Abstract
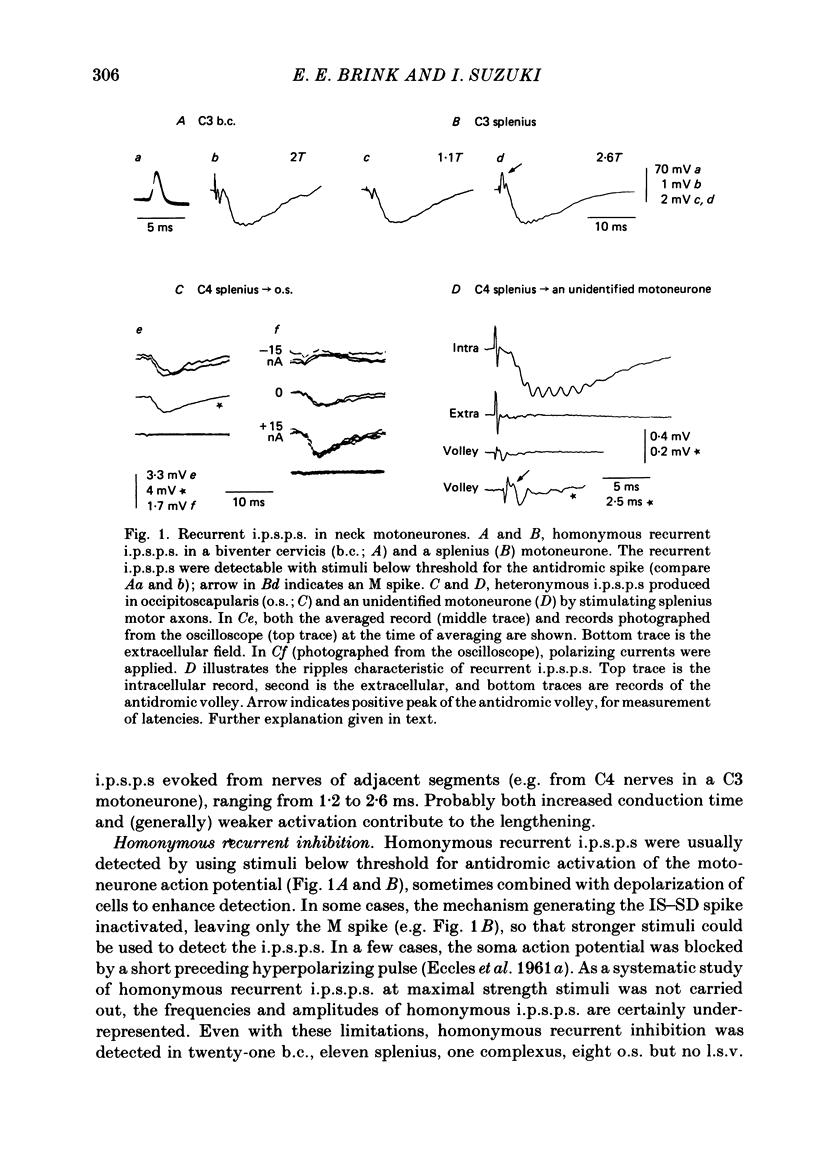
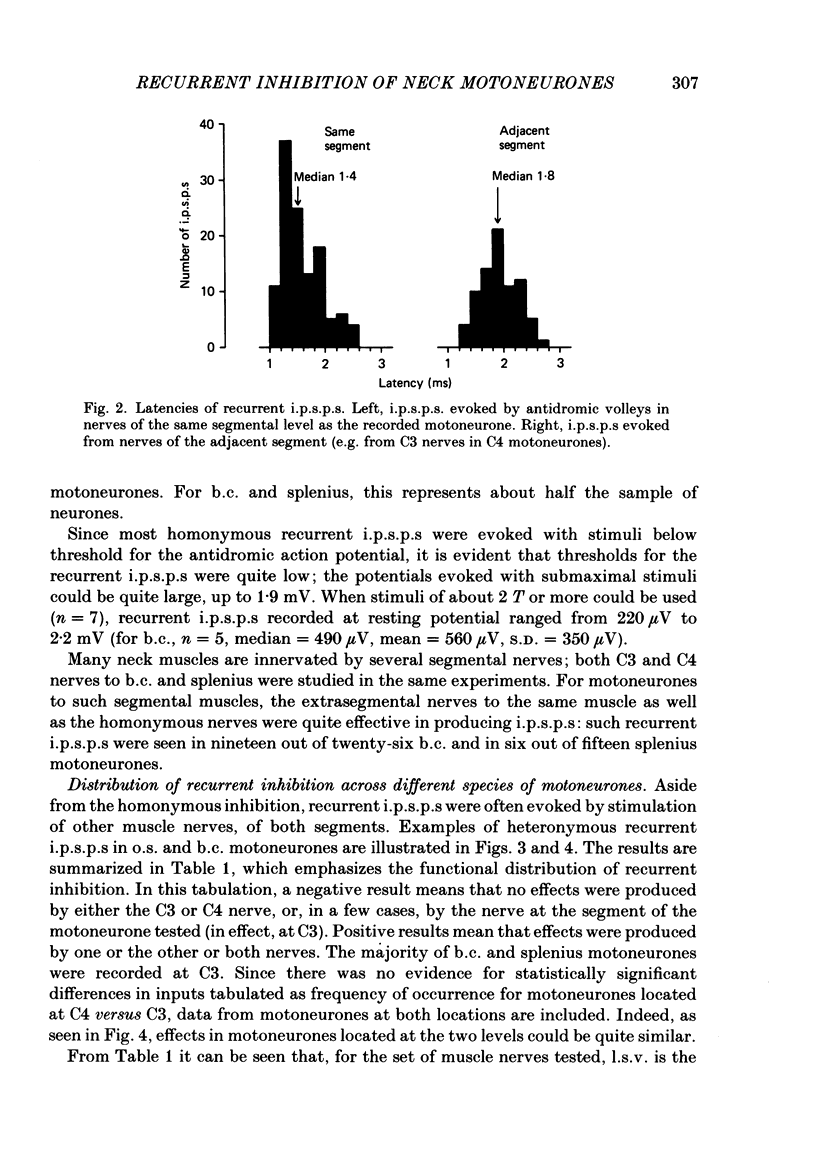
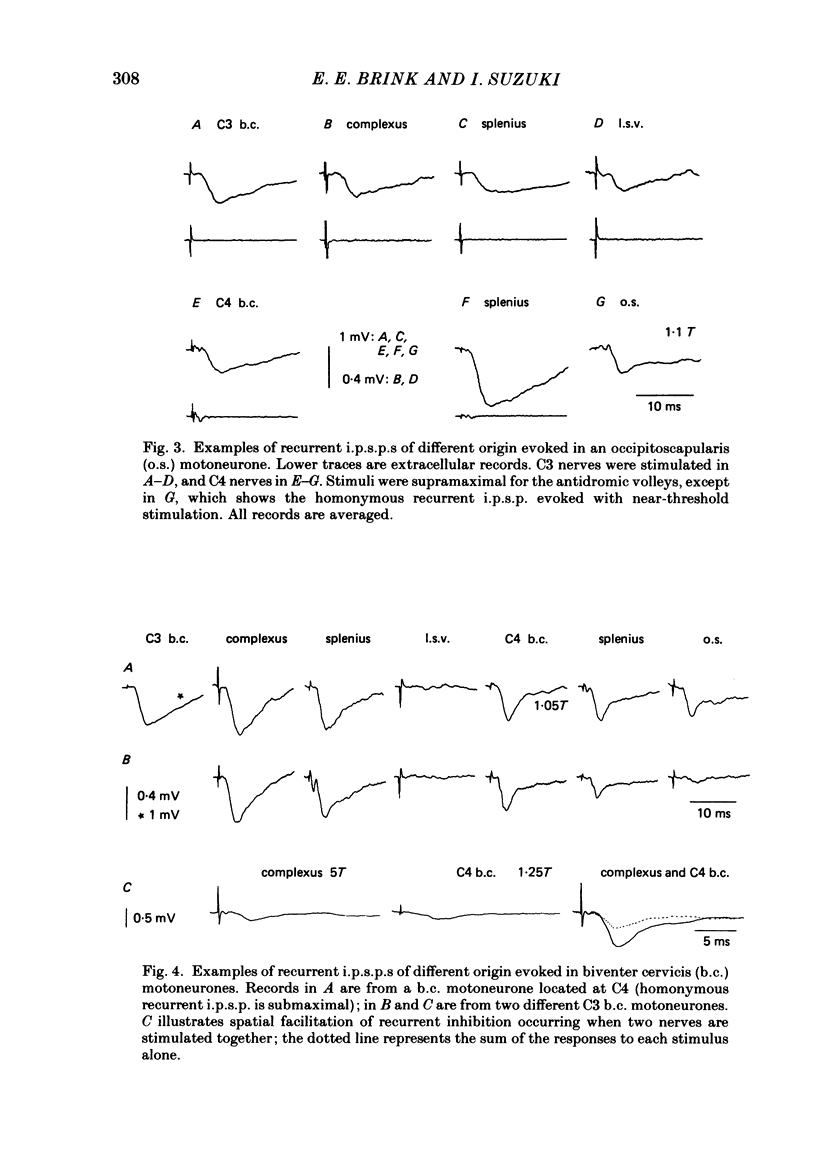
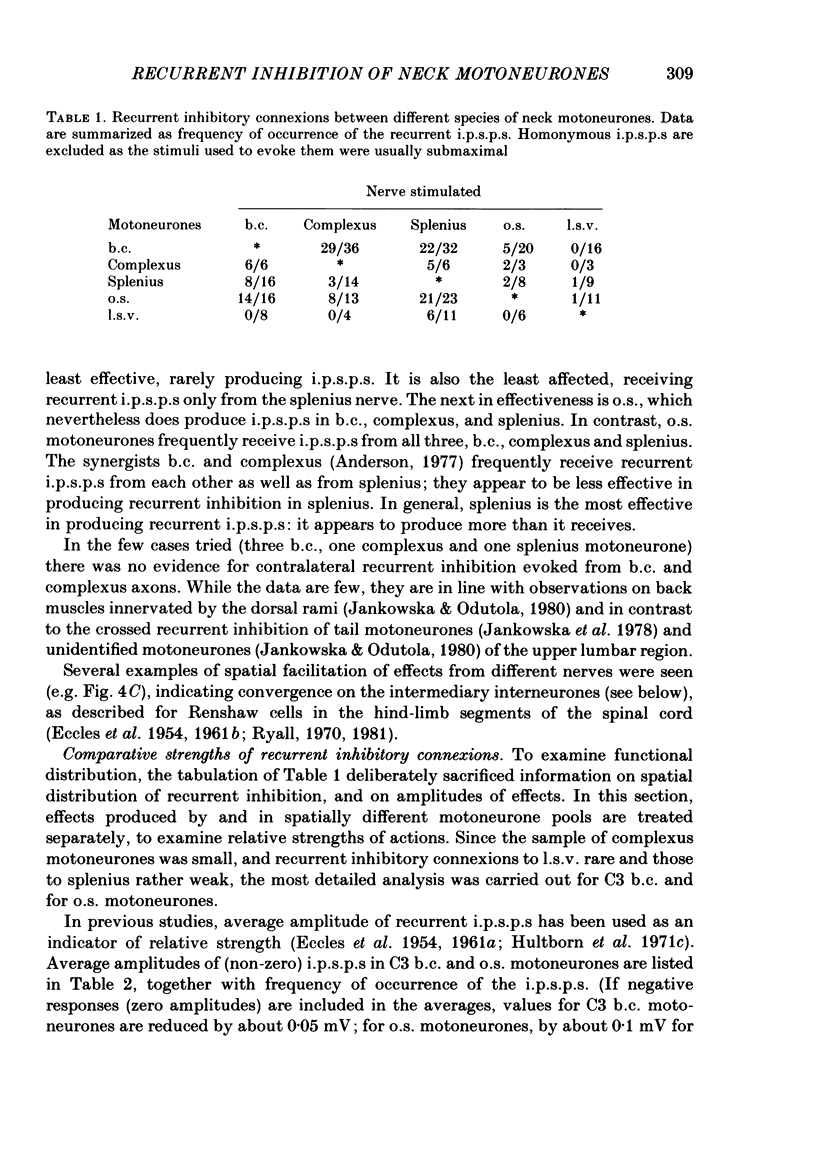
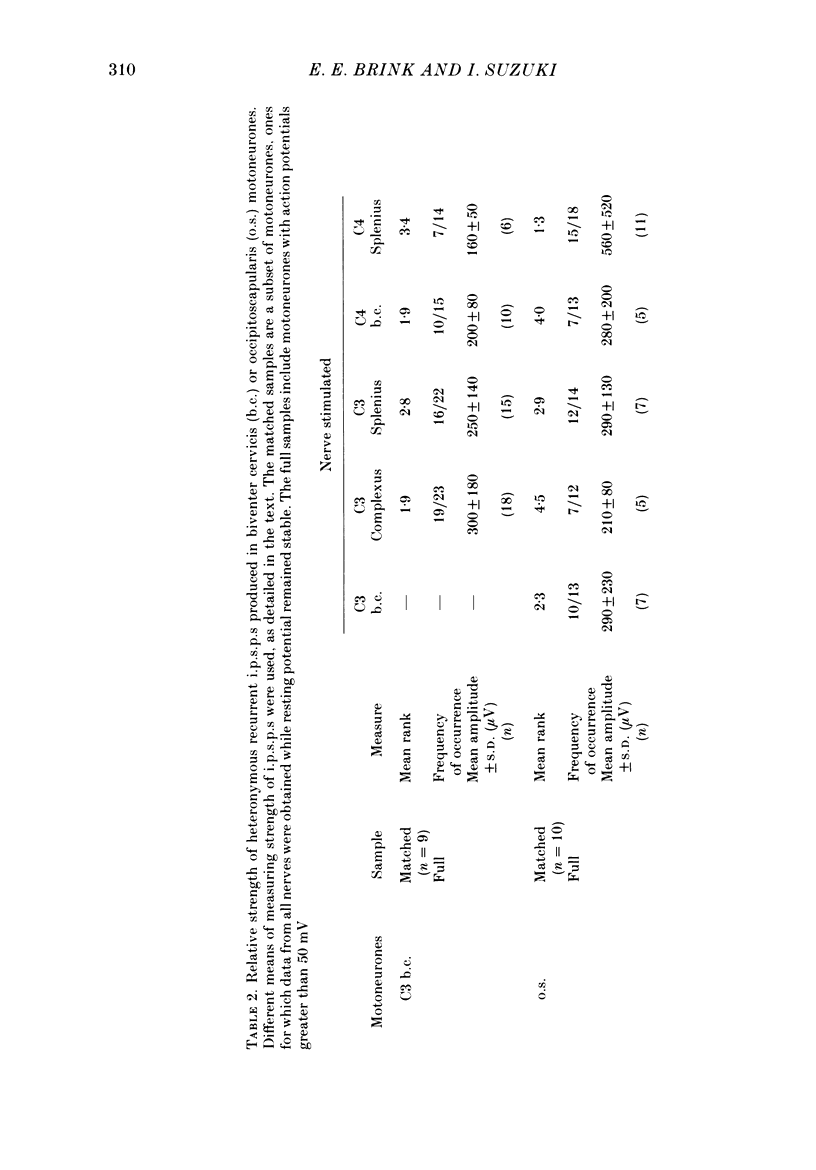
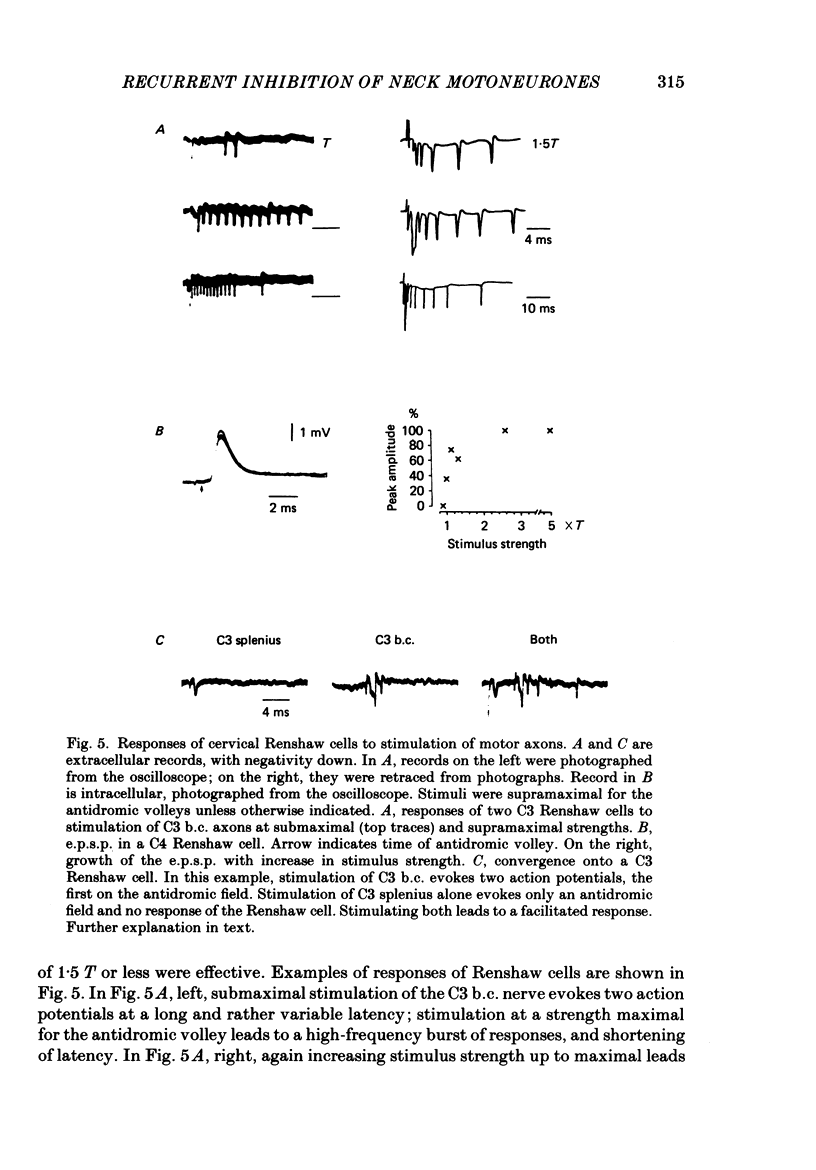
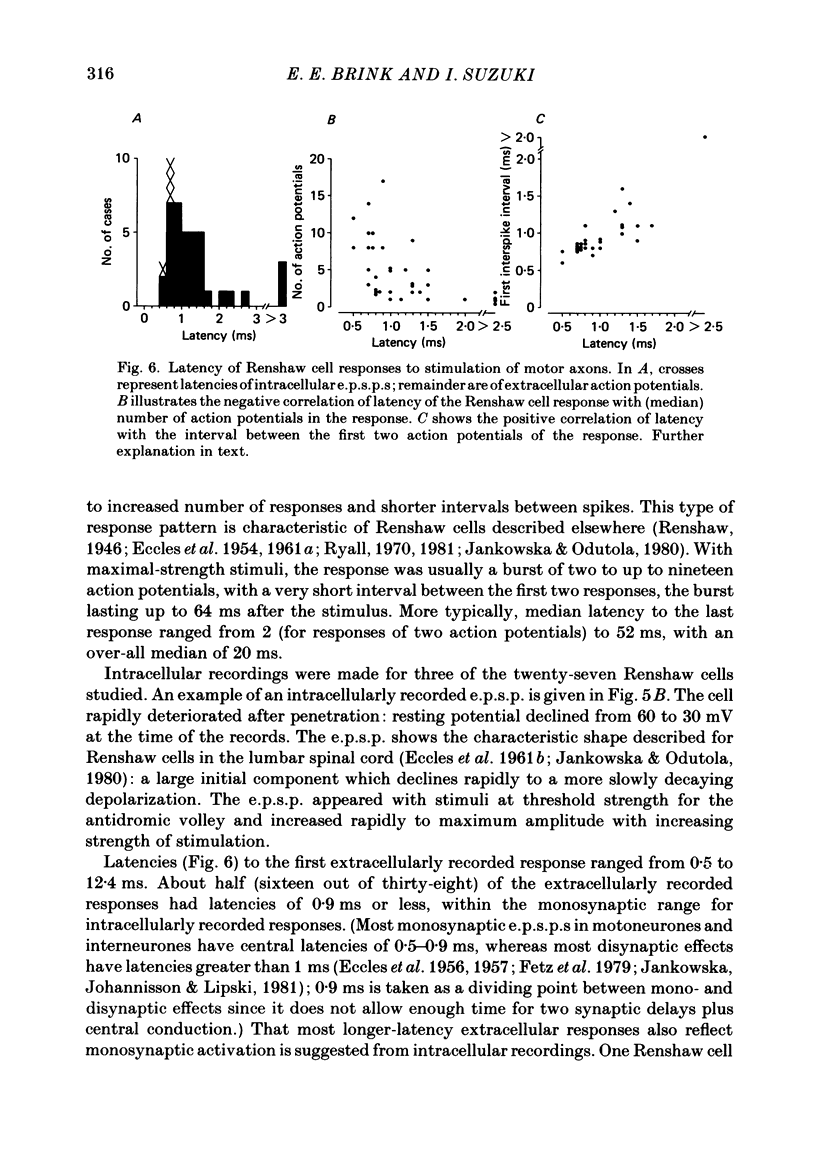
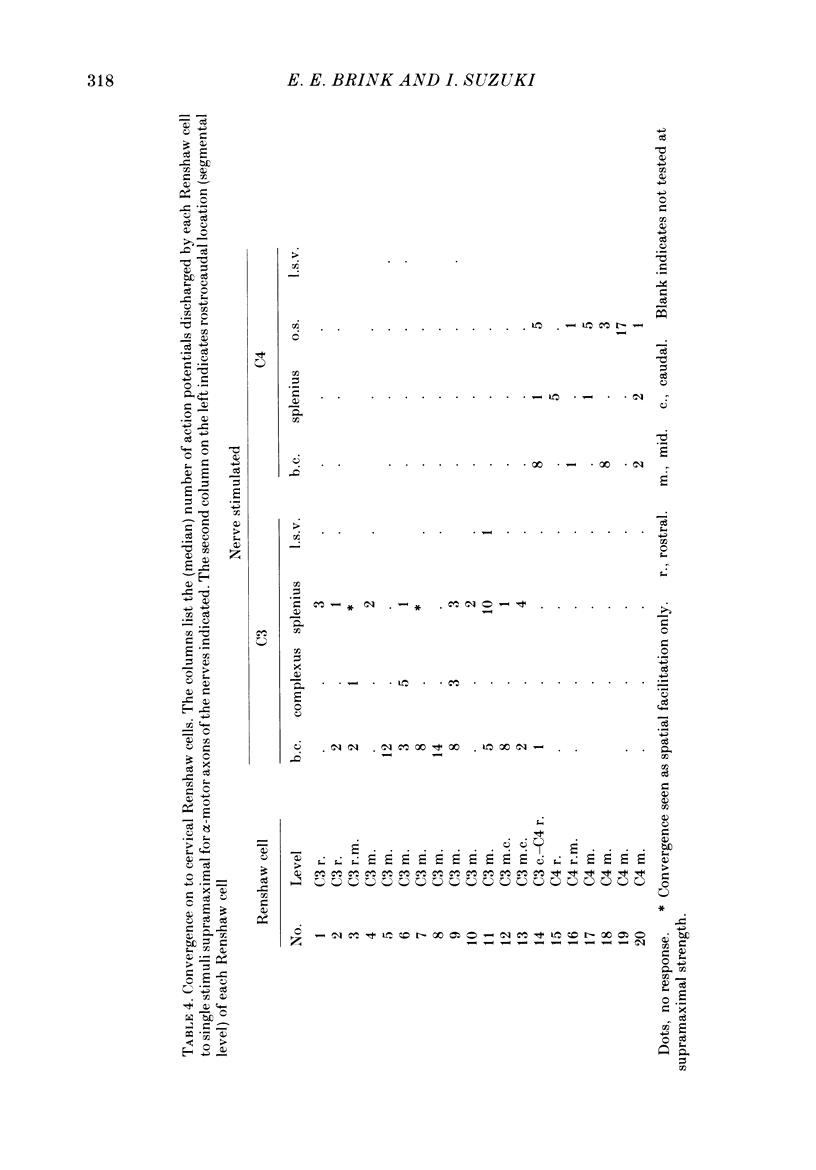
1. Intracellular recordings were made from motoneurones innervating neck muscles in the cat. Dorsal roots were cut and muscle nerves electrically stimulated to activate alpha motor axons. 2. Recurrent inhibitory post-synaptic potentials (i.p.s.p.s) evoked by antidromic volleys in homonymous or heteronymous nerves were found in the majority of motoneurones studied, including those to dorsal neck muscles (biventer cervicis, splenius and complexus) as well as to occipitoscapularis and levator scapulae ventralis. 3. Central latencies of the recurrent i.p.s.p.s indicate disynaptic transmission. Amplitudes ranged from 100 microV (criterion level) to 2.2 mV. Average amplitudes were less than 0.6 mV. 4. The recurrent i.p.s.p.s were distributed to non-synergistic as well as to synergistic motoneurones. Analysis of relative strength of recurrent inhibition indicates influence of proximity of motoneurone pools, functional relatedness of muscles, as well as other factors. Variation in intrinsic motoneuronal properties probably underlies positive correlations (independent of variation in resting potential) between recurrent i.p.s.p.s evoked from different sources in motoneurones of a single pool. 5. Recordings (mainly extracellular) were also made from interneurones (Renshaw cells), located