Abstract
1. The mechanism of twitch potentiation by caffeine was studied in single muscle fibres dissected from m. tibialis anterior of Rana temporaria. Fibres were injected with a Ca2+-sensitive photo-protein, aequorin, and the resultant light signal and tension were simultaneously recorded. 2. In twitch responses triggered every 60 s, peaks of light and tension were maintained at a constant level. Low concentrations of caffeine (0.2-0.4 mM) potentiated twitch tension accompanied by a slight increase in light signal. 3. Although peak twitch tension was dose-dependently potentiated by caffeine, light peaks were suppressed at relatively higher concentrations of caffeine (0.6-1.5 mM) with prolonged decay of the light and tension signals. 4. Light intensity in the resting muscle (resting glow) was elevated by caffeine (0.2-1.5 mM) dose-dependently without detectable tension development. This effect of caffeine was suppressed by 0.5-1.0 mM-procaine or 10 mM-adenine, inhibitors of Ca2+-induced Ca2+ release. 5. In caffeine-treated preparations, peaks of light and tension were augmented by application of procaine (0.5-1.0 mM). Adenine (10 mM) affected the light signal in essentially the same way as procaine, but the effect varied from fibre to fibre. 6. From these results, the following hypothesis is proposed: low concentrations of caffeine directly induce Ca2+ release from sarcoplasmic reticulum (s.r.) in in the resting state, and facilitates Ca2+ release from s.r. induced by the action potential during twitch. At relatively higher concentrations of caffeine (0.6-1.5 mM), rise of resting [Ca2+]i (intracellular free Ca2+ concentration) before activation might be an important factor in twitch potentiation by caffeine. If the rise of resting [Ca2+]i induced by caffeine is inhibited by procaine and the content of Ca2+ in s.r. is well maintained, caffeine could facilitate Ca2+ release by depolarization and thus potentiate twitch tension.
