Abstract
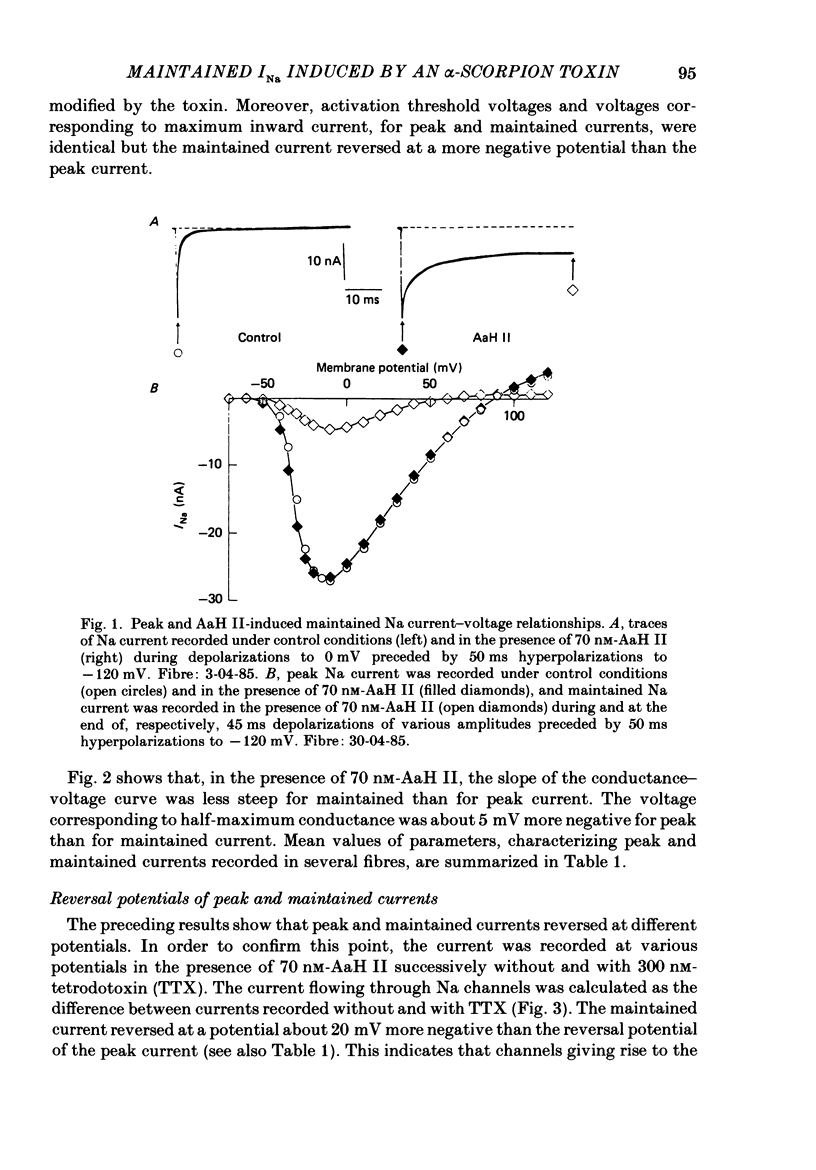
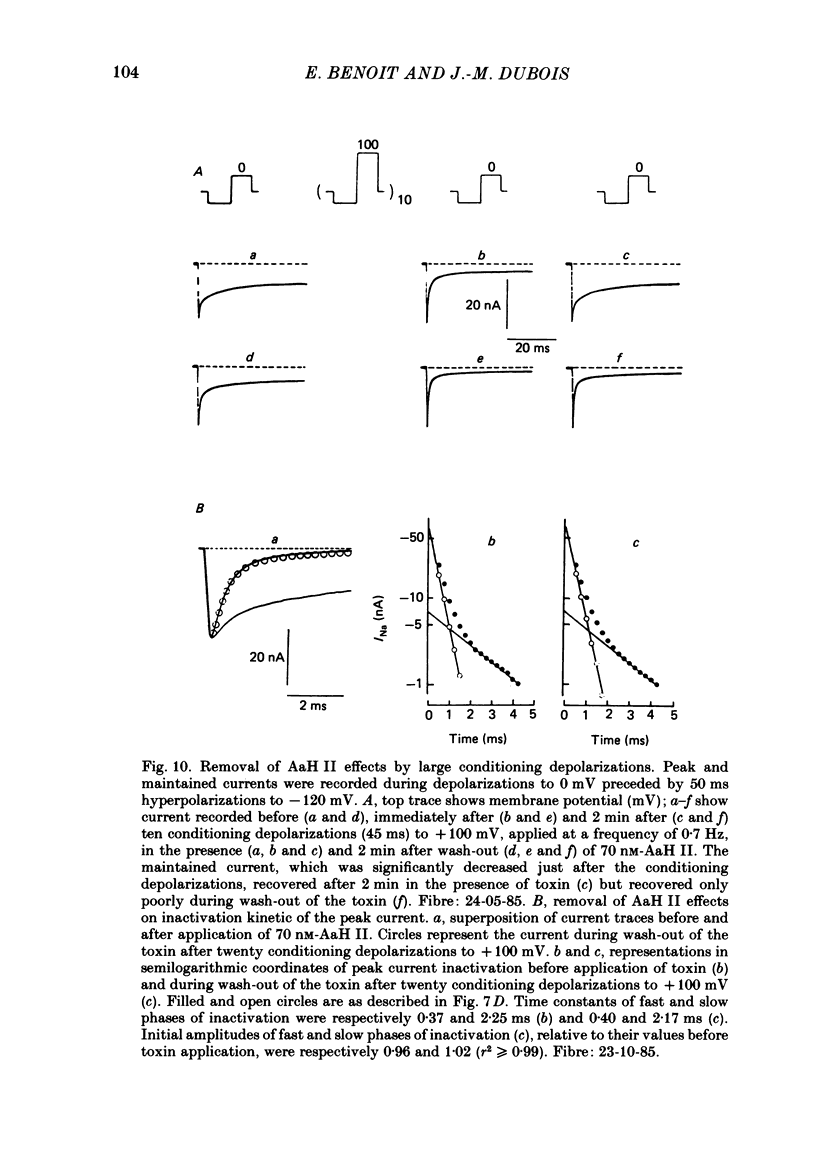
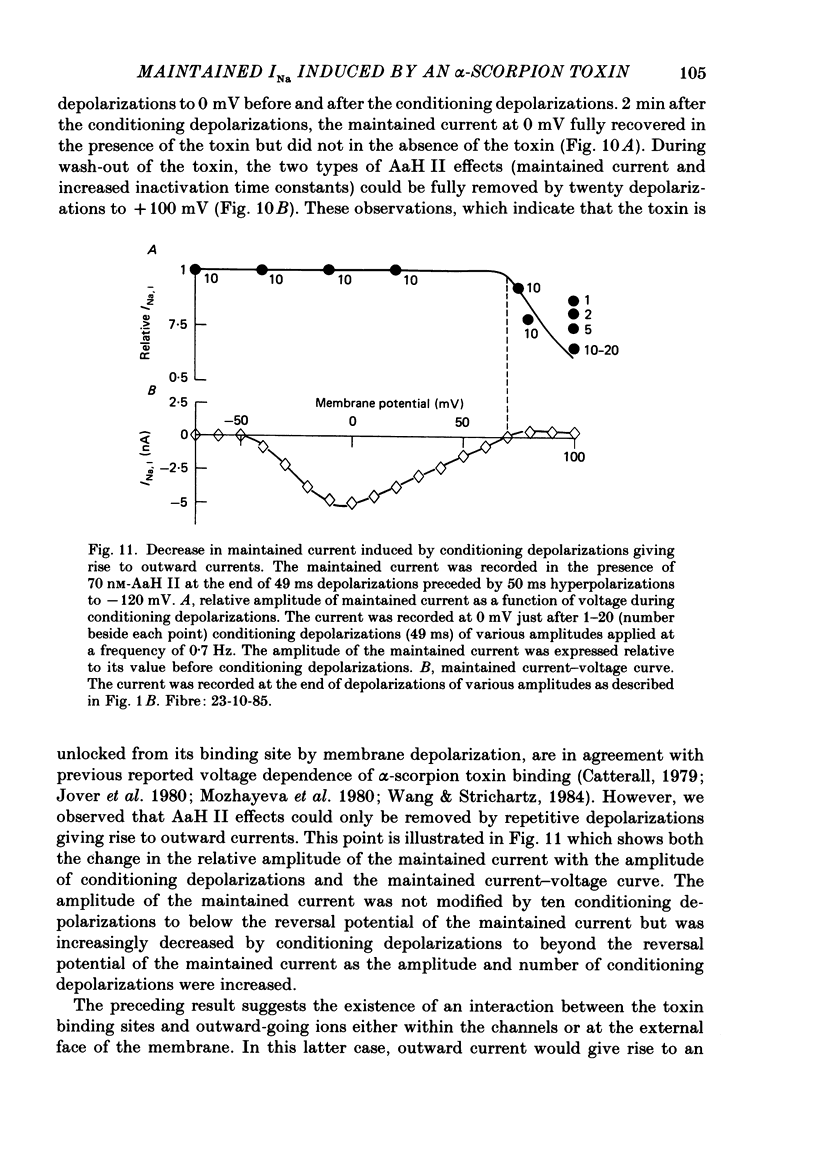
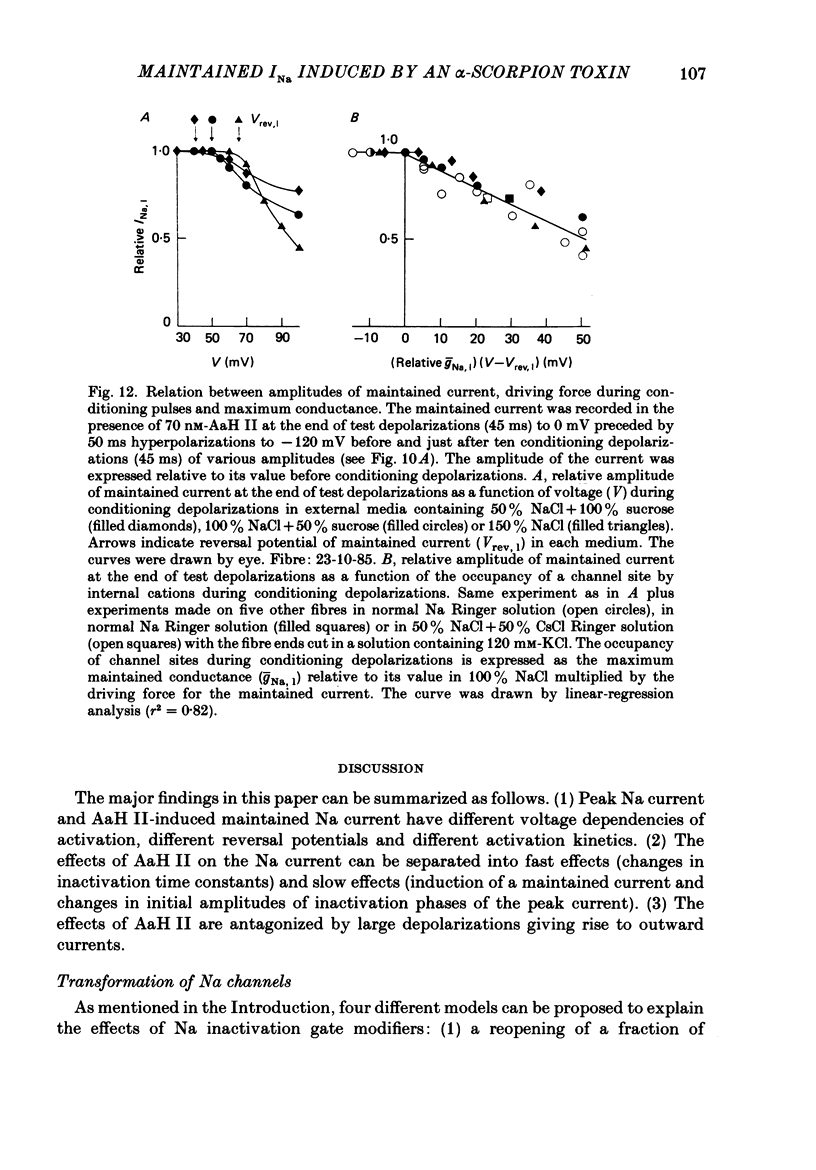
1. The effects of toxin II from scorpion Androctonus australis Hector (AaH II) on the Na current of frog myelinated nerve fibres were analysed under voltage-clamp conditions. 2. Like other alpha-scorpion toxins and Anemonia toxin II, AaH II both increased the inactivation time constants of peak Na current and induced a non-inactivatable Na current (maintained current). 3. In the presence of AaH II, the slope of the maintained conductance-voltage curve was less steep than that corresponding to the peak conductance and the maintained current reversed at a voltage about 20 mV more negative than the peak current. 4. When the peak current was inactivated by pre-depolarizations, 'on' and 'off' relaxation kinetics of the maintained current were an exponential function whose time constant changed with voltage in a bell-shaped manner. At 0 mV, the time constant was about 10 ms. 5. The effects of AaH II could be decomposed into fast effects (increase in inactivation time constants of the peak current) which developed within about 5 s and slow effects (increase in maintained current and changes in initial amplitudes of fast and slow phases of peak current inactivation) which developed within about 30 s. 6. These two types of AaH II effects could be completely removed by conditioning depolarizations giving rise to outward currents. 