in the C3 and C4 segments of the spinal cord, that were excited by antidromic volleys in muscle nerves. The response varied from a single action potential to a burst of up to nineteen action potentials. Central latencies to the first response indicate monosynaptic transmission. Many Renshaw cells were excited by antidromic volleys in several muscle nerves, though this was restricted to nerves of the same segmental level as the Renshaw cell. All the muscle nerves studied were effective in activating Renshaw cells. 6. The results indicate that in many ways the recurrent i.p.s.p.s and the responses of Renshaw cells recorded in the neck segments resemble those in the hind-limb segments. Thus, the basic organization of recurrent inhibition in the neck segments resembles that occurring elsewhere in the spinal cord. A difference is the tendency for recurrent i.p.s.p.s in neck motoneurones to be relatively small in amplitude and Renshaw cell responses to be less strong than those recorded in the hind-limb segments. It is suggested that this is related to the segmentation of neck muscles and their motoneurone pools. 7. It is concluded that recurrent inhibition is a prominent feature of spinal organization governing neck muscles. It can therefore be expected to participate in control of head movements.
Full text
PDF

















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. E. Segmental reflex inputs to motoneurons innervating dorsal neck musculature in the cat. Exp Brain Res. 1977 May 23;28(1-2):175–187. doi: 10.1007/BF00237095. [DOI] [PubMed] [Google Scholar]
