Abstract
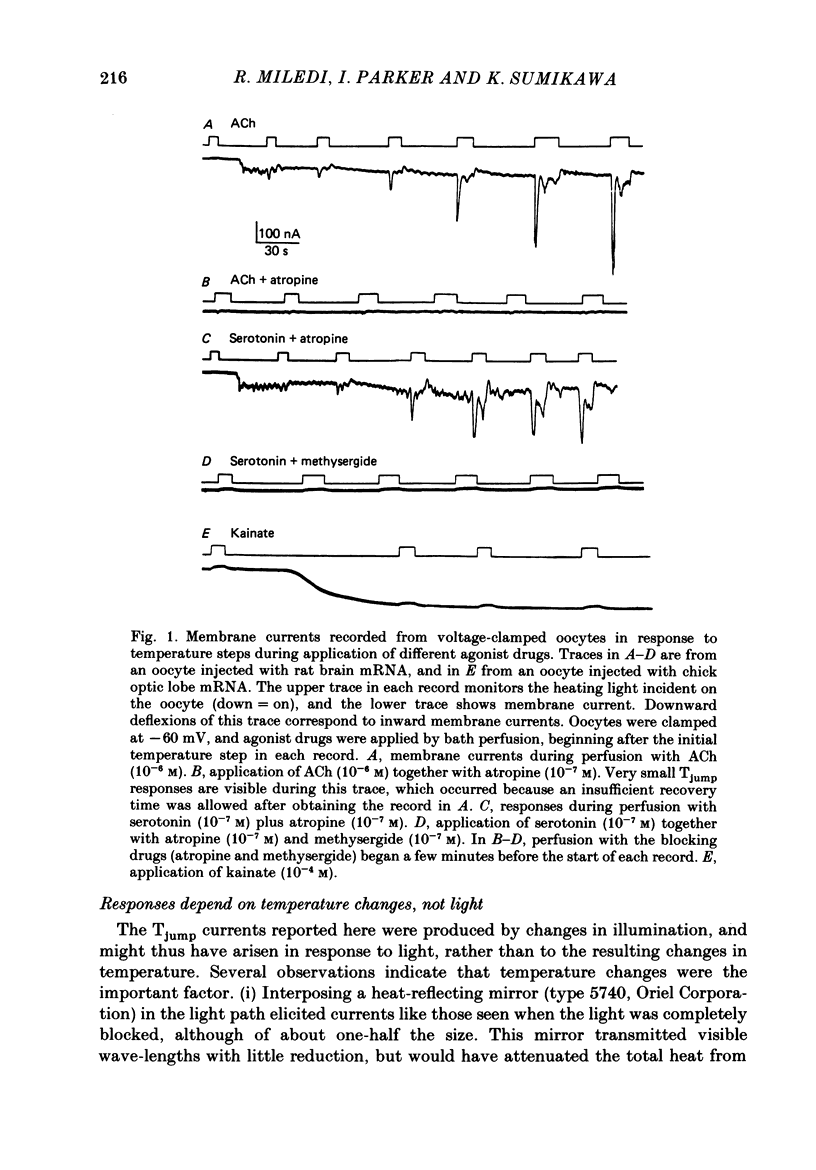
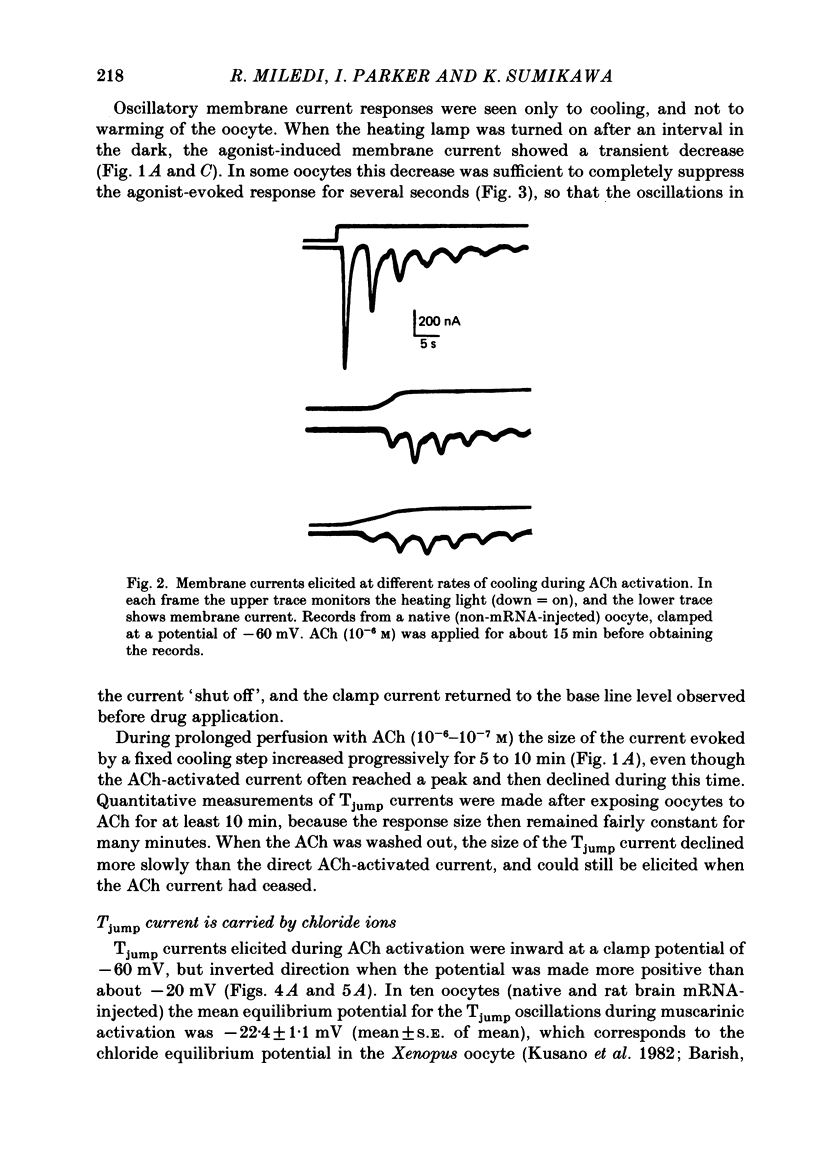
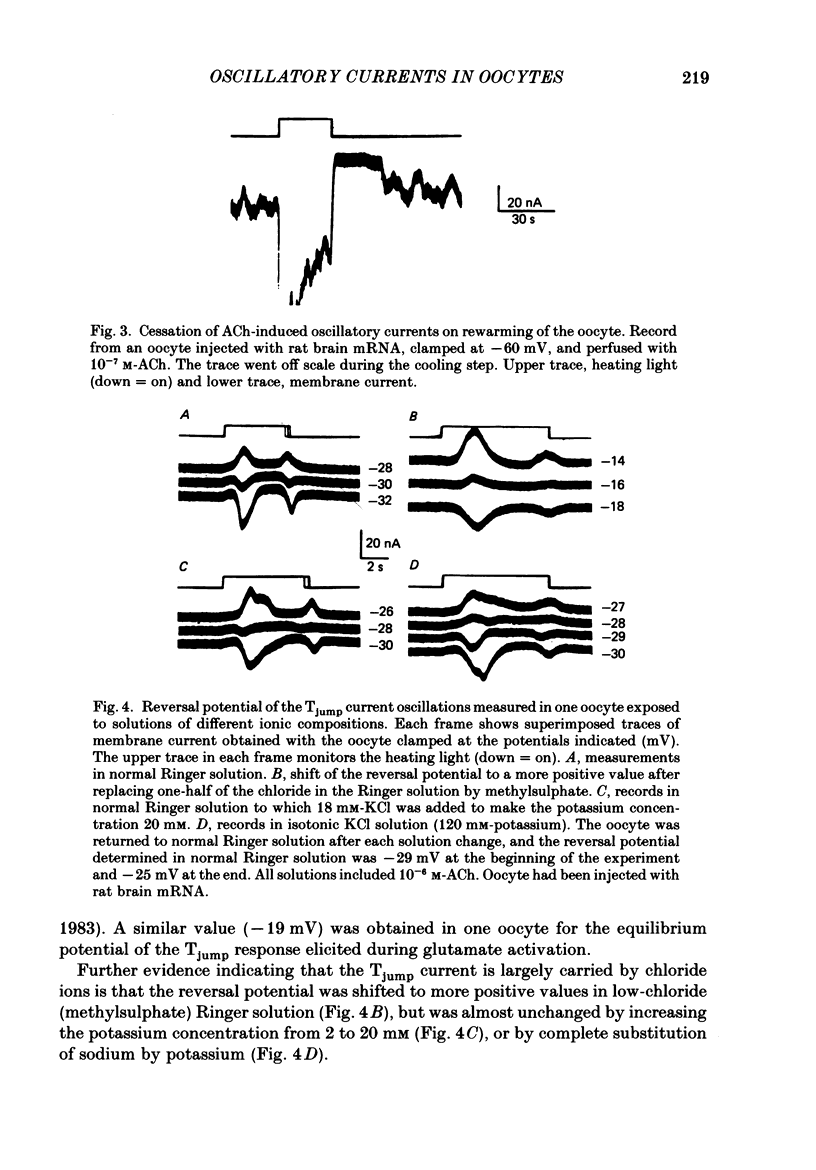
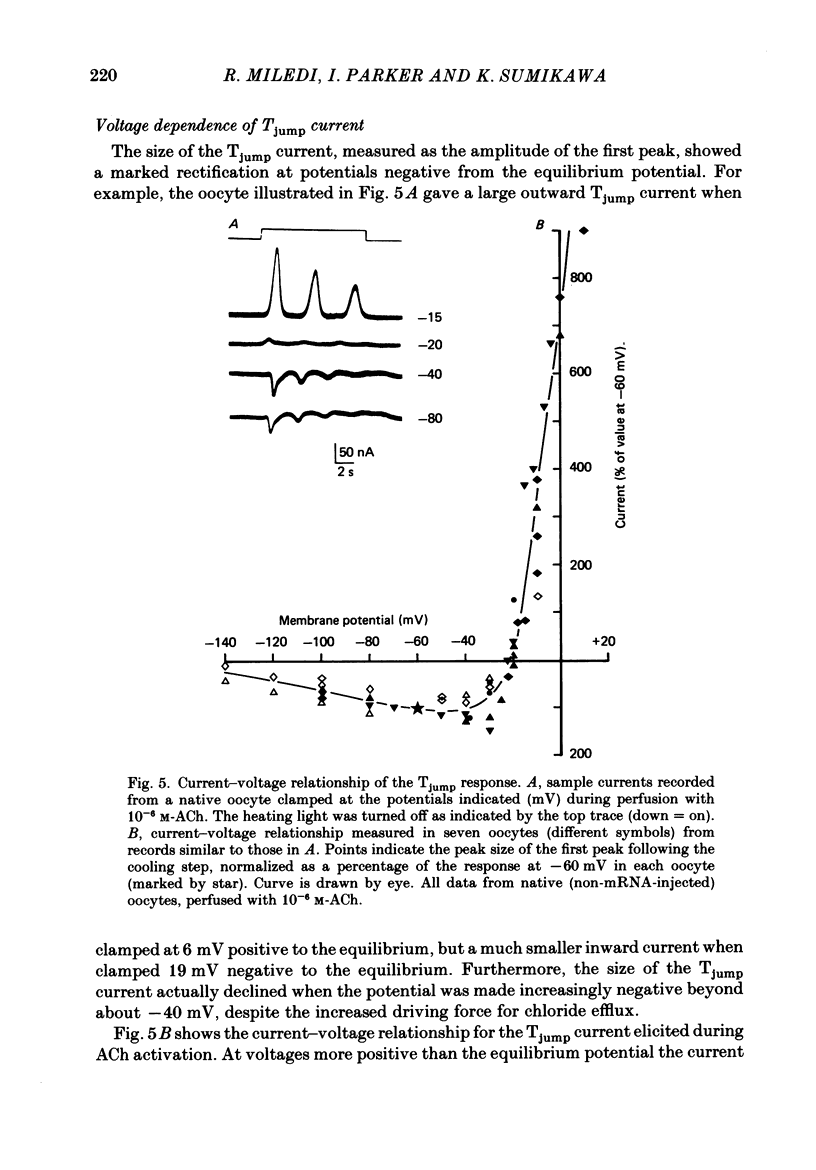
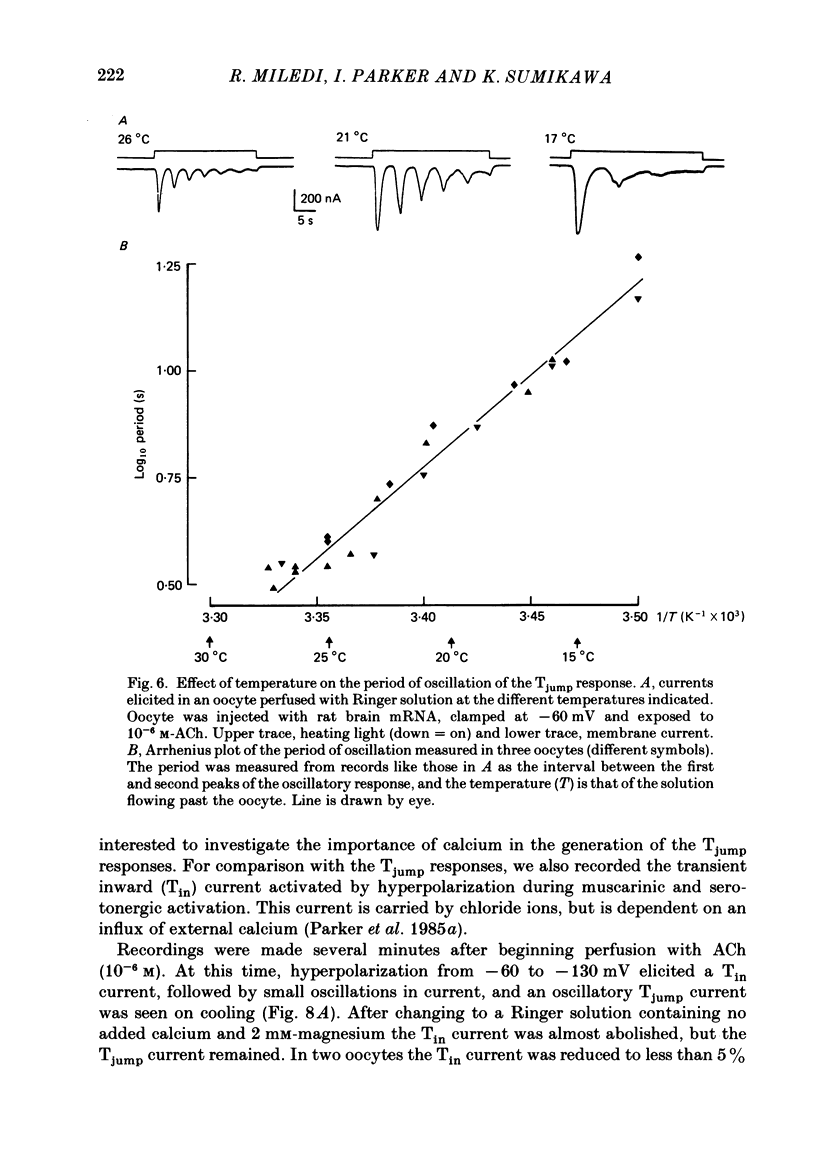
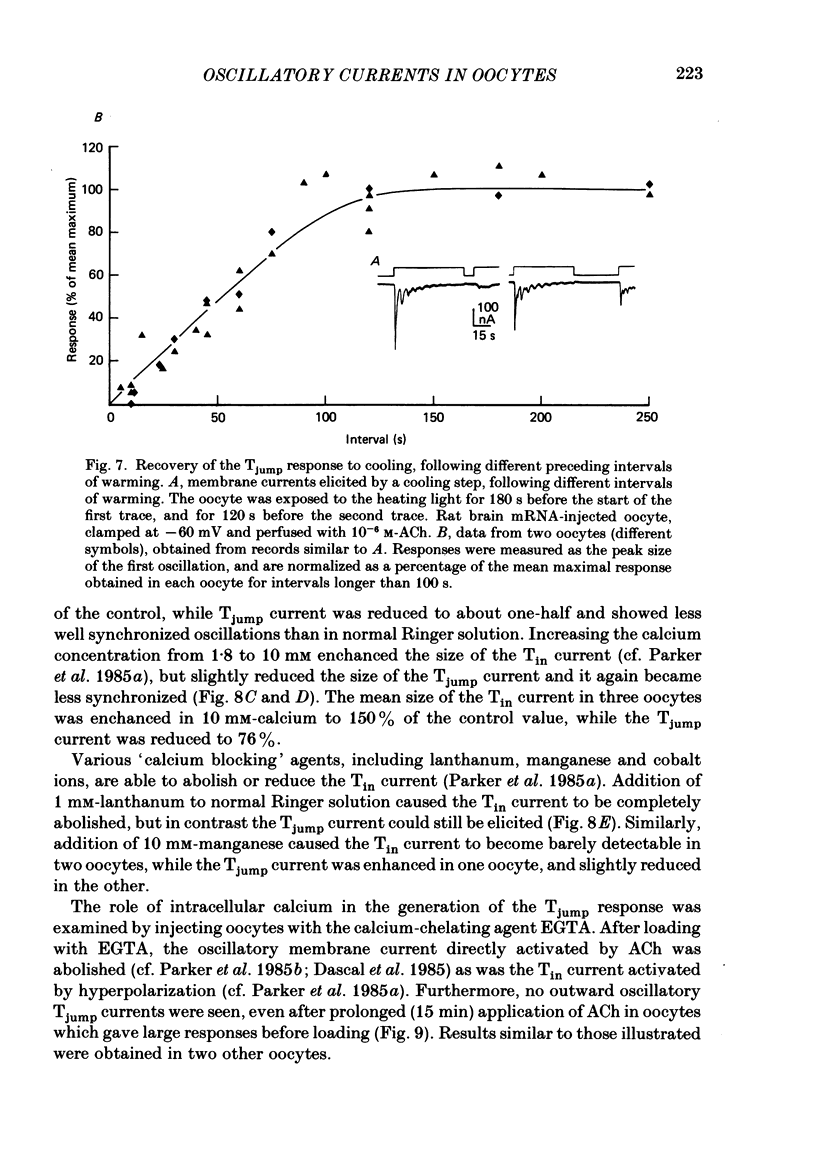
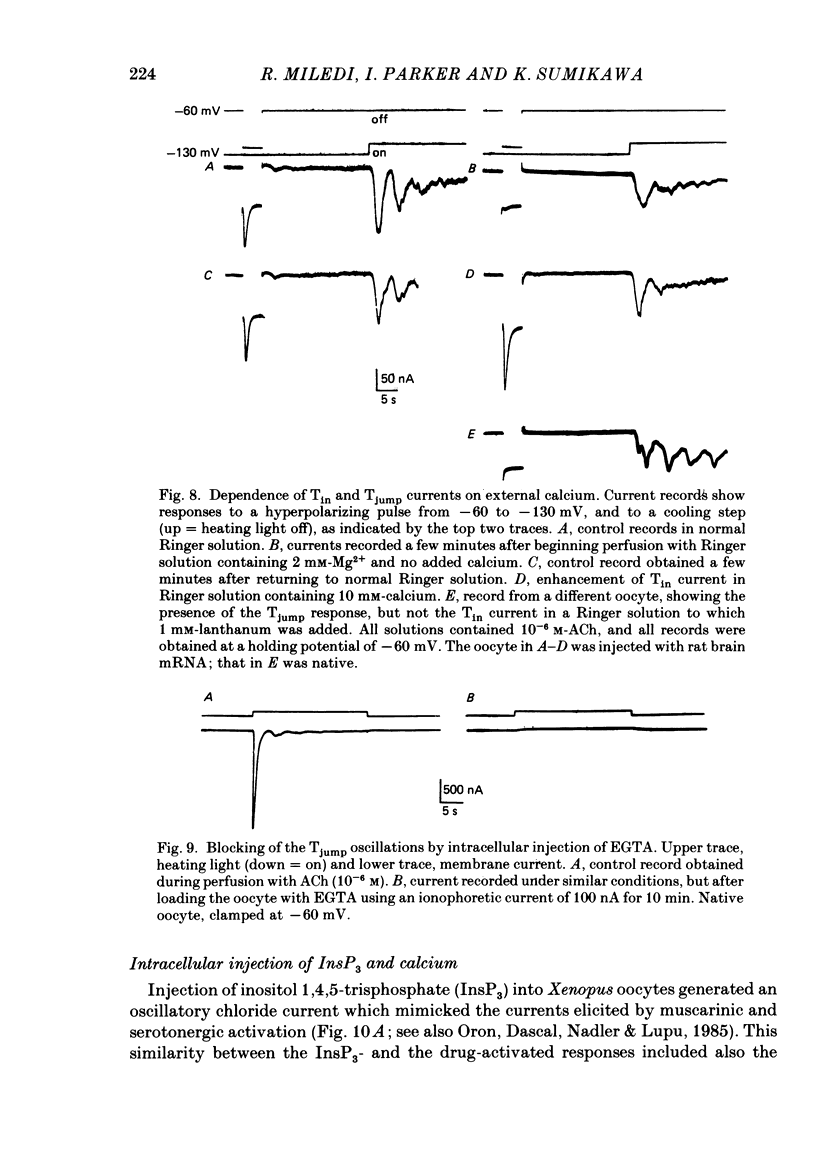
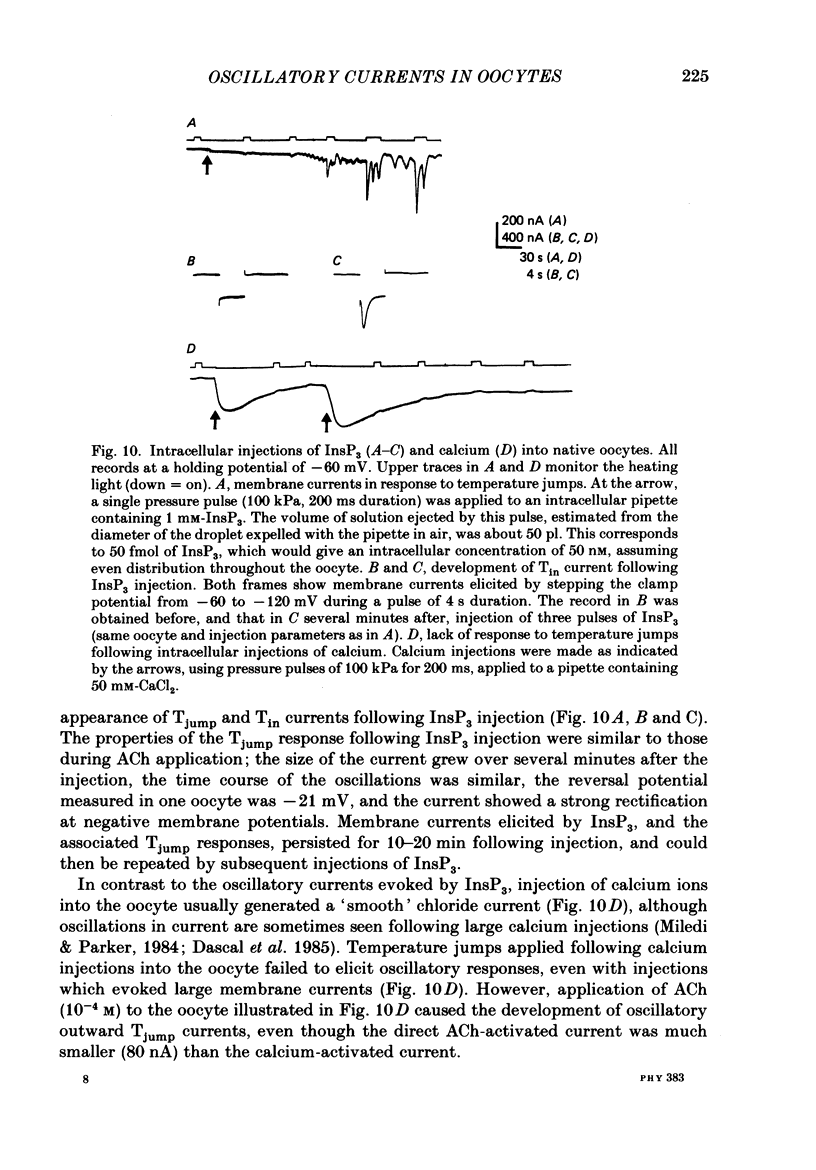
1. Membrane currents were recorded from voltage-clamped oocytes of Xenopus laevis, during temperature jumps imposed by a heating light. Resting oocytes usually showed little response, but large oscillatory membrane currents developed in response to cooling steps applied during activation of 'native' muscarinic receptors. 2. Similar temperature jump (Tjump) currents were seen during activation of oscillatory chloride currents mediated by muscarinic acetylcholine (ACh), serotonin, glutamate and noradrenaline receptors, expressed in the oocyte following injection with messenger ribonucleic acid (mRNA) from rat brain. The Tjump response during muscarinic activation was selectively blocked by atropine, and that during serotonergic activation by methysergide. In contrast, the 'smooth' membrane currents elicited by nicotinic ACh, kainate and gamma-aminobutyric acid (GABA) were not accompanied by Tjump responses. 