7. A model is proposed in which the binding of the toxin with its receptor is modulated by membrane potential and internal cations, the appearance of the maintained current is modulated by the environment of channels and the change in inactivation time constants is modulated by membrane potential. The maintained current would correspond to the transformation of a fraction of channels into a non-inactivable (late) form.
Full text
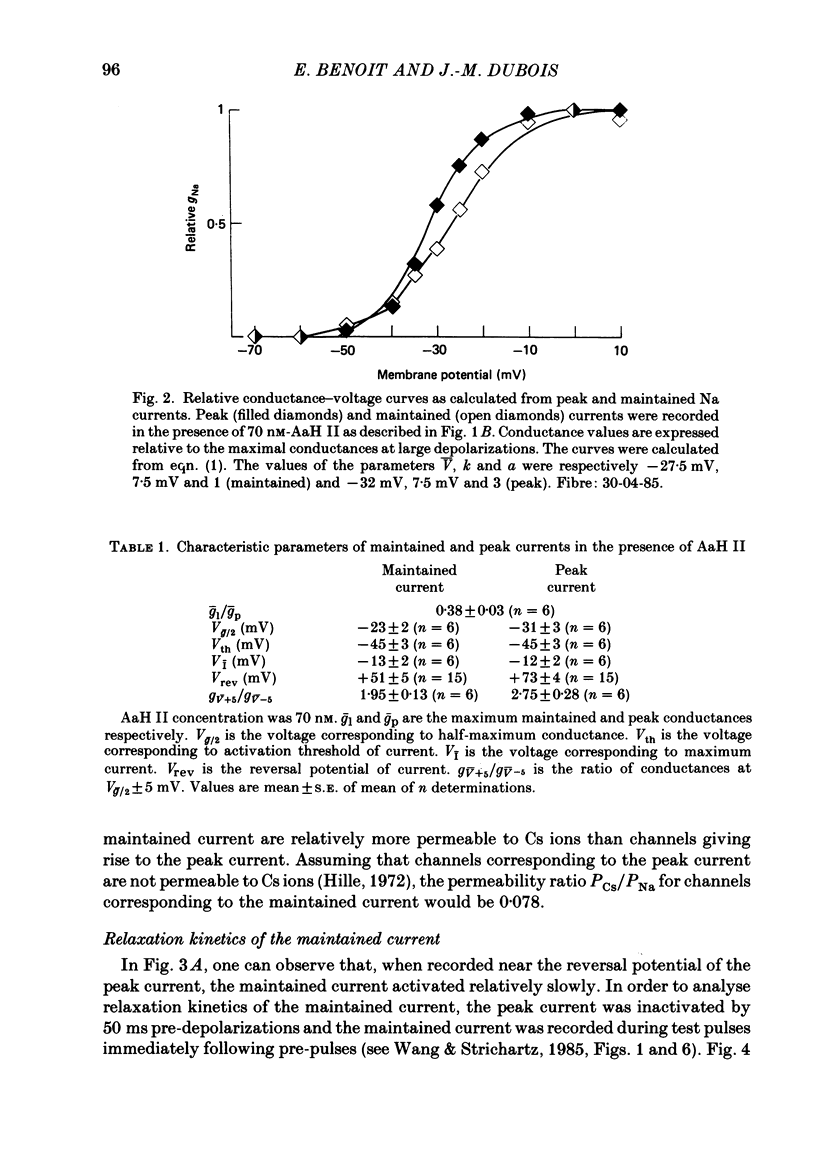
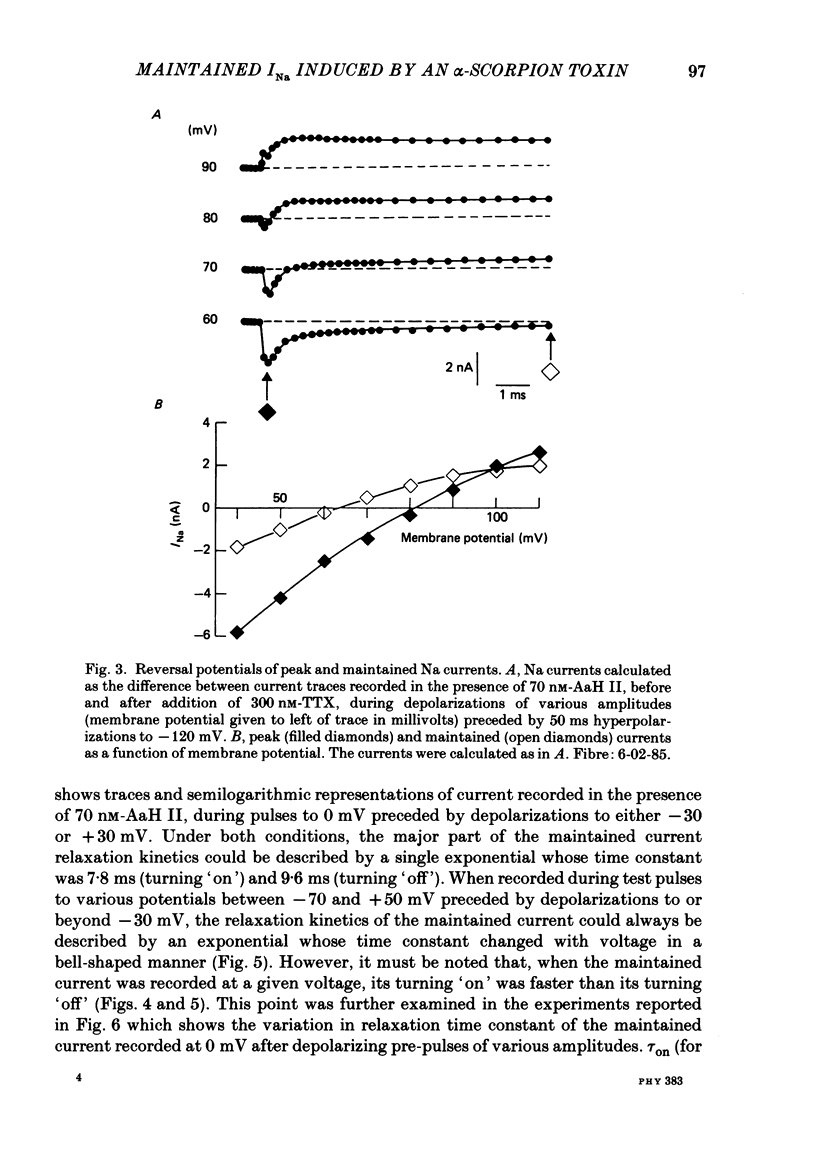
PDF

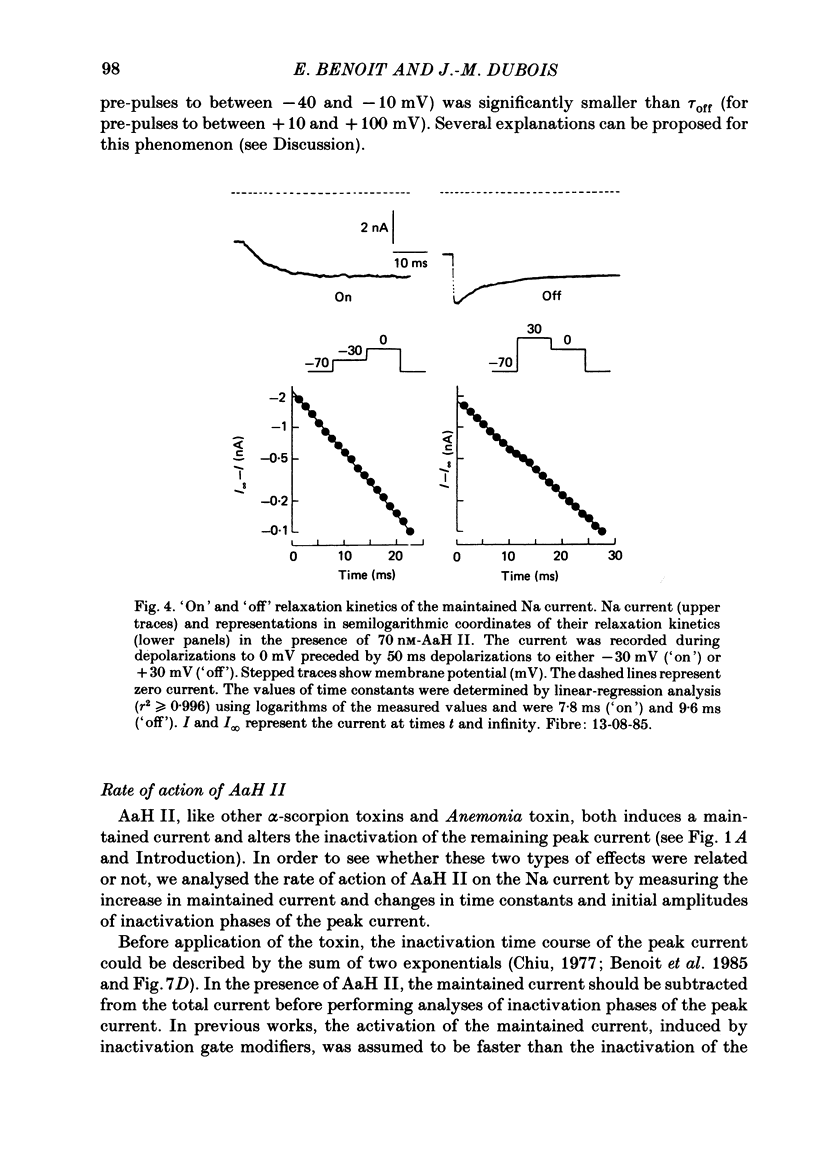
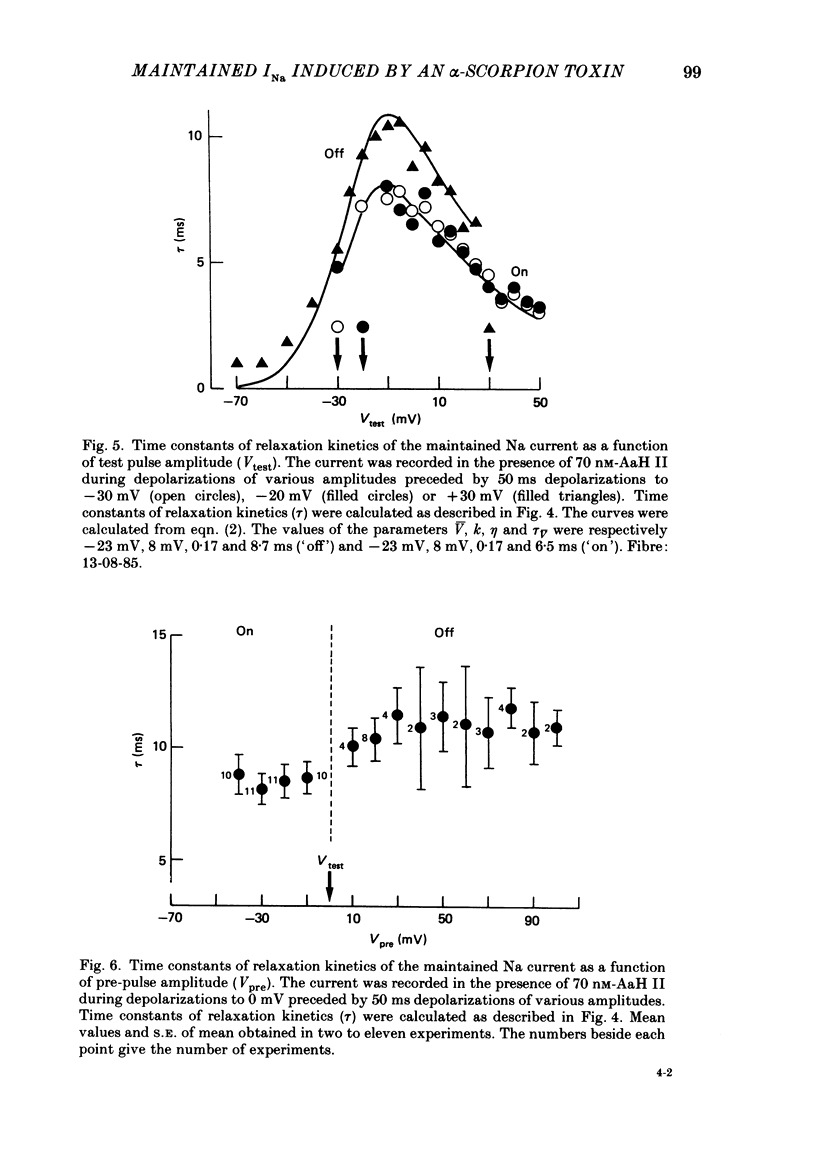

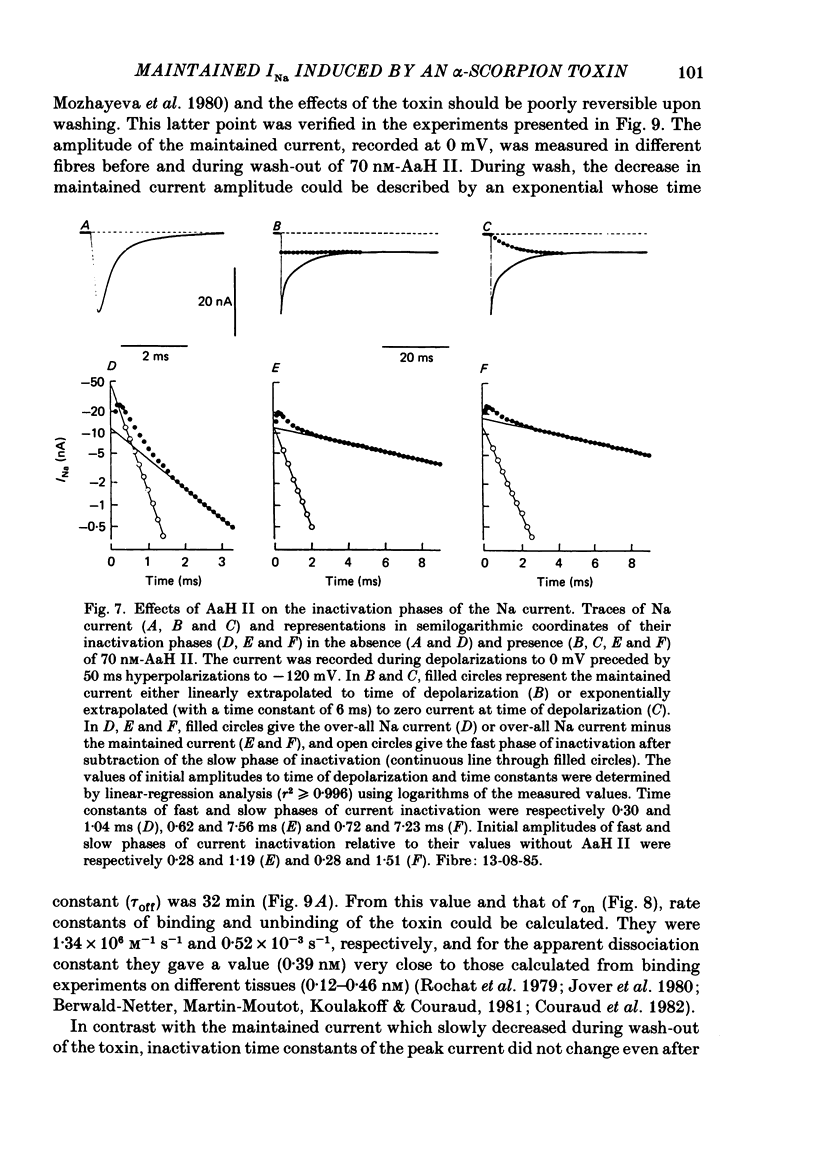
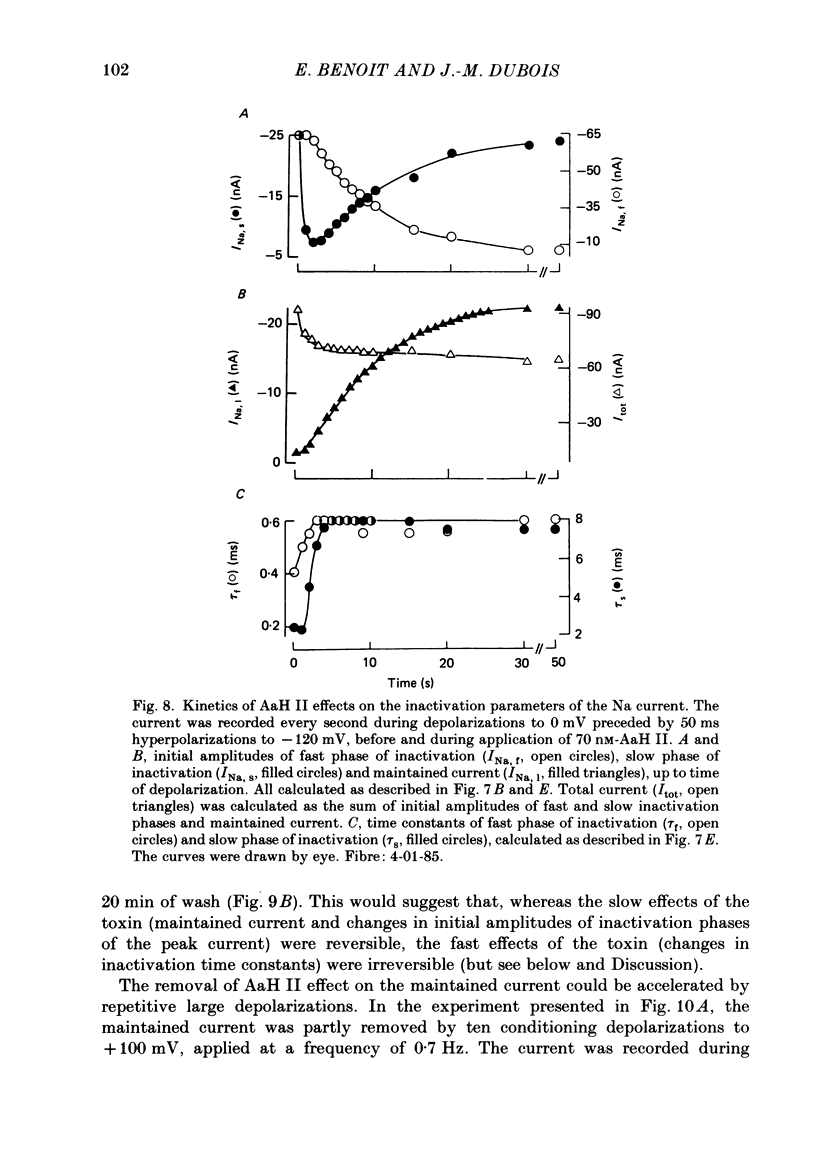
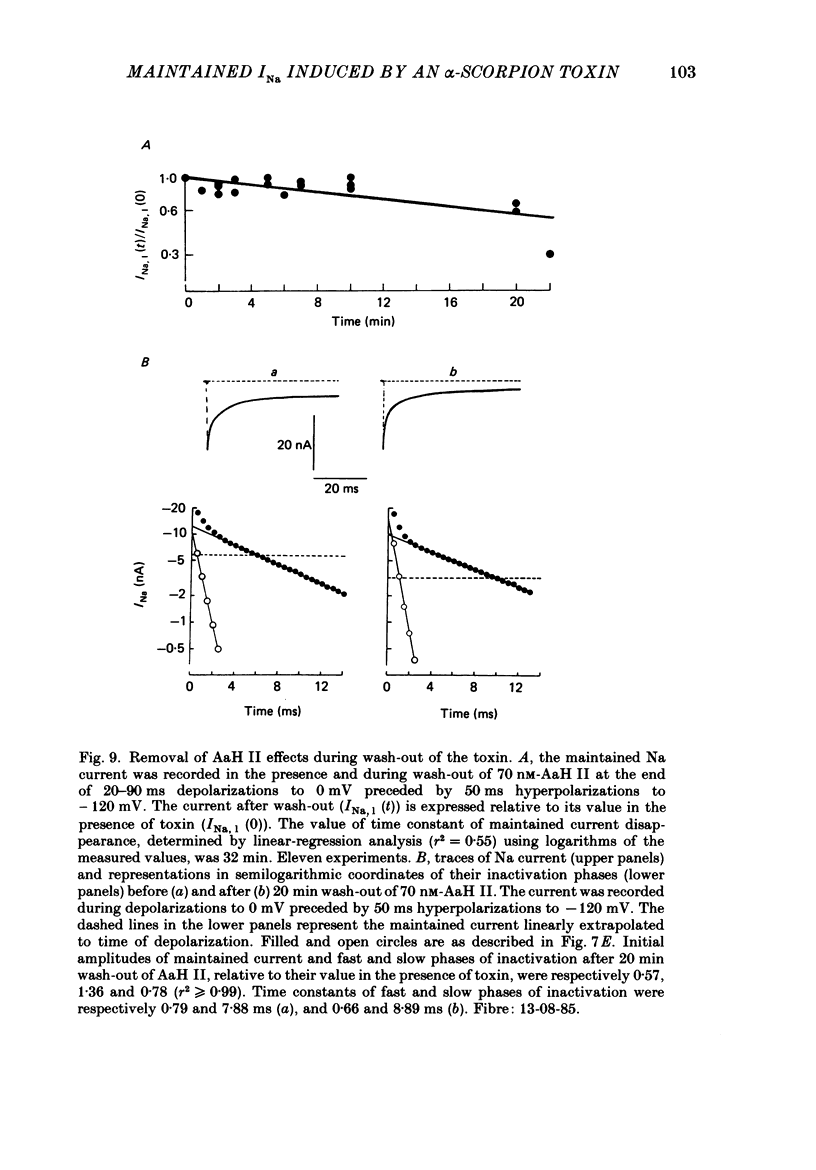







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian R. H. Charge movement in the membrane of striated muscle. Annu Rev Biophys Bioeng. 1978;7:85–112. doi: 10.1146/annurev.bb.07.060178.000505. [DOI] [PubMed] [Google Scholar]
- Armstrong C. M., Hille B. The inner quaternary ammonium ion receptor in potassium channels of the node of Ranvier. J Gen Physiol. 1972 Apr;59(4):388–400. doi: 10.1085/jgp.59.4.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit E., Corbier A., Dubois J. M. Evidence for two transient sodium currents in the frog node of Ranvier. J Physiol. 1985 Apr;361:339–360. doi: 10.1113/jphysiol.1985.sp015649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit E., Dubois J. M. Cooperativity of tetrodotoxin action in the frog node of Ranvier. Pflugers Arch. 1985 Oct;405(3):237–243. doi: 10.1007/BF00582567. [DOI] [PubMed] [Google Scholar]