- Baker J., Goldberg J., Peterson B. Spatial and temporal response properties of the vestibulocollic reflex in decerebrate cats. J Neurophysiol. 1985 Sep;54(3):735–756. doi: 10.1152/jn.1985.54.3.735. [DOI] [PubMed] [Google Scholar]
- Burke R. E., Fedina L., Lundberg A. Spatial synaptic distribution of recurrent and group Ia inhibitory systems in cat spinal motoneurones. J Physiol. 1971 Apr;214(2):305–326. doi: 10.1113/jphysiol.1971.sp009434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coggeshall R. E., Coulter J. D., Willis W. D., Jr Unmyelinated axons in the ventral roots of the cat lumbosacral enlargement. J Comp Neurol. 1974 Jan 1;153(1):39–58. doi: 10.1002/cne.901530105. [DOI] [PubMed] [Google Scholar]
- Cullheim S., Kellerth J. O. A morphological study of the axons and recurrent axon collaterals of cat alpha-motoneurones supplying different functional types of muscle unit. J Physiol. 1978 Aug;281:301–313. doi: 10.1113/jphysiol.1978.sp012423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullheim S., Kellerth J. O. A morphological study of the axons and recurrent axon collaterals of cat alpha-motoneurones supplying different hind-limb muscles. J Physiol. 1978 Aug;281:285–299. doi: 10.1113/jphysiol.1978.sp012422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., IGGO A., ITO M. Distribution of recurrent inhibition among motoneurones. J Physiol. 1961 Dec;159:479–499. doi: 10.1113/jphysiol.1961.sp006822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., LUNDBERG A. Synaptic actions on motoneurones caused by impulses in Golgi tendon organ afferents. J Physiol. 