Full text
PDF


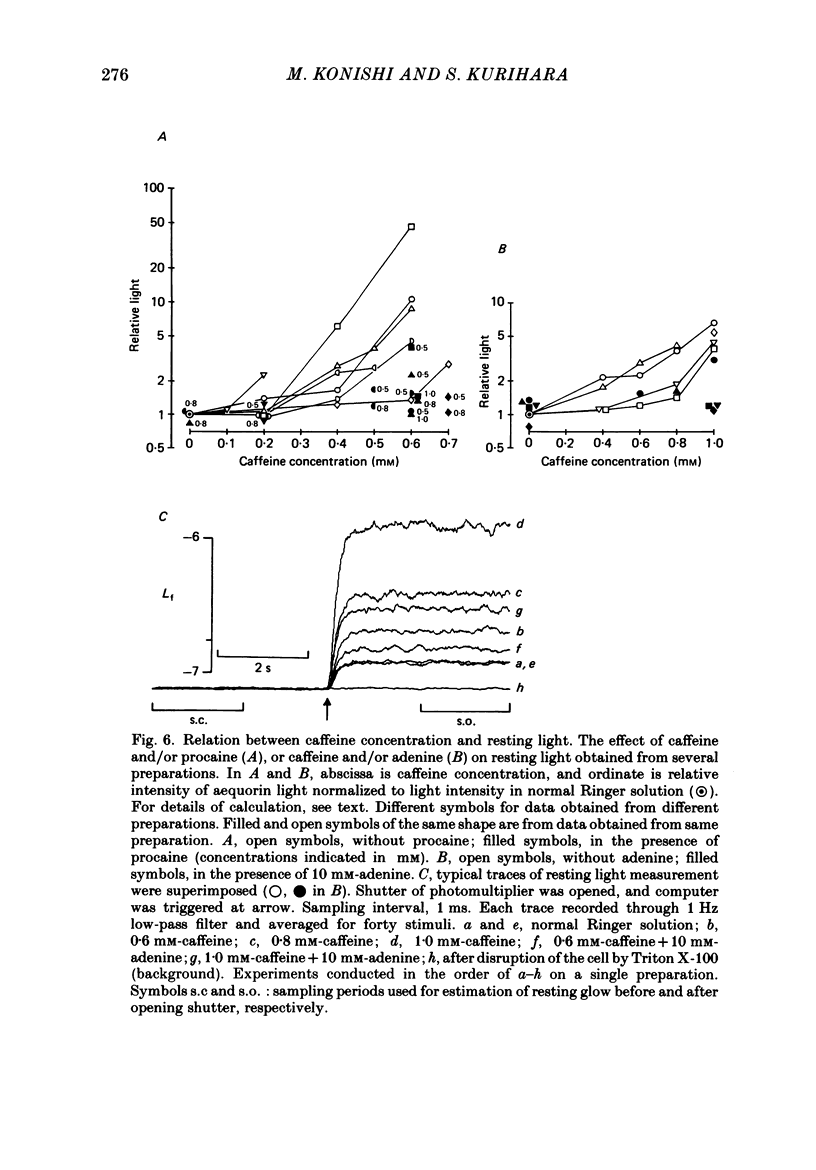
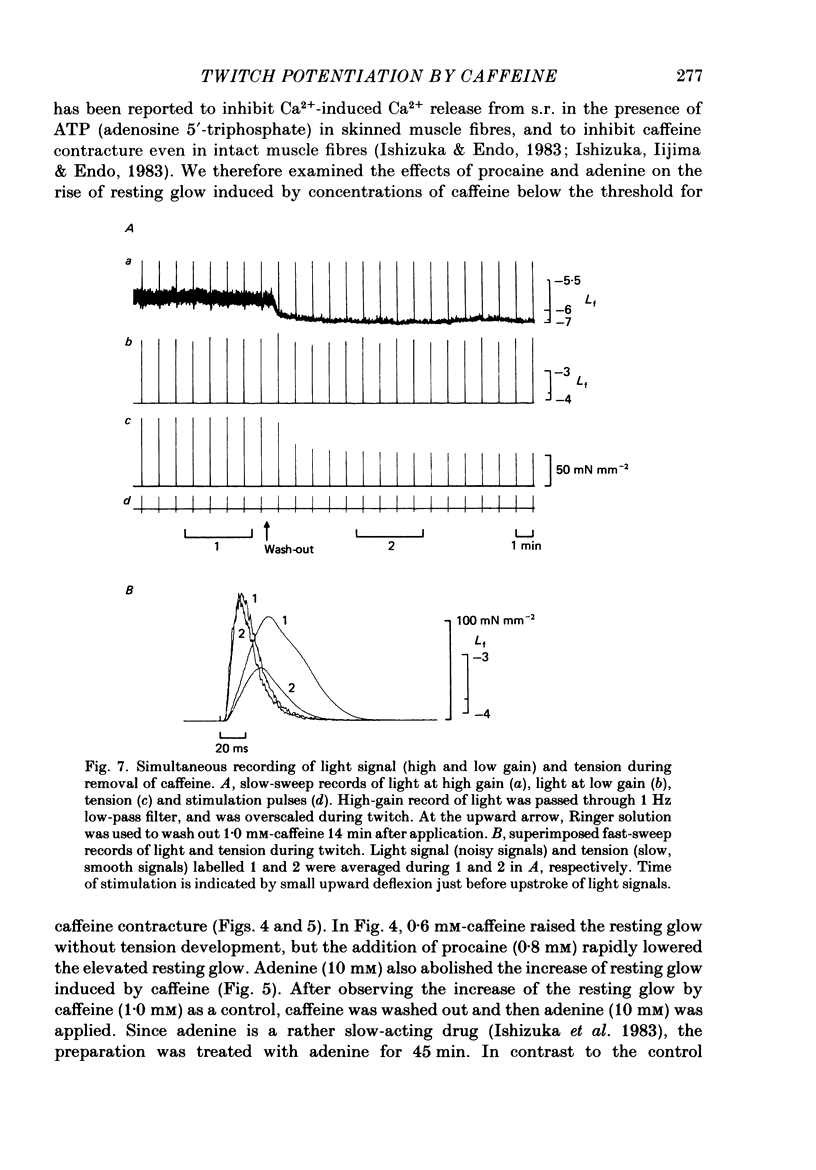
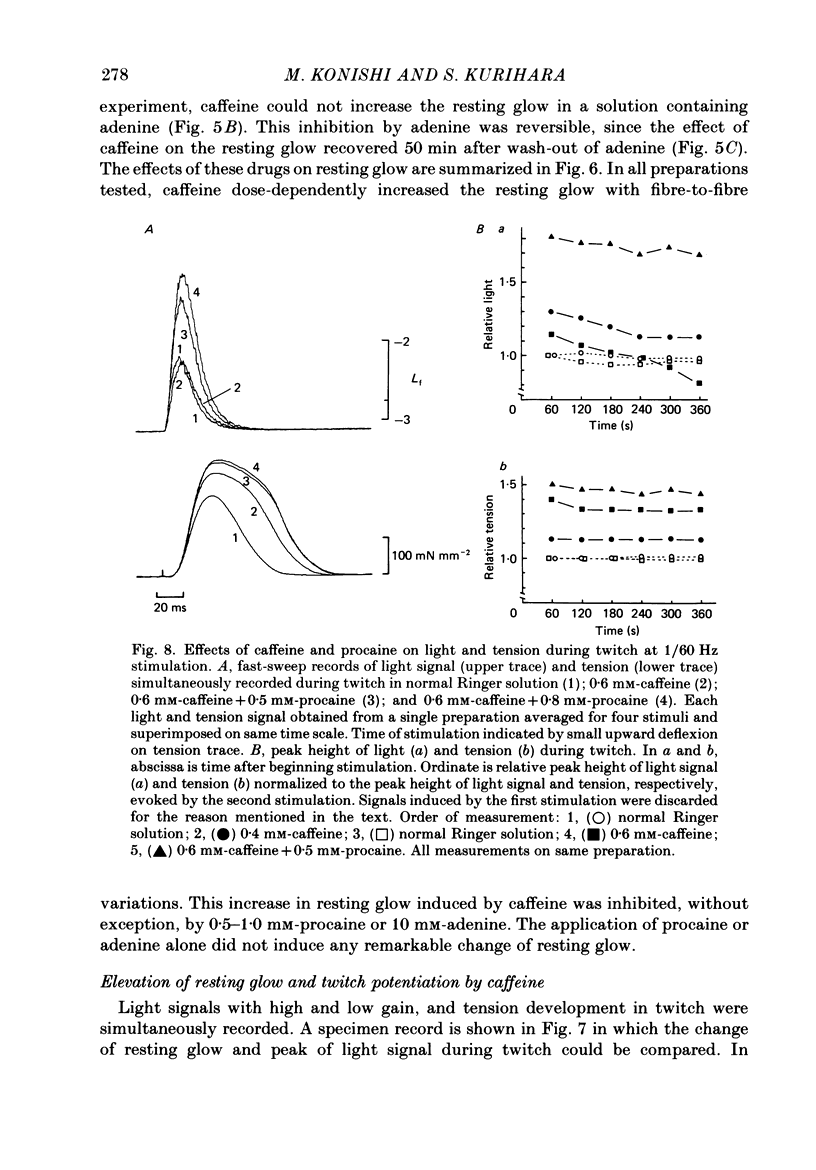





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AXELSSON J., THESLEFF S. Activation of the contractile mechanism in striated muscle. Acta Physiol Scand. 1958 Oct 28;44(1):55–66. doi: 10.1111/j.1748-1716.1958.tb01608.x. [DOI] [PubMed] [Google Scholar]
- Abercrombie R. F., Roos A. The intracellular pH of frog skeletal muscle: its regulation in hypertonic solutions. J Physiol. 1983 Dec;345:189–204. doi: 10.1113/jphysiol.1983.sp014974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen D. G., Blinks J. R., Prendergast F. G. Aequorin luminescence: relation of light emission to calcium concentration--a calcium-independent component. Science. 1977 Mar 11;195(4282):996–998. doi: 10.1126/science.841325. [DOI] [PubMed] [Google Scholar]
- Allen D. G., Orchard C. H. The effects of changes of pH on intracellular calcium transients in mammalian cardiac muscle. J Physiol. 1983 Feb;335:555–567. doi: 10.1113/jphysiol.1983.sp014550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastian J., Nakajima S. Action potential in the transverse tubules and its role in the activation of skeletal muscle. J Gen Physiol. 1974 Feb;63(2):257–278. doi: 10.1085/jgp.63.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blinks J. R., Rüdel R., Taylor S. R. Calcium transients in isolated amphibian skeletal muscle fibres: detection with aequorin. J Physiol. 1978 Apr;277:291–323. doi: 10.1113/jphysiol.1978.sp012273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blinks J. R., Wier W. G., Hess P., Prendergast F. G. Measurement of Ca2+ concentrations in living cells. Prog Biophys Mol Biol. 1982;40(1-2):1–114. doi: 10.1016/0079-6107(82)90011-6. [DOI] [PubMed] [Google Scholar]