- Bergman C., Dubois J. M., Rojas E., Rathmayer W. Decreased rate of sodium conductance inactivation in the node of Ranvier induced by a polypeptide toxin from sea anemone. Biochim Biophys Acta. 1976 Nov 11;455(1):173–184. doi: 10.1016/0005-2736(76)90162-0. [DOI] [PubMed] [Google Scholar]
- Berwald-Netter Y., Martin-Moutot N., Koulakoff A., Couraud F. Na+-channel-associated scorpion toxin receptor sites as probes for neuronal evolution in vivo and in vitro. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1245–1249. doi: 10.1073/pnas.78.2.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall W. A. Binding of scorpion toxin to receptor sites associated with sodium channels in frog muscle. Correlation of voltage-dependent binding with activation. J Gen Physiol. 1979 Sep;74(3):375–391. doi: 10.1085/jgp.74.3.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler W. K., Meves H. Evidence for two types of sodium conductance in axons perfused with sodium fluoride solution. J Physiol. 1970 Dec;211(3):653–678. doi: 10.1113/jphysiol.1970.sp009298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu S. Y. Inactivation of sodium channels: second order kinetics in myelinated nerve. J Physiol. 1977 Dec;273(3):573–596. doi: 10.1113/jphysiol.1977.sp012111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti F., Hille B., Neumcke B., Nonner W., Stämpfli R. Conductance of the sodium channel in myelinated nerve fibres with modified sodium inactivation. J Physiol. 