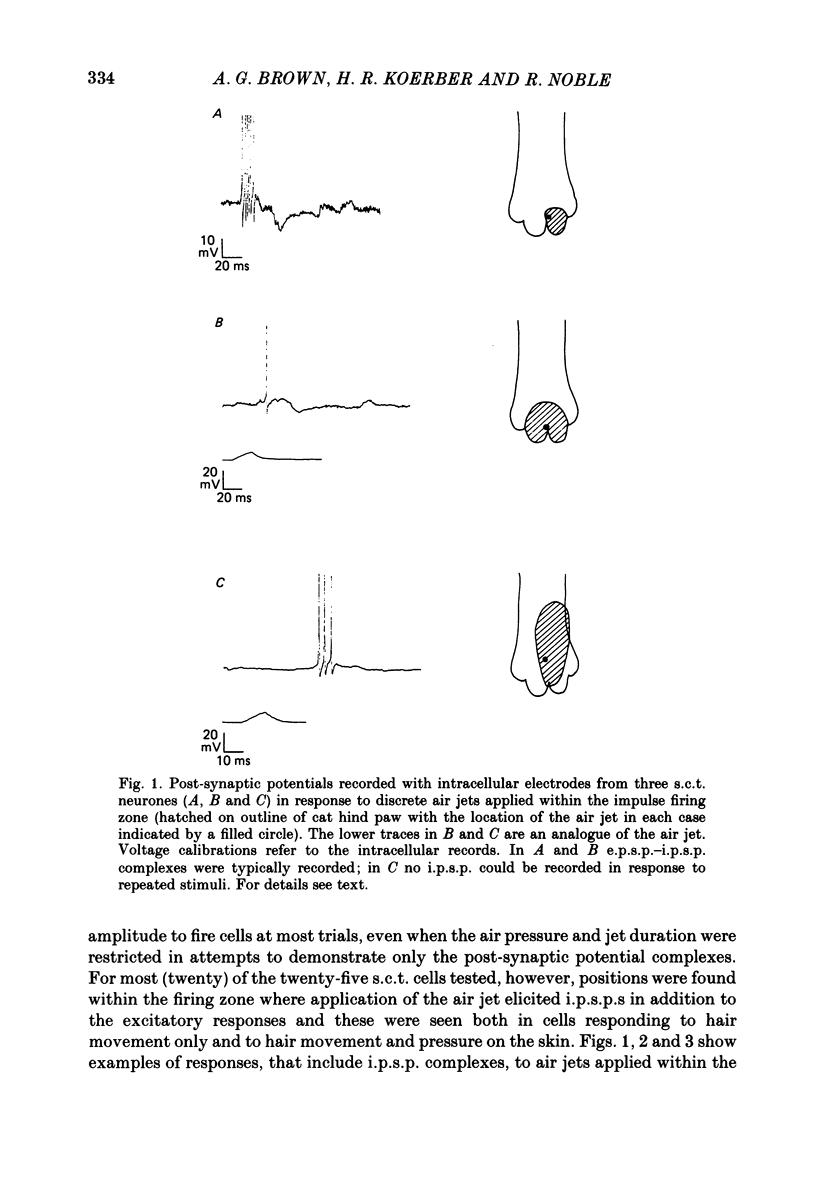
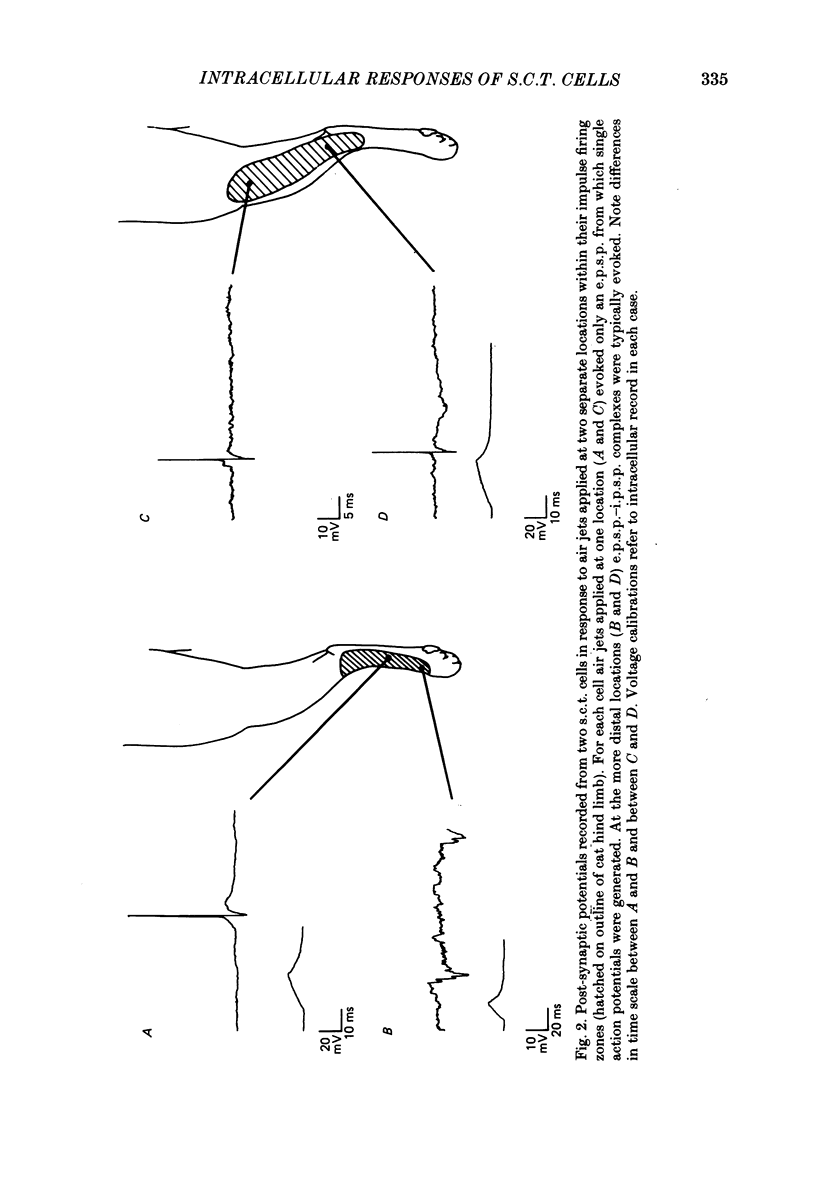
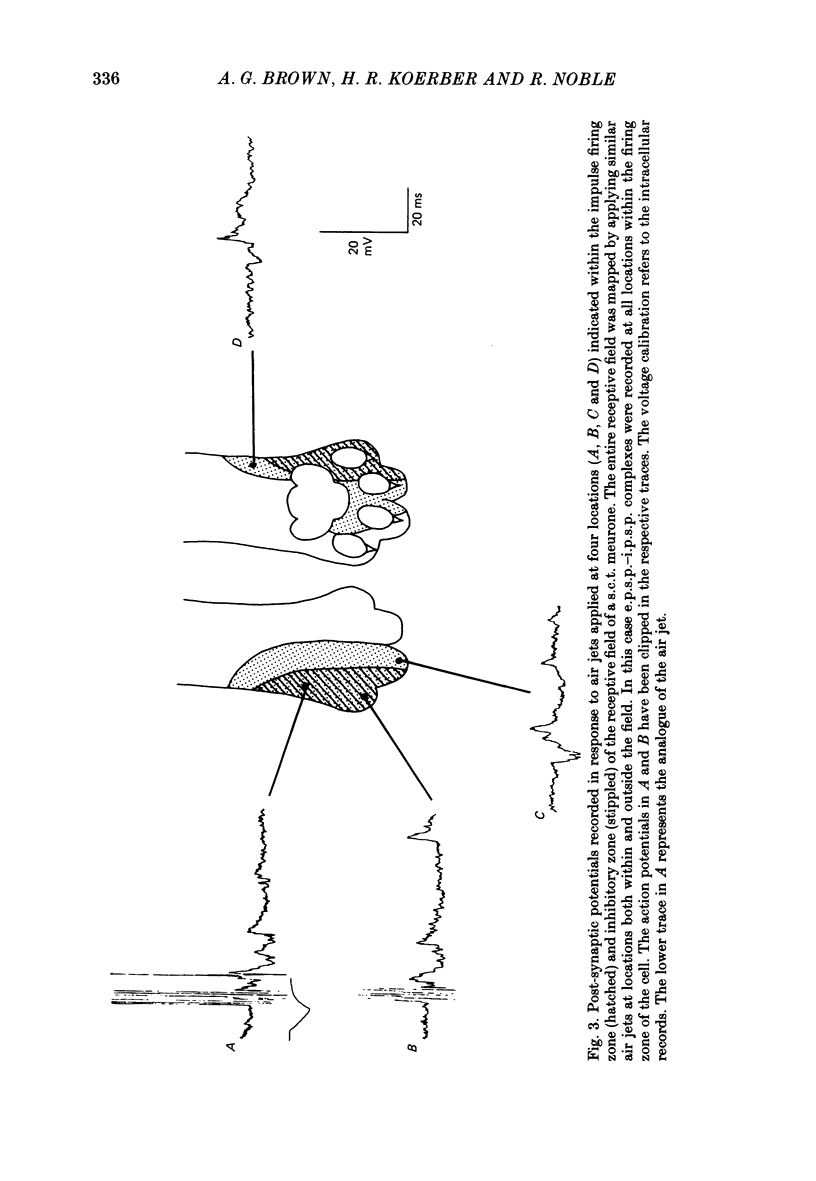
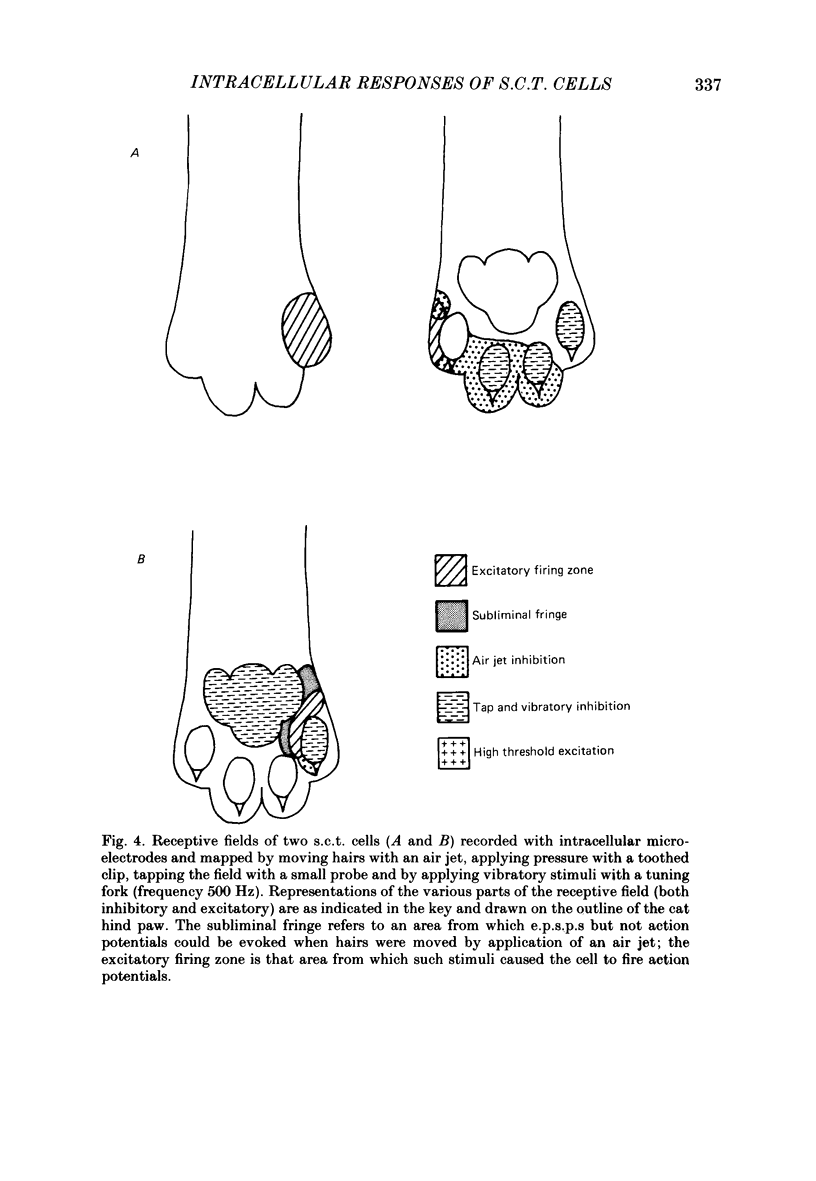
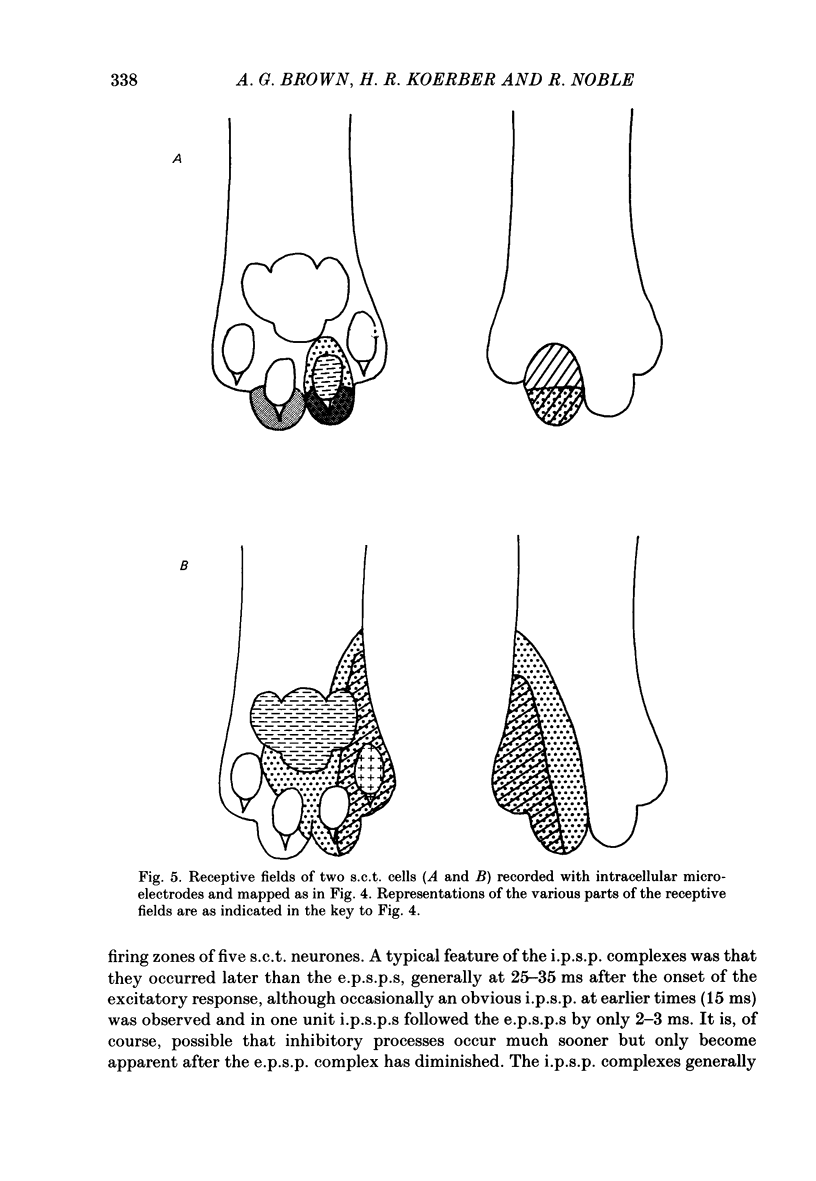
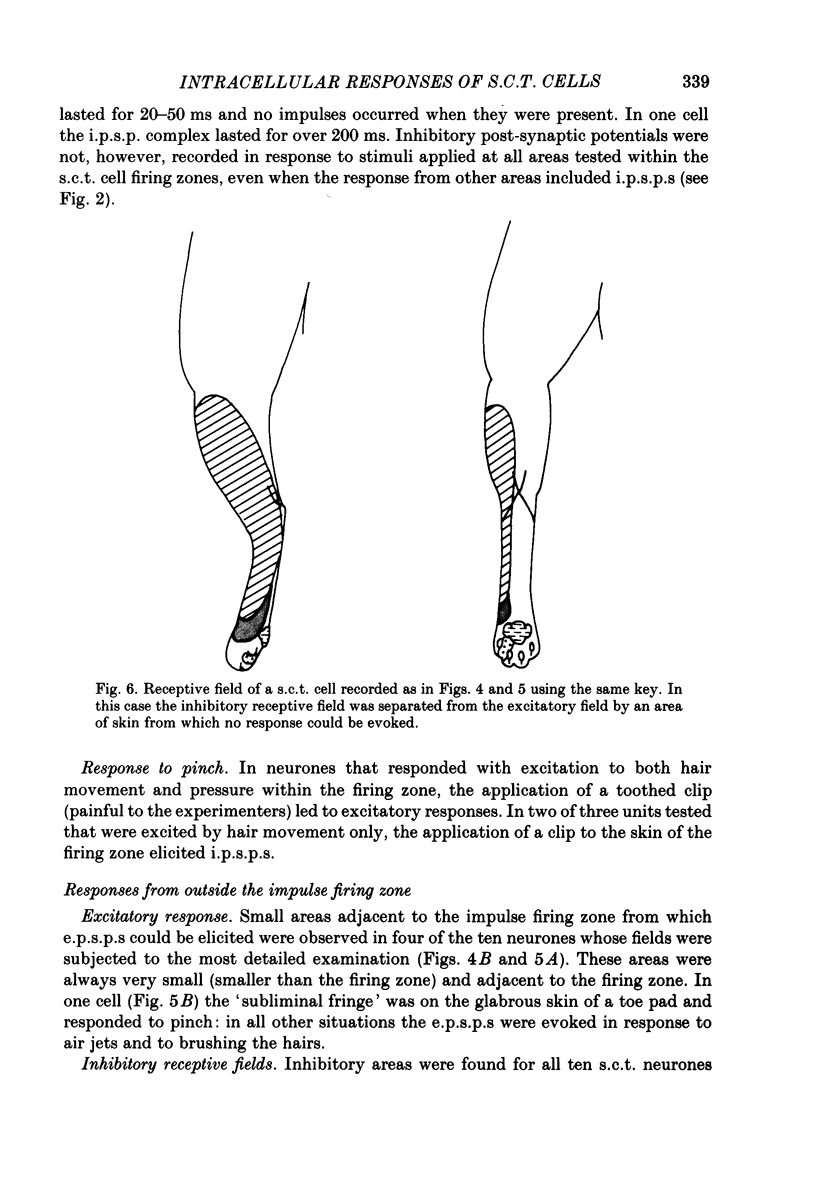
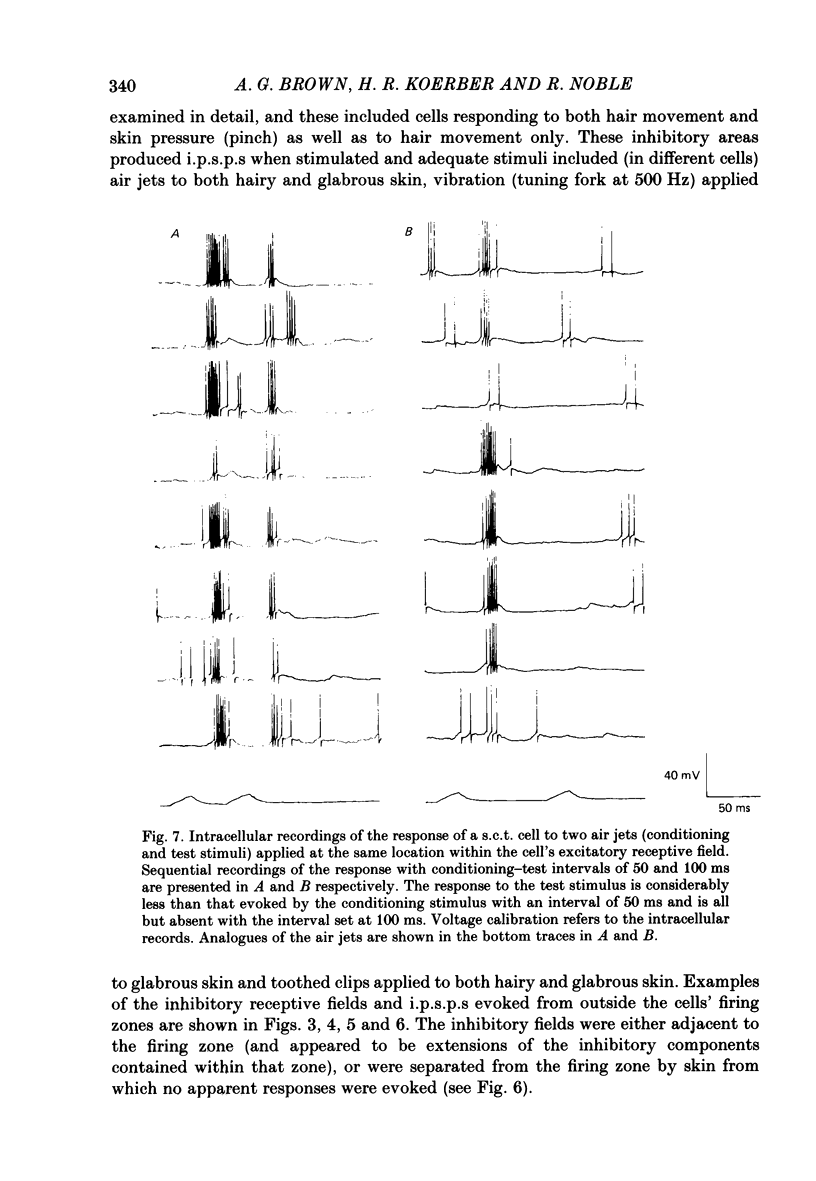
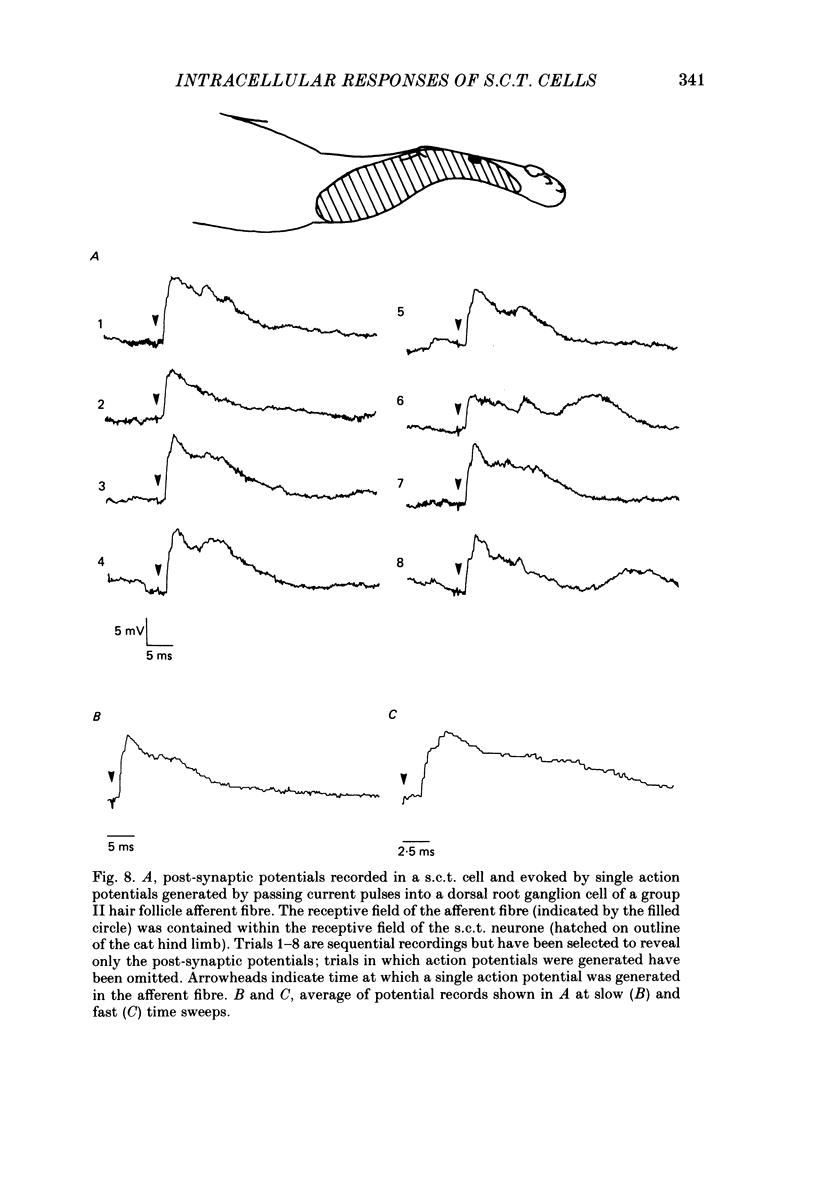
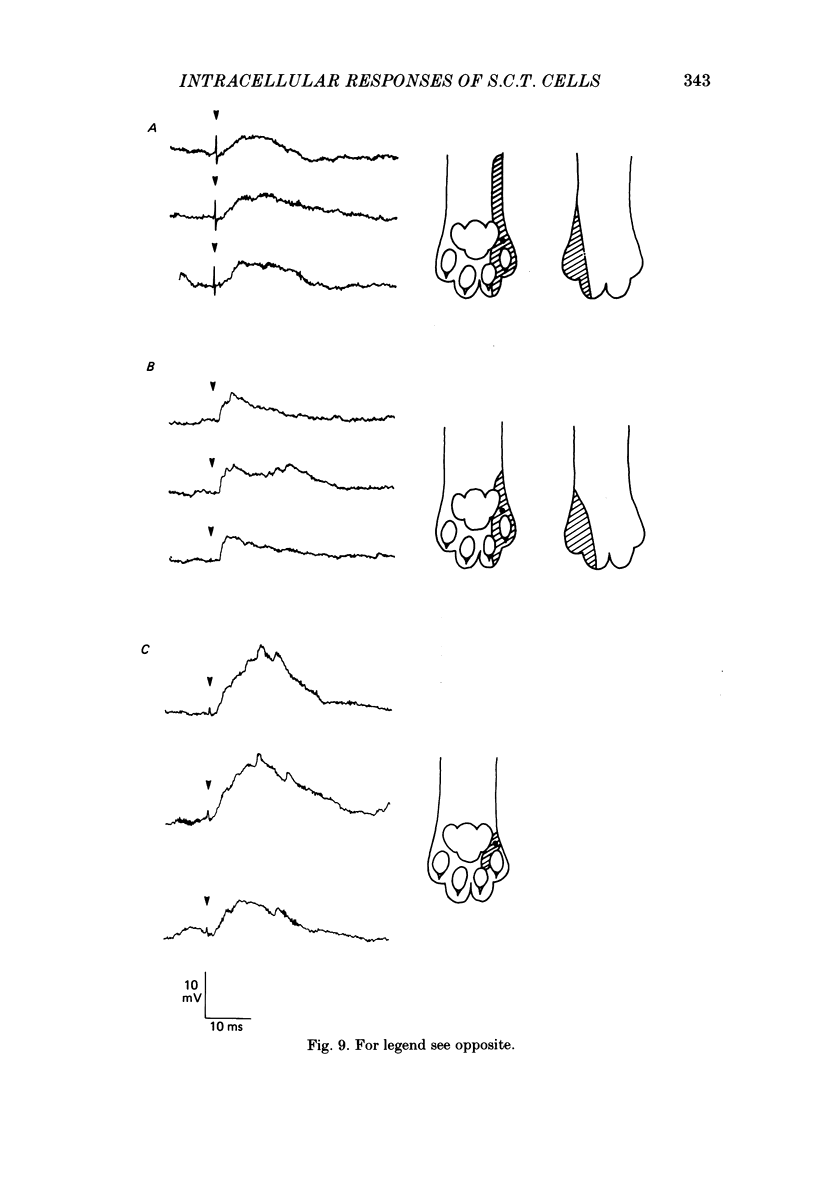
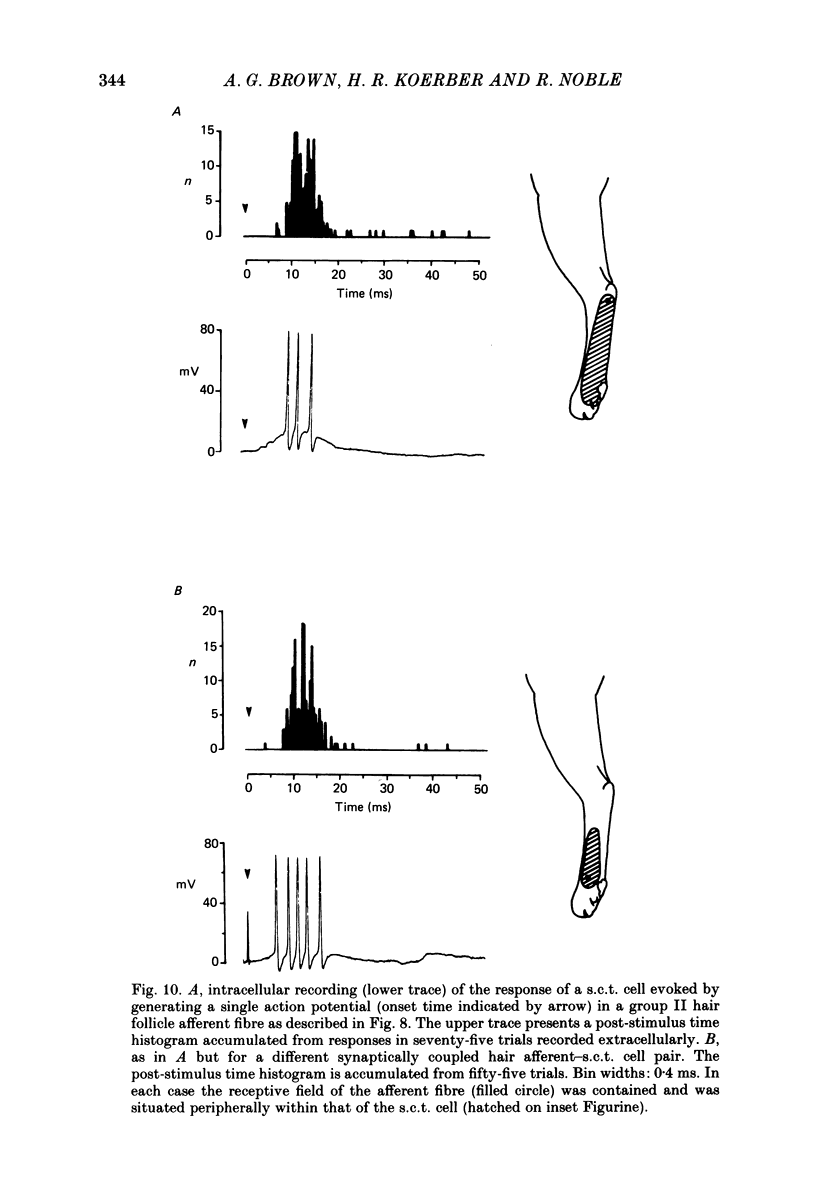
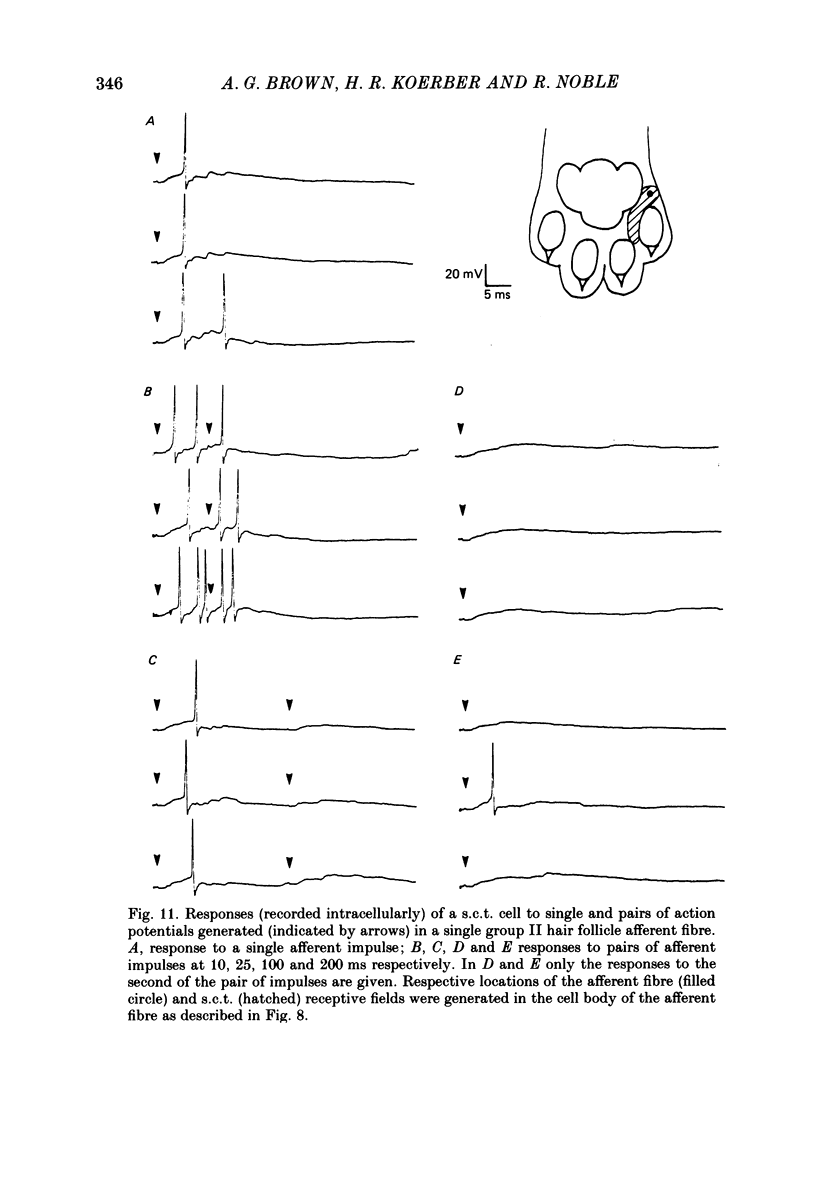
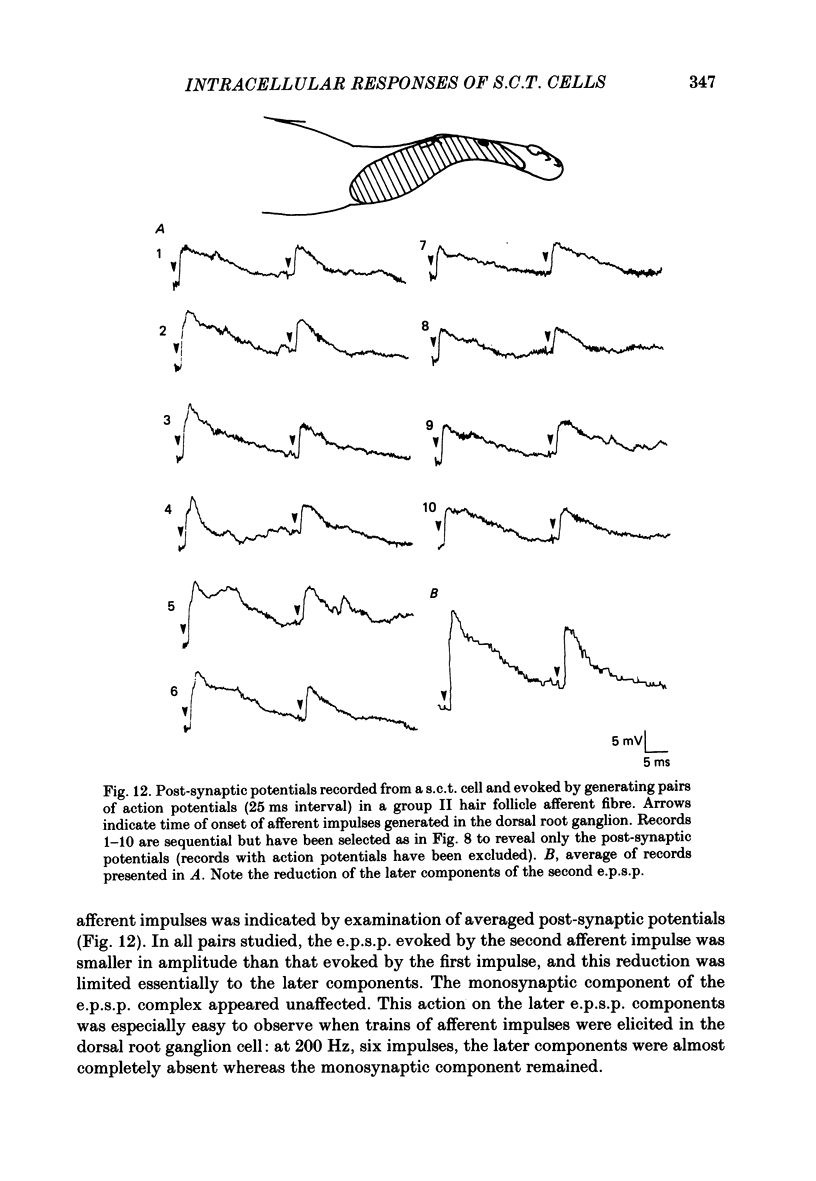
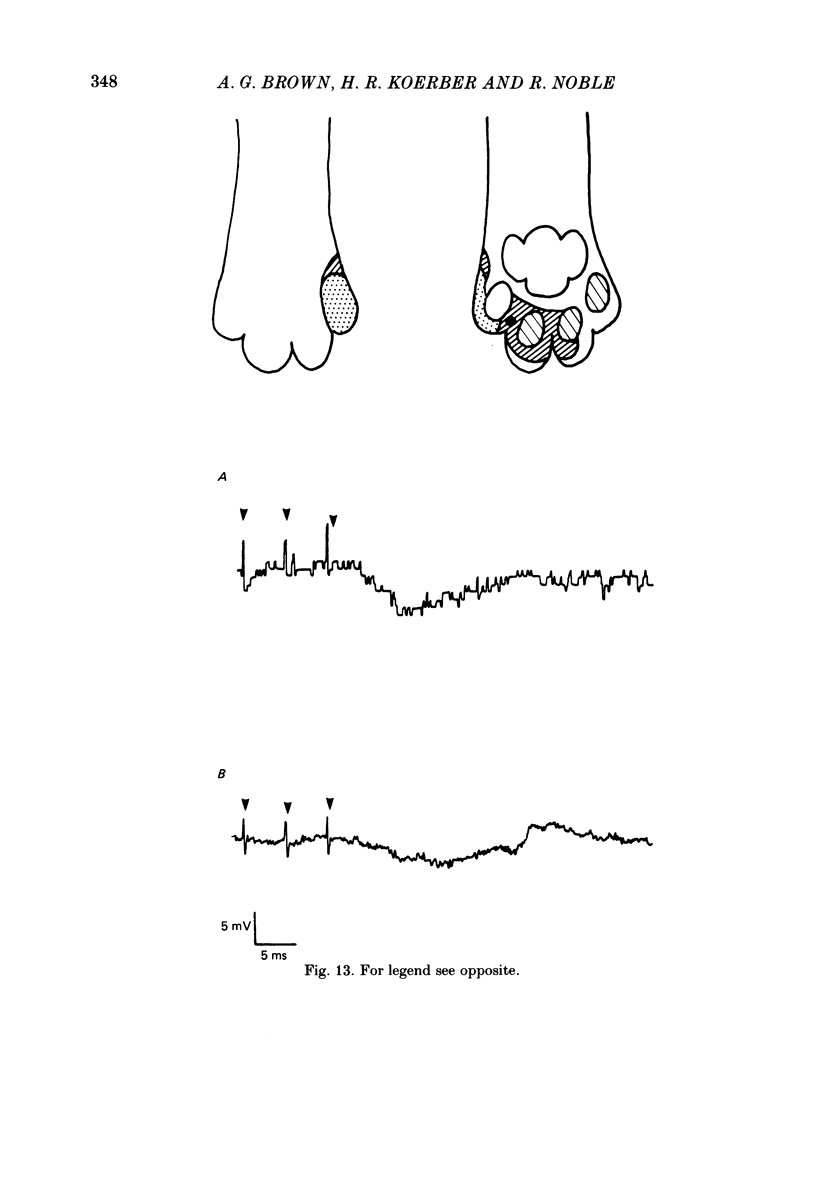
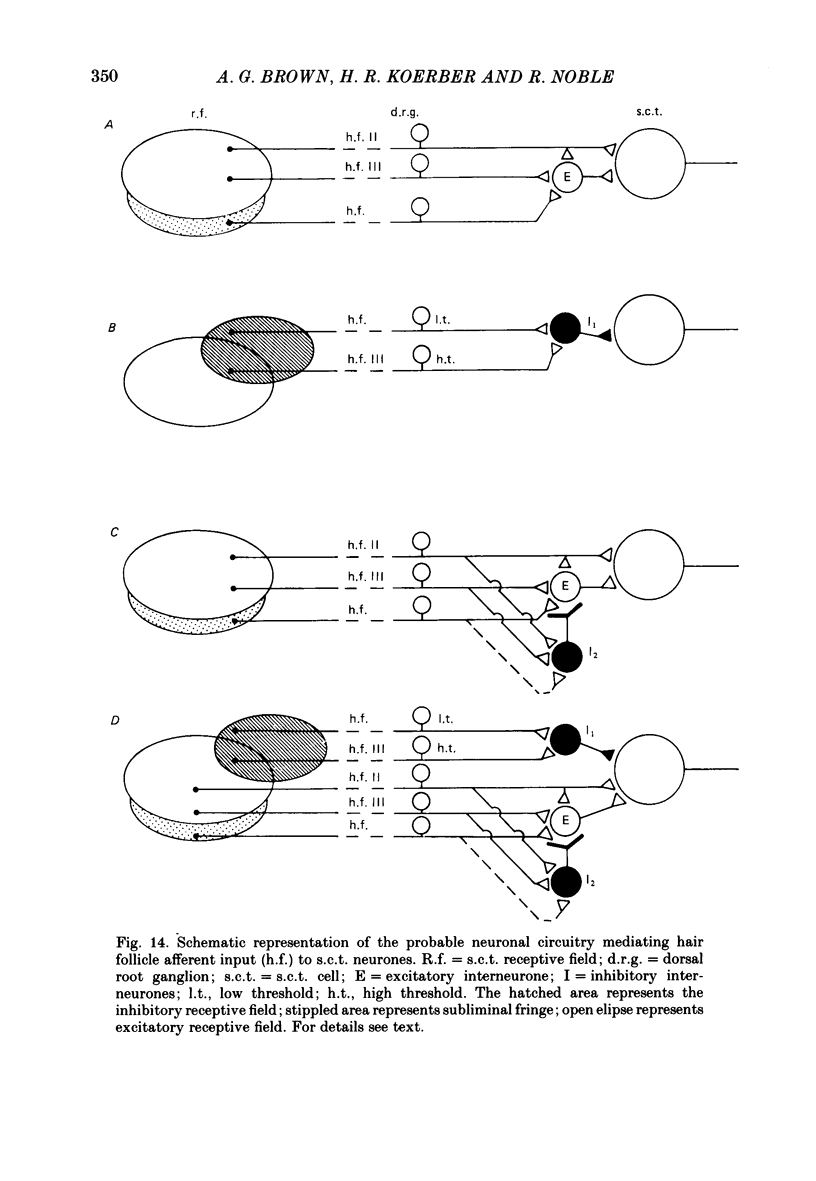
1. Intracellular recordings were made from spinocervical tract (s.c.t.) neurones in cats anaesthetized with chloralose and paralysed with gallamine triethiodide. 