- Gillis J. M., Thomason D., Lefèvre J., Kretsinger R. H. Parvalbumins and muscle relaxation: a computer simulation study. J Muscle Res Cell Motil. 1982 Dec;3(4):377–398. doi: 10.1007/BF00712090. [DOI] [PubMed] [Google Scholar]
- Hess P., Metzger P., Weingart R. Free magnesium in sheep, ferret and frog striated muscle at rest measured with ion-selective micro-electrodes. J Physiol. 1982 Dec;333:173–188. doi: 10.1113/jphysiol.1982.sp014447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi M., Kurihara S., Sakai T. Change in intracellular calcium ion concentration induced by caffeine and rapid cooling in frog skeletal muscle fibres. J Physiol. 1985 Aug;365:131–146. doi: 10.1113/jphysiol.1985.sp015763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovács L., Szücs G. Effect of caffeine on intramembrane charge movement and calcium transients in cut skeletal muscle fibres of the frog. J Physiol. 1983 Aug;341:559–578. doi: 10.1113/jphysiol.1983.sp014824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara S., Konishi M., Miyagishima T., Sakai T. Effects of enflurane on excitation-contraction coupling in frog skeletal muscle fibers. Pflugers Arch. 1984 Dec;402(4):345–352. doi: 10.1007/BF00583934. [DOI] [PubMed] [Google Scholar]
- López J. R., Alamo L., Caputo C., DiPolo R., Vergara S. Determination of ionic calcium in frog skeletal muscle fibers. Biophys J. 1983 Jul;43(1):1–4. doi: 10.1016/S0006-3495(83)84316-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüttgau H. C., Oetliker H. The action of caffeine on the activation of the contractile mechanism in straited muscle fibres. J Physiol. 1968 Jan;194(1):51–74. doi: 10.1113/jphysiol.1968.sp008394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto H., Racker E. Mechanism of calcium release from skeletal sarcoplasmic reticulum. J Membr Biol. 1982;66(3):193–201. doi: 10.1007/BF01868494. [DOI] [PubMed] [Google Scholar]
