Abstract
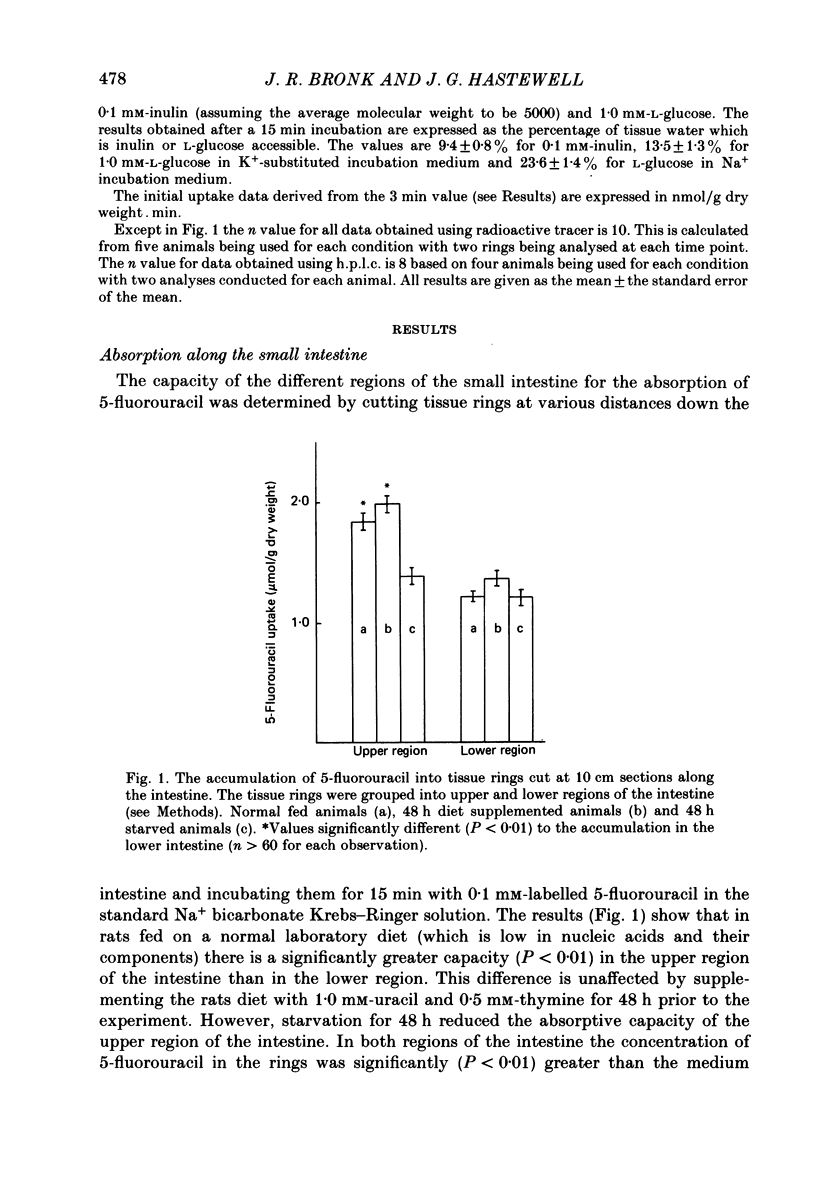
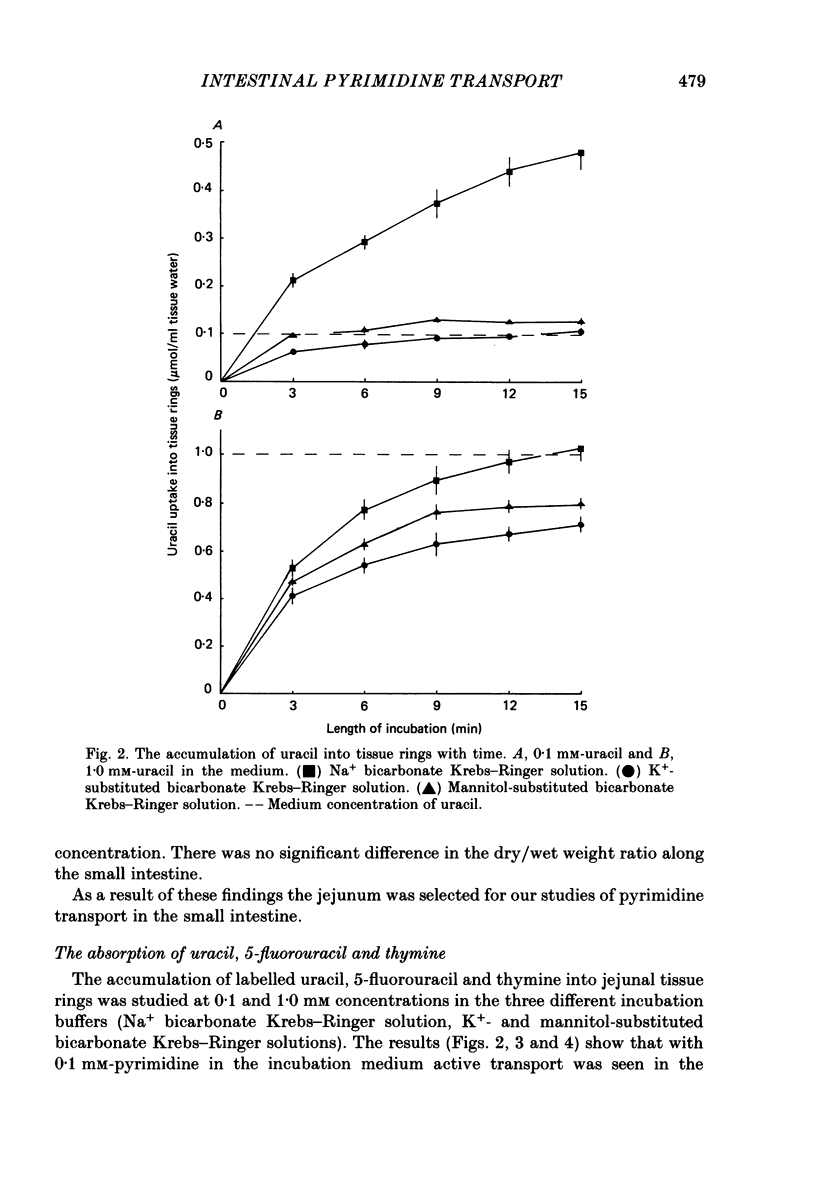
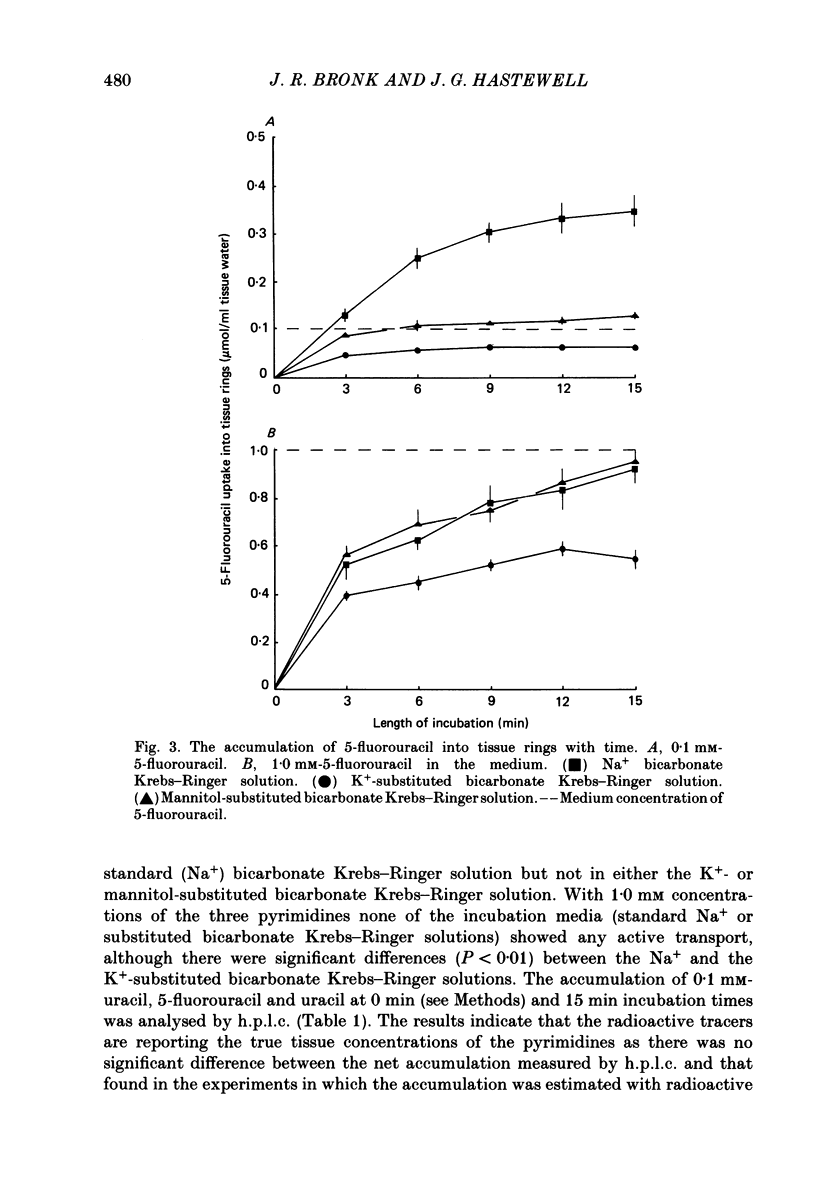
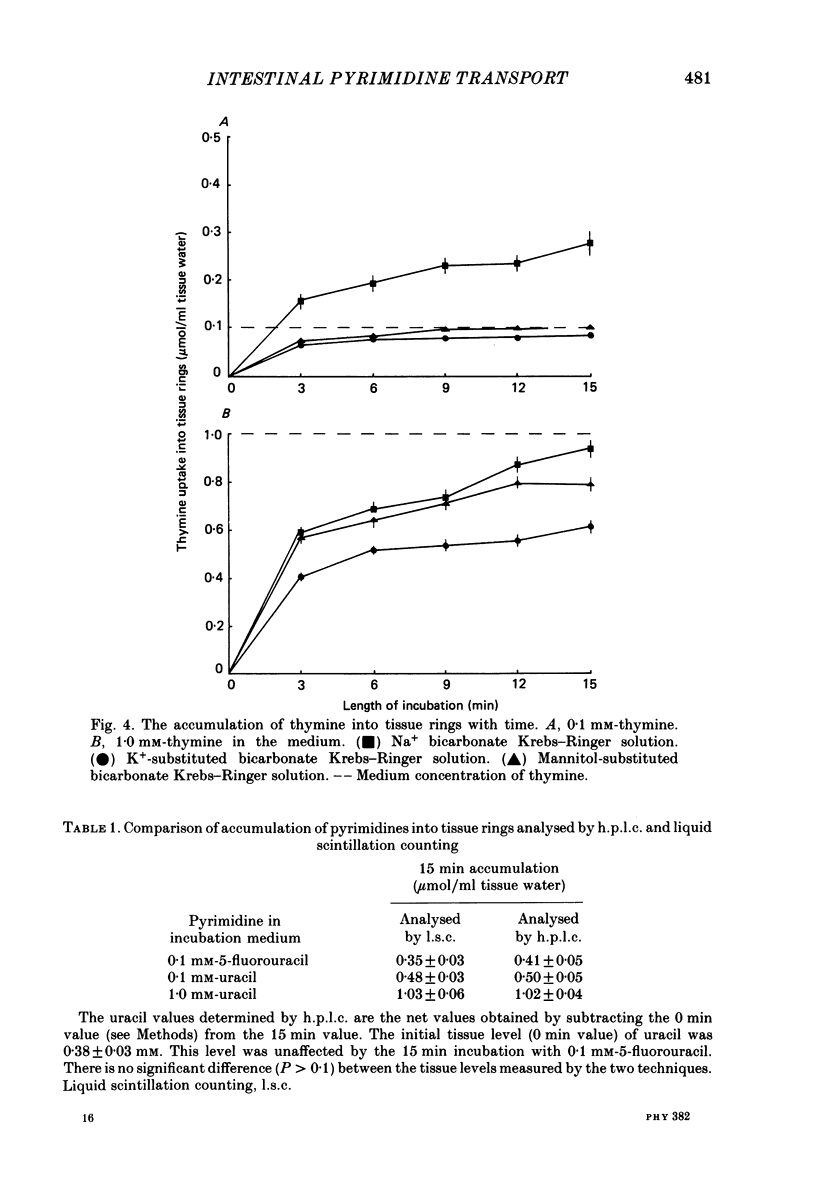
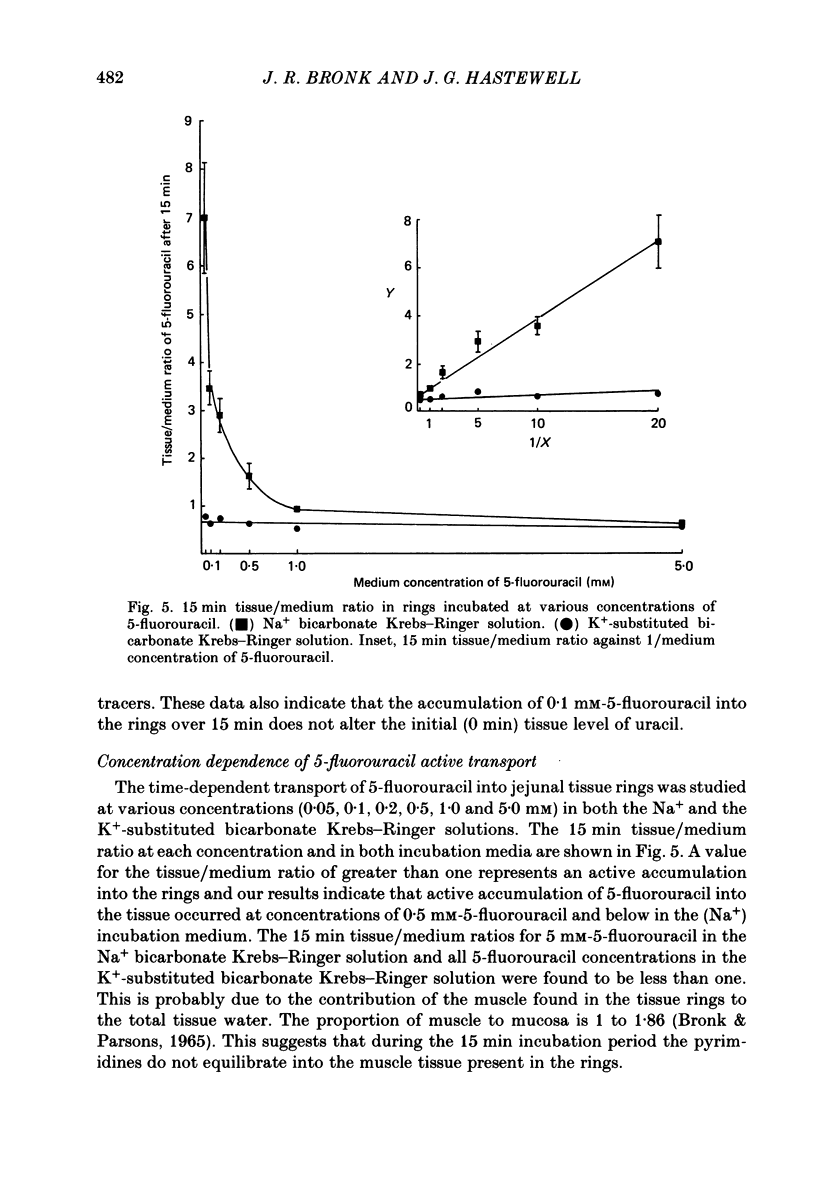
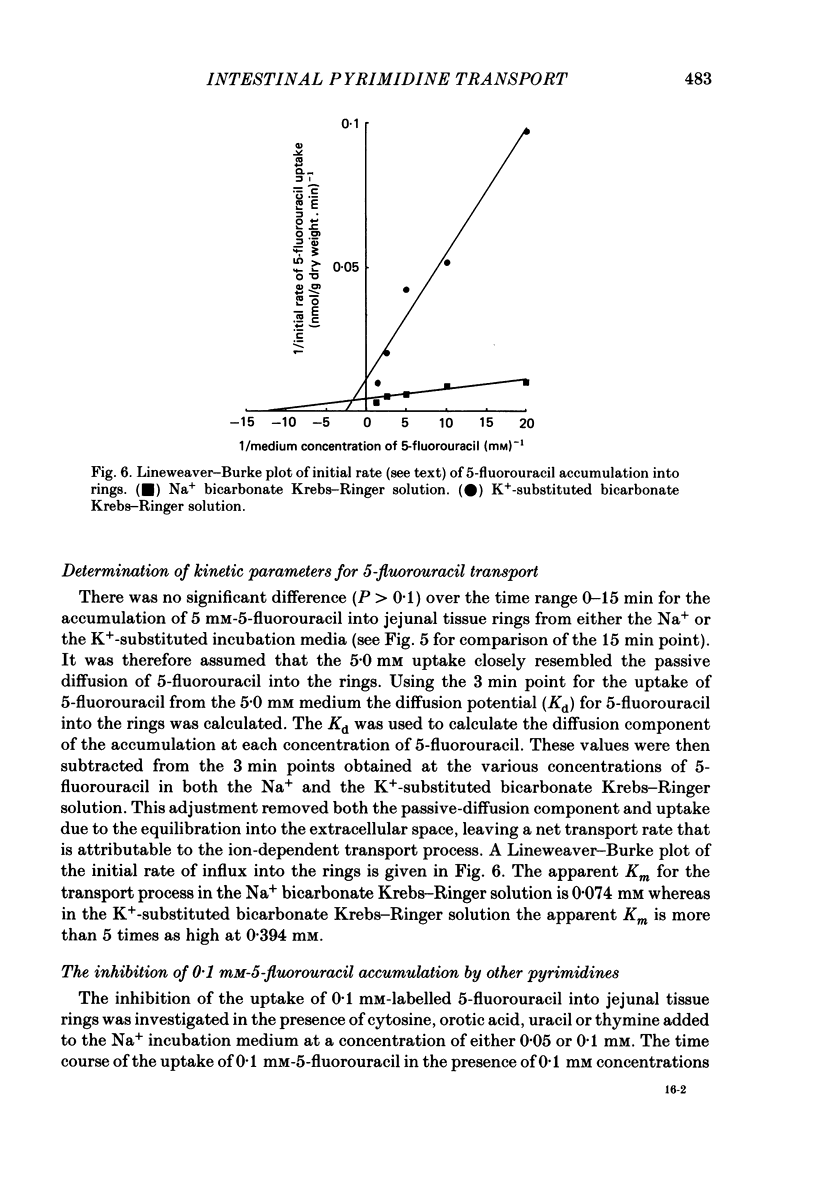
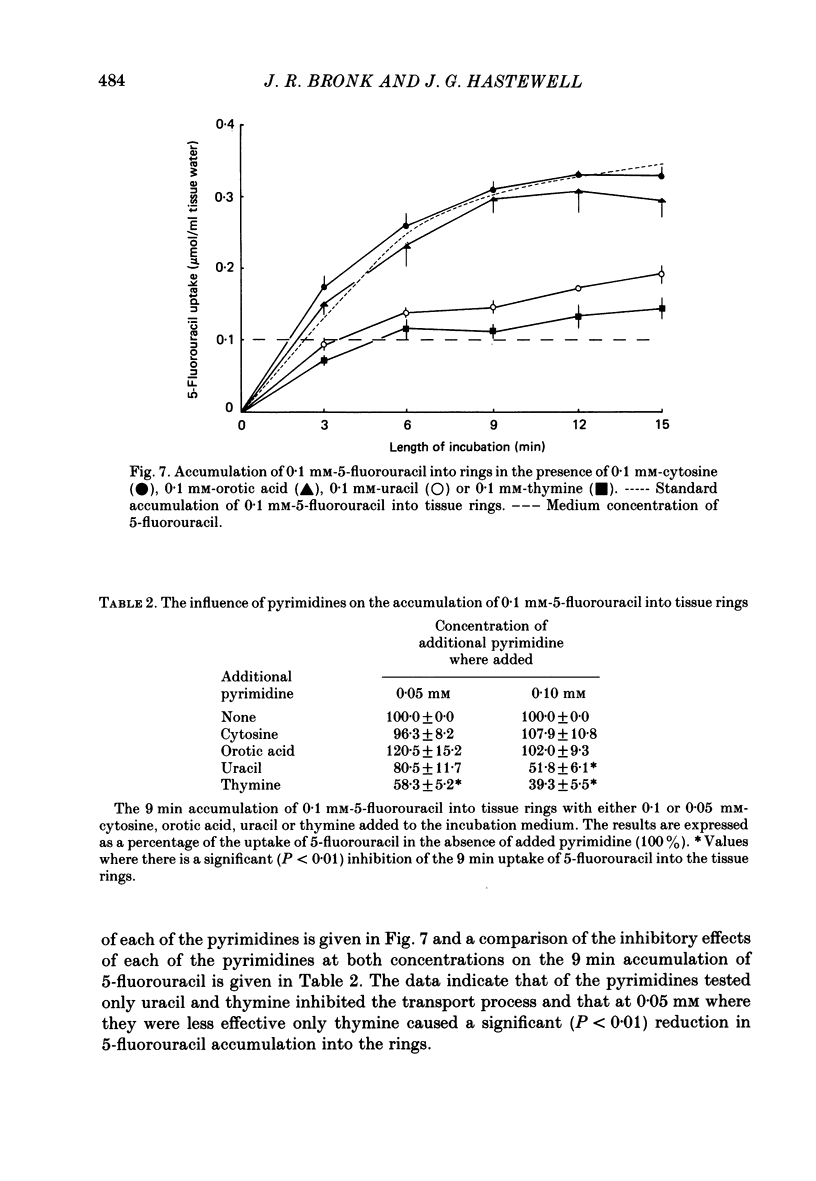
1. At low concentrations (0.1 mM) the transport of uracil, 5-fluorouracil and thymine into jejunal tissue rings is an active process. 2. The transport of 5-fluorouracil into tissue rings cut from the duodenum and jejunum was greater than the transport into rings cut from the ileum. This difference was abolished by starving the rats for 48 h before the experiment. 