3. Rapid cooling of the oocyte gave larger Tjump currents than a gradual cooling over a few seconds. The size of the Tjump current elicited by a fixed cooling step increased linearly with the preceding time of warming, becoming maximal at intervals greater than about 100 s. 4. The Tjump current was inward at a clamp potential of -60 mV and reversed direction at about -22 mV, which corresponds to the chloride equilibrium potential in the oocyte. In low-chloride solution the reversal potential was shifted to more positive potentials, but it was almost unchanged by changes in potassium and sodium concentration. The size of the Tjump current decreased as the membrane potential was made more negative than about -40 mV. 5. The period of oscillation of the Tjump current increased with decreasing temperature, following a Q10 of 3.15. Depolarization also caused a small increase in period. 6. The Tjump current was not abolished in calcium-free solution, or by addition of manganese or lanthanum to the bathing solution. However, it was abolished by intracellular injection of the calcium-chelating agent EGTA. 7. Intracellular injection of inositol 1,4,5-trisphosphate evoked an oscillatory membrane current, during which Tjump responses developed similar to those after muscarinic activation. Intracellular injection of calcium evoked a chloride current, but this was not accompanied by Tjump responses. 8. We conclude that the oscillatory currents evoked by temperature jumps arise from chloride channels activated by intracellular calcium. This calcium is probably mobilized from intracellular stores by inositol trisphosphate which is liberated as a result of activation of muscarinic receptors, and also receptors for serotonin and glutamate.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. G., Eisner D. A., Orchard C. H. Characterization of oscillations of intracellular calcium concentration in ferret ventricular muscle. J Physiol. 1984 Jul;352:113–128. doi: 10.1113/jphysiol.1984.sp015281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barish M. E. A transient calcium-dependent chloride current in the immature Xenopus oocyte. J Physiol. 