1976 Nov;262(3):729–742. doi: 10.1113/jphysiol.1976.sp011617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couraud F., Jover E., Dubois J. M., Rochat H. Two types of scorpion receptor sites, one related to the activation, the other to the inactivation of the action potential sodium channel. Toxicon. 1982;20(1):9–16. doi: 10.1016/0041-0101(82)90138-6. [DOI] [PubMed] [Google Scholar]
- Couraud F., Rochat H., Lissitzky S. Binding of scorpion and sea anemone neurotoxins to a common site related to the action potential Na+ ionophore in neuroblastoma cells. Biochem Biophys Res Commun. 1978 Aug 29;83(4):1525–1530. doi: 10.1016/0006-291x(78)91394-3. [DOI] [PubMed] [Google Scholar]
- Dubois J. M., Bergman C. Late sodium current in the node of Ranvier. Pflugers Arch. 1975;357(1-2):145–148. doi: 10.1007/BF00584552. [DOI] [PubMed] [Google Scholar]
- Dubois J. M., Bergman C. Variation de la conductance sodium de la membrane nodale en fonction de la concentration en ions Na+. C R Acad Sci Hebd Seances Acad Sci D. 1971 Jun 2;272(22):2796–2799. [PubMed] [Google Scholar]
- Dubois J. M., Coulombe A. Current-dependent inactivation induced by sodium depletion in normal and batrachotoxin-treated frog node of Ranvier. J Gen Physiol. 1984 Jul;84(1):25–48. doi: 10.1085/jgp.84.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois J. M., Schneider M. F. Kinetics of intramembrane charge movement and conductance activation of batrachotoxin-modified sodium channels in frog node of Ranvier. J Gen Physiol. 1985 Sep;86(3):381–394. doi: 10.1085/jgp.86.