3. The active transport can be abolished by replacing the Na+ in the incubation medium with either K+ or mannitol, or by increasing the concentration of the pyrimidine to 1.0 mM. 4. The accumulation of uracil or 5-fluorouracil into the jejunal rings was identical when determined by radioactive tracer or by high-performance liquid chromatography. 5. The apparent Michaelis constant (Km) for 5-fluorouracil transport into jejunal rings was 0.074 mM in the standard Na+ bicarbonate Krebs-Ringer solution and 0.394 mM in the K+-substituted bicarbonate Krebs-Ringer solution. 6. Both thymine and uracil inhibited the transport of 5-fluorouracil into jejunal tissue rings: however, cytosine and orotic acid did not.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AGAR W. T., HIRD F. J., SIDHU G. S. The uptake of amino acids by the intestine. Biochim Biophys Acta. 1954 May;14(1):80–84. doi: 10.1016/0006-3002(54)90134-1. [DOI] [PubMed] [Google Scholar]
- Alvarado F., Lherminier M., Phan H. H. Hamster intestinal disaccharide absorption: extracellular hydrolysis precedes transport of the monosaccharide products. J Physiol. 1984 Oct;355:493–507. doi: 10.1113/jphysiol.1984.sp015434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronk J. R., Parsons D. S. The polarographic determination of the respiration of the small intestine of the rat. Biochim Biophys Acta. 1965 Oct 18;107(3):397–404. doi: 10.1016/0304-4165(65)90183-2. [DOI] [PubMed] [Google Scholar]