1983 Sep;342:309–325. doi: 10.1113/jphysiol.1983.sp014852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard E. A., Miledi R., Sumikawa K. Translation of exogenous messenger RNA coding for nicotinic acetylcholine receptors produces functional receptors in Xenopus oocytes. Proc R Soc Lond B Biol Sci. 1982 May 22;215(1199):241–246. doi: 10.1098/rspb.1982.0040. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Busa W. B., Ferguson J. E., Joseph S. K., Williamson J. R., Nuccitelli R. Activation of frog (Xenopus laevis) eggs by inositol trisphosphate. I. Characterization of Ca2+ release from intracellular stores. J Cell Biol. 1985 Aug;101(2):677–682. doi: 10.1083/jcb.101.2.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbertson K. S., Cobbold P. H. Phorbol ester and sperm activate mouse oocytes by inducing sustained oscillations in cell Ca2+. Nature. 1985 Aug 8;316(6028):541–542. doi: 10.1038/316541a0. [DOI] [PubMed] [Google Scholar]
- Dascal N., Gillo B., Lass Y. Role of calcium mobilization in mediation of acetylcholine-evoked chloride currents in Xenopus laevis oocytes. J Physiol. 1985 Sep;366:299–313. doi: 10.1113/jphysiol.1985.sp015799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo M. Calcium release from the sarcoplasmic reticulum. Physiol Rev. 1977 Jan;57(1):71–108. doi: 10.1152/physrev.1977.57.1.71. [DOI] [PubMed] [Google Scholar]