2. In one series of experiments the cells' receptive fields were examined with the use of natural stimuli. Hair movement within the impulse firing zone of the cell evoked excitatory post-synaptic potentials (e.p.s.p.s) from which impulses were generated; in addition, in the majority of s.c.t. cells tested, areas were found within the impulse firing zone where hair movement elicited both e.p.s.p.s and inhibitory post-synaptic potentials (i.p.s.p.s). Outside the firing zones, both regions evoking e.p.s.p.s and regions evoking i.p.s.p.s were observed in all neurones examined in detail (ten cells). The responses of these neurones to a variety of natural stimuli showed the receptive fields of s.c.t. cells to be more complex than previously thought. 3. In a second series of experiments, intracellular recordings from s.c.t. cells were combined with intracellular recording and stimulation of single dorsal root ganglion cells belonging to group II hair follicle afferent fibres. When the afferent fibres innervated skin within the impulse firing zone of the s.c.t. cell, single afferent impulses evoked e.p.s.p. complexes consisting of both mono- and polysynaptic components; no i.p.s.p.s were observed in response to single hair follicle afferent impulses or to trains. Although the monosynaptic e.p.s.p. component was often large and had a fast rise time, s.c.t. cell impulses usually arose from the later components. Afferent fibres innervating the central region of the s.c.t. cell firing zones tended to evoke relatively large e.p.s.p.s with fast rise times. The rise times and amplitudes of the e.p.s.p.s evoked by afferent fibres from the periphery, however, varied between afferent fibres but included the slowest and smallest in the total sample of synaptically coupled pairs. Afferent fibres from outside the s.c.t. cell's firing zone were usually ineffective in setting up post-synaptic potentials, but one group III hair follicle afferent fibre, from an inhibitory receptive field component, gave rise to i.p.s.p.s. 4. The effects of pairs and trains of afferent impulses at intervals of 10, 25, 50, 100 and 200 ms were examined. At 25 ms the response to the second afferent impulse