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois J. M., Schneider M. F. Kinetics of intramembrane charge movement and sodium current in frog node of Ranvier. J Gen Physiol. 1982 Apr;79(4):571–602. doi: 10.1085/jgp.79.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B. Quantitative description of sodium currents in myelinated nerve fibres of Xenopus laevis. J Physiol. 1960 Jun;151:491–501. doi: 10.1113/jphysiol.1960.sp006455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonoi T., Hille B., Catterall W. A. Voltage clamp analysis of sodium channels in normal and scorpion toxin-resistant neuroblastoma cells. J Neurosci. 1984 Nov;4(11):2836–2842. doi: 10.1523/JNEUROSCI.04-11-02836.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B., Schwarz W. Potassium channels as multi-ion single-file pores. J Gen Physiol. 1978 Oct;72(4):409–442. doi: 10.1085/jgp.72.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. The permeability of the sodium channel to metal cations in myelinated nerve. J Gen Physiol. 1972 Jun;59(6):637–658. doi: 10.1085/jgp.59.6.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowicz P., Schneider M. F. Membrane charge moved at contraction thresholds in skeletal muscle fibres. J Physiol. 1981 May;314:595–633. doi: 10.1113/jphysiol.1981.sp013726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacques Y., Fosset M., Lazdunski M. Molecular properties of the action potential Na+ ionophore in neuroblastoma cells. Interactions with neurotoxins. J Biol Chem. 1978 Oct 25;253(20):7383–7392. [PubMed] [Google Scholar]