- Nagasaki K., Kasai M. Calcium-induced calcium release from sarcoplasmic reticulum vesicles. J Biochem. 1981 Sep;90(3):749–755. doi: 10.1093/oxfordjournals.jbchem.a133529. [DOI] [PubMed] [Google Scholar]
- Ochi K. Effects of twitch potentiators and repetitive stimulation on arsenazo III Ca-transients in Xenopus skeletal muscle fibers. Jpn J Physiol. 1984;34(5):857–870. doi: 10.2170/jjphysiol.34.857. [DOI] [PubMed] [Google Scholar]
- Ogawa Y. Some properties of fragmented frog sarcoplasmic reticulum with particular reference to its response to caffeine. J Biochem. 1970 May;67(5):667–683. doi: 10.1093/oxfordjournals.jbchem.a129295. [DOI] [PubMed] [Google Scholar]
- SANDOW A., TAYLOR S. R., ISAASON A., SEGUIN J. J. ELECTROCHEMICAL COUPLING IN POTENTIATION OF MUSCULAR CONTRACTION. Science. 1964 Feb 7;143(3606):577–579. doi: 10.1126/science.143.3606.577. [DOI] [PubMed] [Google Scholar]
- Weber A., Herz R. The relationship between caffeine contracture of intact muscle and the effect of caffeine on reticulum. J Gen Physiol. 1968 Nov;52(5):750–759. doi: 10.1085/jgp.52.5.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendt I. R., Stephenson D. G. Effects of caffeine on Ca-activated force production in skinned cardiac and skeletal muscle fibres of the rat. Pflugers Arch. 1983 Aug;398(3):210–216. doi: 10.1007/BF00657153. [DOI] [PubMed] [Google Scholar]
- Yoshioka T., Somlyo A. P. Calcium and magnesium contents and volume of the terminal cisternae in caffeine-treated skeletal muscle. J Cell Biol. 1984 Aug;99(2):558–568. doi: 10.1083/jcb.99.2.558. [DOI] [PMC free article] [PubMed] [Google Scholar]