was profoundly less than that evoked by the first and was still substantially reduced at 200 ms interval. In all synaptically coupled pairs studied, the e.p.s.p. complex evoked by the second afferent impulse was smaller in amplitude than that evoked by the first.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown A. G. Effects of descending impulses on transmission through the spinocervical tract. J Physiol. 1971 Dec;219(1):103–125. doi: 10.1113/jphysiol.1971.sp009652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., Franz D. N. Responses of spinocervical tract neurones to natural stimulation of identified cutaneous receptors. Exp Brain Res. 1969;7(3):231–249. doi: 10.1007/BF00239031. [DOI] [PubMed] [Google Scholar]
- Brown A. G., Kirk E. J., Martin H. F., 3rd Descending and segmental inhibition of transmission through the spinocervical tract. J Physiol. 1973 May;230(3):689–705. doi: 10.1113/jphysiol.1973.sp010212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., Koerber H. R., Noble R. Actions of trains and pairs of impulses from single primary afferent fibres on single spinocervical tract cells in cat. J Physiol. 1987 Jan;382:313–329. doi: 10.1113/jphysiol.1987.sp016369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., Koerber H. R., Noble R. Excitatory actions of single impulses in single hair follicle afferent fibres on spinocervical tract neurones in the cat. J Physiol. 