1957 Sep 30;138(2):227–252. doi: 10.1113/jphysiol.1957.sp005849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., FATT P., KOKETSU K. Cholinergic and inhibitory synapses in a pathway from motor-axon collaterals to motoneurones. J Physiol. 1954 Dec 10;126(3):524–562. doi: 10.1113/jphysiol.1954.sp005226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., FATT P., LANDGREN S. Central pathway for direct inhibitory action of impulses in largest afferent nerve fibres to muscle. J Neurophysiol. 1956 Jan;19(1):75–98. doi: 10.1152/jn.1956.19.1.75. [DOI] [PubMed] [Google Scholar]
- Fetz E. E., Jankowska E., Johannisson T., Lipski J. Autogenetic inhibition of motoneurones by impulses in group Ia muscle spindle afferents. J Physiol. 1979 Aug;293:173–195. doi: 10.1113/jphysiol.1979.sp012884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman W. A., Sypert G. W., Munson J. B., Fleshman J. W. Recurrent inhibition in type-identified motoneurons. J Neurophysiol. 1981 Dec;46(6):1349–1359. doi: 10.1152/jn.1981.46.6.1349. [DOI] [PubMed] [Google Scholar]
- GILL P. K., KUNO M. PROPERTIES OF PHRENIC MOTONEURONES. J Physiol. 1963 Sep;168:258–273. doi: 10.1113/jphysiol.1963.sp007191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRANIT R., PASCOE J. E., STEG G. The behaviour of tonic alpha and gamma motoneurones during stimulation of recurrent collaterals. J Physiol. 1957 Oct 30;138(3):381–400. doi: 10.1113/jphysiol.1957.sp005857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase J., Cleveland S., Ross H. G. Problems of postsynaptic autogenous and recurrent inhibition in the mammalian spinal cord. Rev Physiol Biochem Pharmacol. 1975;73:73–129. doi: 10.1007/BFb0034660. [DOI] [PubMed] [Google Scholar]