- CRANE R. K. Intestinal absorption of sugars. Physiol Rev. 1960 Oct;40:789–825. doi: 10.1152/physrev.1960.40.4.789. [DOI] [PubMed] [Google Scholar]
- CRANE R. K., MANDELSTAM P. The active transport of sugars by various preparations of hamster intestine. Biochim Biophys Acta. 1960 Dec 18;45:460–476. doi: 10.1016/0006-3002(60)91482-7. [DOI] [PubMed] [Google Scholar]
- CSAKAY T. Z. A possible link between active transport of electrolytes and nonelectrolyes. Fed Proc. 1963 Jan-Feb;22:3–7. [PubMed] [Google Scholar]
- Hopfer U., Sigrist-Nelson K., Groseclose R. Jejunal and ileal D-glucose transport in isolated brush border membranes. Biochim Biophys Acta. 1976 Mar 5;426(2):349–353. doi: 10.1016/0005-2736(76)90344-8. [DOI] [PubMed] [Google Scholar]
- Leese H. J., Mansford K. R. The effect of insulin and insulin deficiency on the transport and metabolism of glucose by rat small intestine. J Physiol. 1971 Feb;212(3):819–838. doi: 10.1113/jphysiol.1971.sp009358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M., Levine S. Kinetics of induced uphill transport of sugars in human erythrocytes. J Theor Biol. 1969 Jul;24(1):85–107. doi: 10.1016/s0022-5193(69)80008-1. [DOI] [PubMed] [Google Scholar]
- Parsons D. S., Shaw M. I. Use of high performance liquid chromatography to study absorption and metabolism of purines by rat jejunum in vitro. Q J Exp Physiol. 1983 Jan;68(1):53–67. doi: 10.1113/expphysiol.1983.sp002702. [DOI] [PubMed] [Google Scholar]