- Gundersen C. B., Miledi R., Parker I. Glutamate and kainate receptors induced by rat brain messenger RNA in Xenopus oocytes. Proc R Soc Lond B Biol Sci. 1984 Apr 24;221(1223):127–143. doi: 10.1098/rspb.1984.0027. [DOI] [PubMed] [Google Scholar]
- Gundersen C. B., Miledi R., Parker I. Properties of human brain glycine receptors expressed in Xenopus oocytes. Proc R Soc Lond B Biol Sci. 1984 Apr 24;221(1223):235–244. doi: 10.1098/rspb.1984.0032. [DOI] [PubMed] [Google Scholar]
- Gundersen C. B., Miledi R., Parker I. Serotonin receptors induced by exogenous messenger RNA in Xenopus oocytes. Proc R Soc Lond B Biol Sci. 1983 Aug 22;219(1214):103–109. doi: 10.1098/rspb.1983.0062. [DOI] [PubMed] [Google Scholar]
- Kusano K., Miledi R., Stinnakre J. Cholinergic and catecholaminergic receptors in the Xenopus oocyte membrane. J Physiol. 1982 Jul;328:143–170. doi: 10.1113/jphysiol.1982.sp014257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R. A calcium-dependent transient outward current in Xenopus laevis oocytes. Proc R Soc Lond B Biol Sci. 1982 Jul 22;215(1201):491–497. doi: 10.1098/rspb.1982.0056. [DOI] [PubMed] [Google Scholar]