- Hilaire G., Khatib M., Monteau R. Spontaneous respiratory activity of phrenic and intercostal Renshaw cells. Neurosci Lett. 1983 Dec 23;43(1):97–101. doi: 10.1016/0304-3940(83)90135-0. [DOI] [PubMed] [Google Scholar]
- Hultborn H., Jankowska E., Lindström S. Recurrent inhibition from motor axon collaterals of transmission in the Ia inhibitory pathway to motoneurones. J Physiol. 1971 Jul;215(3):591–612. doi: 10.1113/jphysiol.1971.sp009487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H., Jankowska E., Lindström S. Recurrent inhibition of interneurones monosynaptically activated from group Ia afferents. J Physiol. 1971 Jul;215(3):613–636. doi: 10.1113/jphysiol.1971.sp009488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H., Jankowska E., Lindström S. Relative contribution from different nerves to recurrent depression of Ia IPSPs in motoneurones. J Physiol. 1971 Jul;215(3):637–664. doi: 10.1113/jphysiol.1971.sp009489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E., Johannisson T., Lipski J. Common interneurones in reflex pathways from group 1a and 1b afferents of ankle extensors in the cat. J Physiol. 1981 Jan;310:381–402. doi: 10.1113/jphysiol.1981.sp013556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E., Odutola A. Crosses and uncrossed synaptic actions on motoneurones of back muscles in the cat. Brain Res. 1980 Jul 21;194(1):65–78. doi: 10.1016/0006-8993(80)91319-0. [DOI] [PubMed] [Google Scholar]