- Jover E., Martin-Moutot N., Couraud F., Rochat H. Binding of scorpion toxins to rat brain synaptosomal fraction. Effects of membrane potential, ions, and other neurotoxins. Biochemistry. 1980 Feb 5;19(3):463–467. doi: 10.1021/bi00544a010. [DOI] [PubMed] [Google Scholar]
- Keynes R. D., Rojas E. Kinetics and steady-state properties of the charged system controlling sodium conductance in the squid giant axon. J Physiol. 1974 Jun;239(2):393–434. doi: 10.1113/jphysiol.1974.sp010575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landowne D., Scruggs V. Effects of internal and external sodium on the sodium current-voltage relationship in the Squid giant axon. J Membr Biol. 1981 Apr 15;59(2):79–89. doi: 10.1007/BF01875706. [DOI] [PubMed] [Google Scholar]
- Meves H., Rubly N., Watt D. D. Effect of toxins isolated from the venom of the scorpion Centruroides sculpturatus on the Na currents of the node of Ranvier. Pflugers Arch. 1982 Mar;393(1):56–62. doi: 10.1007/BF00582392. [DOI] [PubMed] [Google Scholar]
- Meves H., Rubly N., Watt D. D. Voltage-dependent effect of a scorpion toxin on sodium current inactivation. Pflugers Arch. 1984 Sep;402(1):24–33. doi: 10.1007/BF00584827. [DOI] [PubMed] [Google Scholar]
- Mozhayeva G. N., Naumov A. P., Nosyreva E. D., Grishin E. V. Potential-dependent interaction of toxin from venom of the scorpion Buthus eupeus with sodium channels in myelinated fibre: voltage clamp experiments. Biochim Biophys Acta. 1980 Apr 24;597(3):587–602. doi: 10.1016/0005-2736(80)90230-8. [DOI] [PubMed] [Google Scholar]