- Miledi R., Parker I. Chloride current induced by injection of calcium into Xenopus oocytes. J Physiol. 1984 Dec;357:173–183. doi: 10.1113/jphysiol.1984.sp015495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Parker I., Sumikawa K. Properties of acetylcholine receptors translated by cat muscle mRNA in Xenopus oocytes. EMBO J. 1982;1(11):1307–1312. doi: 10.1002/j.1460-2075.1982.tb01315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Parker I., Sumikawa K. Synthesis of chick brain GABA receptors by frog oocytes. Proc R Soc Lond B Biol Sci. 1982 Nov 22;216(1205):509–515. doi: 10.1098/rspb.1982.0089. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Turnover of inositol phospholipids and signal transduction. Science. 1984 Sep 21;225(4668):1365–1370. doi: 10.1126/science.6147898. [DOI] [PubMed] [Google Scholar]
- Oron Y., Dascal N., Nadler E., Lupu M. Inositol 1,4,5-trisphosphate mimics muscarinic response in Xenopus oocytes. Nature. 1985 Jan 10;313(5998):141–143. doi: 10.1038/313141a0. [DOI] [PubMed] [Google Scholar]
- Parker I., Gundersen C. B., Miledi R. A transient inward current elicited by hyperpolarization during serotonin activation in Xenopus oocytes. Proc R Soc Lond B Biol Sci. 1985 Jan 22;223(1232):279–292. doi: 10.1098/rspb.1985.0002. [DOI] [PubMed] [Google Scholar]
- Parker I., Gundersen C. B., Miledi R. Intracellular Ca2+-dependent and Ca2+-independent responses of rat brain serotonin receptors transplanted to Xenopus oocytes. Neurosci Res. 1985 Aug;2(6):491–496. doi: 10.1016/0168-0102(85)90021-5. [DOI] [PubMed] [Google Scholar]
- Slack B. E., Bell J. E., Benos D. J. Inositol-1,4,5-trisphosphate injection mimics fertilization potentials in sea urchin eggs. Am J Physiol. 1986 Feb;250(2 Pt 1):C340–C344. doi: 10.1152/ajpcell.1986.250.2.C340. [DOI] [PubMed] [Google Scholar]
- Sladeczek F., Pin J. P., Récasens M., Bockaert J., Weiss S. Glutamate stimulates inositol phosphate formation in striatal neurones. Nature. 1985 Oct 24;317(6039):717–719. doi: 10.1038/317717a0. [DOI] [PubMed] [Google Scholar]