- Jankowska E., Padel Y., Zarzecki P. Crossed disynaptic inhibition of sacral motoneurones. J Physiol. 1978 Dec;285:425–444. doi: 10.1113/jphysiol.1978.sp012580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E., Roberts W. J. Synaptic actions of single interneurones mediating reciprocal Ia inhibition of motoneurones. J Physiol. 1972 May;222(3):623–642. doi: 10.1113/jphysiol.1972.sp009818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E., Smith D. O. Antidromic activation of Renshaw cells and their axonal projections. Acta Physiol Scand. 1973 Jun;88(2):198–214. doi: 10.1111/j.1748-1716.1973.tb05447.x. [DOI] [PubMed] [Google Scholar]
- KUNO M. Excitability following antidromic activation in spinal motoneurones supplying red muscles. J Physiol. 1959 Dec;149:374–393. doi: 10.1113/jphysiol.1959.sp006345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood P. A., Sears T. A., Westgaard R. H. Recurrent inhibition of intercostal motoneurones in the cat. J Physiol. 1981;319:111–130. doi: 10.1113/jphysiol.1981.sp013895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagerbäck P. A., Kellerth J. O. Light microscopic observations on cat Renshaw cells after intracellular staining with horseradish peroxidase. I. The axonal systems. J Comp Neurol. 1985 Oct 22;240(4):359–367. doi: 10.1002/cne.902400404. [DOI] [PubMed] [Google Scholar]
- Lipski J., Fyffe R. E., Jodkowski J. Recurrent inhibition of cat phrenic motoneurons. J Neurosci. 1985 Jun;5(6):1545–1555. doi: 10.1523/JNEUROSCI.05-06-01545.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackel R. Segmental and descending control of the external urethral and anal sphincters in the cat. J Physiol. 