- SCHANKER L. S., JEFFREY J. J., TOCCO D. J. INTERACTION OF PURINES WITH THE PYRIMIDINE TRANSPORT PROCESS OF THE SMALL INTESTINE. Biochem Pharmacol. 1963 Sep;12:1047–1053. doi: 10.1016/0006-2952(63)90028-5. [DOI] [PubMed] [Google Scholar]
- SCHANKER L. S., TOCCO D. J. Active transport of some pyrimidines across the rat intestinal epithelium. J Pharmacol Exp Ther. 1960 Feb;128:115–121. [PubMed] [Google Scholar]
- SCHANKER L. S., TOCCO D. J. Some characteristics of the pyrimidine transport process of the small intestine. Biochim Biophys Acta. 1962 Jan 29;56:469–473. doi: 10.1016/0006-3002(62)90598-x. [DOI] [PubMed] [Google Scholar]
- Scharrer E., Amann B. Active intestinal transport of uracil in sheep. Ann Rech Vet. 1979;10(2-3):467–469. [PubMed] [Google Scholar]
- Schultz S. G., Curran P. F. Coupled transport of sodium and organic solutes. Physiol Rev. 1970 Oct;50(4):637–718. doi: 10.1152/physrev.1970.50.4.637. [DOI] [PubMed] [Google Scholar]
- Wohlhueter R. M., McIvor R. S., Plagemann P. G. Facilitated transport of uracil and 5-fluorouracil, and permeation of orotic acid into cultured mammalian cells. J Cell Physiol. 1980 Sep;104(3):309–319. doi: 10.1002/jcp.1041040305. [DOI] [PubMed] [Google Scholar]
- Yamamoto S., Kawasaki T. Active transport of 5-fluorouracil and its energy coupling in Ehrlich ascites tumor cells. J Biochem. 1981 Sep;90(3):635–642. doi: 10.1093/oxfordjournals.jbchem.a133517. [DOI] [PubMed] [Google Scholar]