- Neumcke B., Nonner W., Stämpfli R. Asymmetrical displacement current and its relation with the activation of sodium current in the membrane of frog myelinated nerve. Pflugers Arch. 1976 Jun 22;363(3):193–203. doi: 10.1007/BF00594601. [DOI] [PubMed] [Google Scholar]
- Neumcke B., Schwarz W., Stämpfli R. Comparison of the effects of Anemonia toxin II on sodium and gating currents in frog myelinated nerve. Biochim Biophys Acta. 1985 Mar 28;814(1):111–119. doi: 10.1016/0005-2736(85)90425-0. [DOI] [PubMed] [Google Scholar]
- Neumcke B., Schwarz W., Stämpfli R. Modification of sodium inactivation in myelinated nerve by Anemonia toxin II and iodate. Analysis of current fluctuations and current relaxations. Biochim Biophys Acta. 1980 Aug 4;600(2):456–466. doi: 10.1016/0005-2736(80)90448-4. [DOI] [PubMed] [Google Scholar]
- Nonner W. A new voltage clamp method for Ranvier nodes. Pflugers Arch. 1969;309(2):176–192. doi: 10.1007/BF00586967. [DOI] [PubMed] [Google Scholar]
- Nonner W. Effects of Leiurus scorpion venom on the "gating" current in myelinated nerve. Adv Cytopharmacol. 1979;3:345–352. [PubMed] [Google Scholar]
- Rochat H., Bernard P., Couraud F. Scorpion toxins: chemistry and mode of action. Adv Cytopharmacol. 1979;3:325–334. [PubMed] [Google Scholar]
- Schmidtmayer J. Behaviour of chemically modified sodium channels in frog nerve supports a three-state model of inactivation. Pflugers Arch. 1985 May;404(1):21–28. doi: 10.1007/BF00581486. [DOI] [PubMed] [Google Scholar]