1979 Sep;294:105–122. doi: 10.1113/jphysiol.1979.sp012918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson J. B., Sypert G. W. Properties of single fibre excitatory post-synaptic potentials in triceps surae motoneurones. J Physiol. 1979 Nov;296:329–342. doi: 10.1113/jphysiol.1979.sp013008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROMANES G. J. The motor cell columns of the lumbo-sacral spinal cord of the cat. J Comp Neurol. 1951 Apr;94(2):313–363. doi: 10.1002/cne.900940209. [DOI] [PubMed] [Google Scholar]
- Rapoport S. Location of sternocleidomastoid and trapezius motoneurons in the cat. Brain Res. 1978 Nov 10;156(2):339–344. doi: 10.1016/0006-8993(78)90515-2. [DOI] [PubMed] [Google Scholar]
- Rapoport S. Reflex connexions of motoneurones of muscles involved in head movement in the cat. J Physiol. 1979 Apr;289:311–327. doi: 10.1113/jphysiol.1979.sp012739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond F. J., Abrahams V. C. Morphology and enzyme histochemistry of dorsal muscles of the cat neck. J Neurophysiol. 1975 Nov;38(6):1312–1321. doi: 10.1152/jn.1975.38.6.1312. [DOI] [PubMed] [Google Scholar]
- Richmond F. J., Anstee G. C., Sherwin E. A., Abrahams V. C. Motor and sensory fibres of neck muscle nerves in the cat. Can J Physiol Pharmacol. 1976 Jun;54(3):294–304. doi: 10.1139/y76-043. [DOI] [PubMed] [Google Scholar]
- Richmond F. J., Scott D. A., Abrahams V. C. Distribution of motoneurones to the neck muscles, biventer cervicis, splenius and complexus in the cat. J Comp Neurol. 1978 Oct 1;181(3):451–463. doi: 10.1002/cne.901810302. [DOI] [PubMed] [Google Scholar]