- Sumikawa K., Parker I., Miledi R. Messenger RNA from rat brain induces noradrenaline and dopamine receptors in Xenopus oocytes. Proc R Soc Lond B Biol Sci. 1984 Dec 22;223(1231):255–260. doi: 10.1098/rspb.1984.0093. [DOI] [PubMed] [Google Scholar]
- Sumikawa K., Parker I., Miledi R. Partial purification and functional expression of brain mRNAs coding for neurotransmitter receptors and voltage-operated channels. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7994–7998. doi: 10.1073/pnas.81.24.7994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicentini L. M., Ambrosini A., Di Virgilio F., Pozzan T., Meldolesi J. Muscarinic receptor-induced phosphoinositide hydrolysis at resting cytosolic Ca2+ concentration in PC12 cells. J Cell Biol. 1985 Apr;100(4):1330–1333. doi: 10.1083/jcb.100.4.1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker M. J., Steinhardt R. A. Ionic regulation of egg activation. Q Rev Biophys. 1982 Nov;15(4):593–666. doi: 10.1017/s0033583500003760. [DOI] [PubMed] [Google Scholar]
- Woods N. M., Cuthbertson K. S., Cobbold P. H. Repetitive transient rises in cytoplasmic free calcium in hormone-stimulated hepatocytes. Nature. 1986 Feb 13;319(6054):600–602. doi: 10.1038/319600a0. [DOI] [PubMed] [Google Scholar]