1987 Jan;382:291–312. doi: 10.1113/jphysiol.1987.sp016368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., Noble R. Connexions between hair follicle afferent fibres and spinocervical tract neurones in the cat: the synthesis of receptive fields. J Physiol. 1982 Feb;323:77–91. doi: 10.1113/jphysiol.1982.sp014062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., Noble R., Rowe M. J. Receptive field profiles and integrative properties of spinocervical tract cells in the cat. J Physiol. 1986 May;374:335–348. doi: 10.1113/jphysiol.1986.sp016082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G. The spinocervical tract. Prog Neurobiol. 1981;17(1-2):59–96. doi: 10.1016/0301-0082(81)90004-6. [DOI] [PubMed] [Google Scholar]
- Cervero F., Iggo A., Molony V. Responses of spinocervical tract neurones to noxious stimulation of the skin. J Physiol. 1977 May;267(2):537–558. doi: 10.1113/jphysiol.1977.sp011825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hongo T., Jankowska E., Lundberg A. Post-synaptic excitation and inhibition from primary afferents in neurones of the spinocervical tract. J Physiol. 1968 Dec;199(3):569–592. doi: 10.1113/jphysiol.1968.sp008669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell D. J., Fyffe R. E., Brown A. G. Fine structure of normal and degenerating primary afferent boutons associated with characterized spinocervical tract neurons in the cat. Neuroscience. 1984 May;12(1):151–163. doi: 10.1016/0306-4522(84)90144-1. [DOI] [PubMed] [Google Scholar]