- Ryall R. W. Patterns of recurrent excitation and mutual inhibition of cat Renshaw cells. J Physiol. 1981 Jul;316:439–452. doi: 10.1113/jphysiol.1981.sp013799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryall R. W., Piercey M. F., Polosa C. Intersegmental and intrasegmental distribution of mutual inhibition of Renshaw cells. J Neurophysiol. 1971 Jul;34(4):700–707. doi: 10.1152/jn.1971.34.4.700. [DOI] [PubMed] [Google Scholar]
- Ryall R. W. Renshaw cell mediated inhibition of Renshaw cells: patterns of excitation and inhibition from impulses in motor axon collaterals. J Neurophysiol. 1970 Mar;33(2):257–270. doi: 10.1152/jn.1970.33.2.257. [DOI] [PubMed] [Google Scholar]
- SEARS T. A. SOME PROPERTIES AND REFLEX CONNEXIONS OF RESPIRATORY MOTONEURONES OF THE CAT'S THORACIC SPINAL CORD. J Physiol. 1964 Dec;175:386–403. doi: 10.1113/jphysiol.1964.sp007523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R. C., Wilson V. J. Recurrent interactions between motoneurons of known location in the cervical cord of the cat. J Neurophysiol. 1967 Jul;30(4):661–674. doi: 10.1152/jn.1967.30.4.661. [DOI] [PubMed] [Google Scholar]
- WILSON V. J., TALBOT W. H., DIECKE F. P. Distribution of recurrent facilitation and inhibition in cat spinal cord. J Neurophysiol. 1960 Mar;23:144–153. doi: 10.1152/jn.1960.23.2.144. [DOI] [PubMed] [Google Scholar]
- Willis W. D. The case for the Renshaw cell. Brain Behav Evol. 1971;4(1):5–52. doi: 10.1159/000125422. [DOI] [PubMed] [Google Scholar]
- van Keulen L. C. Axon trajectories of Renshaw cells in the lumbar spinal cord of the cat, as reconstructed after intracellular staining with horseradish peroxidase. Brain Res. 1979 May 5;167(1):157–162. doi: 10.1016/0006-8993(79)90270-1. [DOI] [PubMed] [Google Scholar]