- Schmidtmayer J., Stoye-Herzog M., Ulbricht W. Rate of action of Anemonia sulcata toxin II on sodium channels in myelinated nerve fibres. Pflugers Arch. 1982 Oct 1;394(4):313–319. doi: 10.1007/BF00583695. [DOI] [PubMed] [Google Scholar]
- Swenson R. P., Jr, Armstrong C. M. K+ channels close more slowly in the presence of external K+ and Rb+. Nature. 1981 Jun 4;291(5814):427–429. doi: 10.1038/291427a0. [DOI] [PubMed] [Google Scholar]
- Ulbricht W., Schmidtmayer J. Modification of sodium channels in myelinated nerve by Anemonia sulcata toxin II. J Physiol (Paris) 1981 May;77(9):1103–1111. [PubMed] [Google Scholar]
- Wang G. K., Strichartz G. Kinetic analysis of the action of Leiurus scorpion alpha-toxin on ionic currents in myelinated nerve. J Gen Physiol. 1985 Nov;86(5):739–762. doi: 10.1085/jgp.86.5.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodhull A. M. Ionic blockage of sodium channels in nerve. J Gen Physiol. 1973 Jun;61(6):687–708. doi: 10.1085/jgp.61.6.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto D., Yeh J. Z., Narahashi T. Interactions of permeant cations with sodium channels of squid axon membranes. Biophys J. 1985 Sep;48(3):361–368. doi: 10.1016/S0006-3495(85)83792-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Laat S. W., van der Saag P. T., Shinitzky M. Microviscosity modulation during the cell cycle of neuroblastoma cells. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4458–4461. doi: 10.1073/pnas.74.10.4458. [DOI] [PMC free article] [PubMed] [Google Scholar]


