Abstract
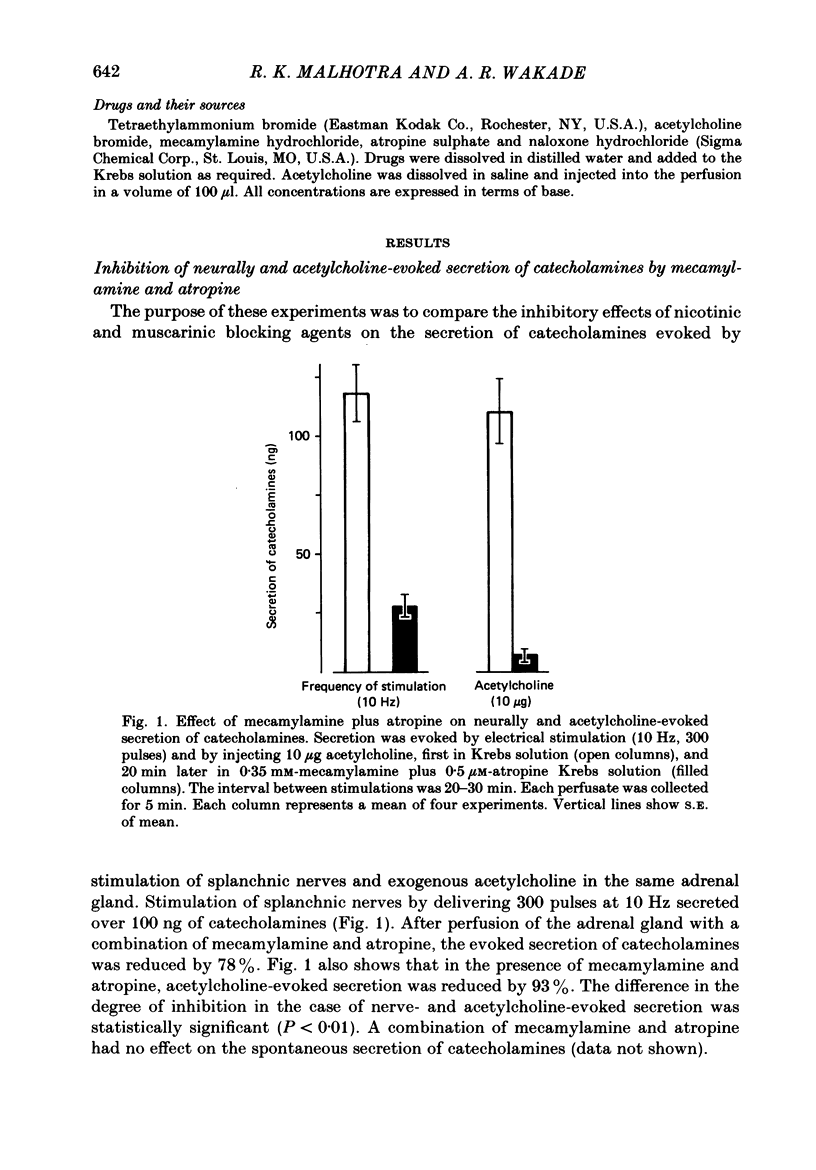
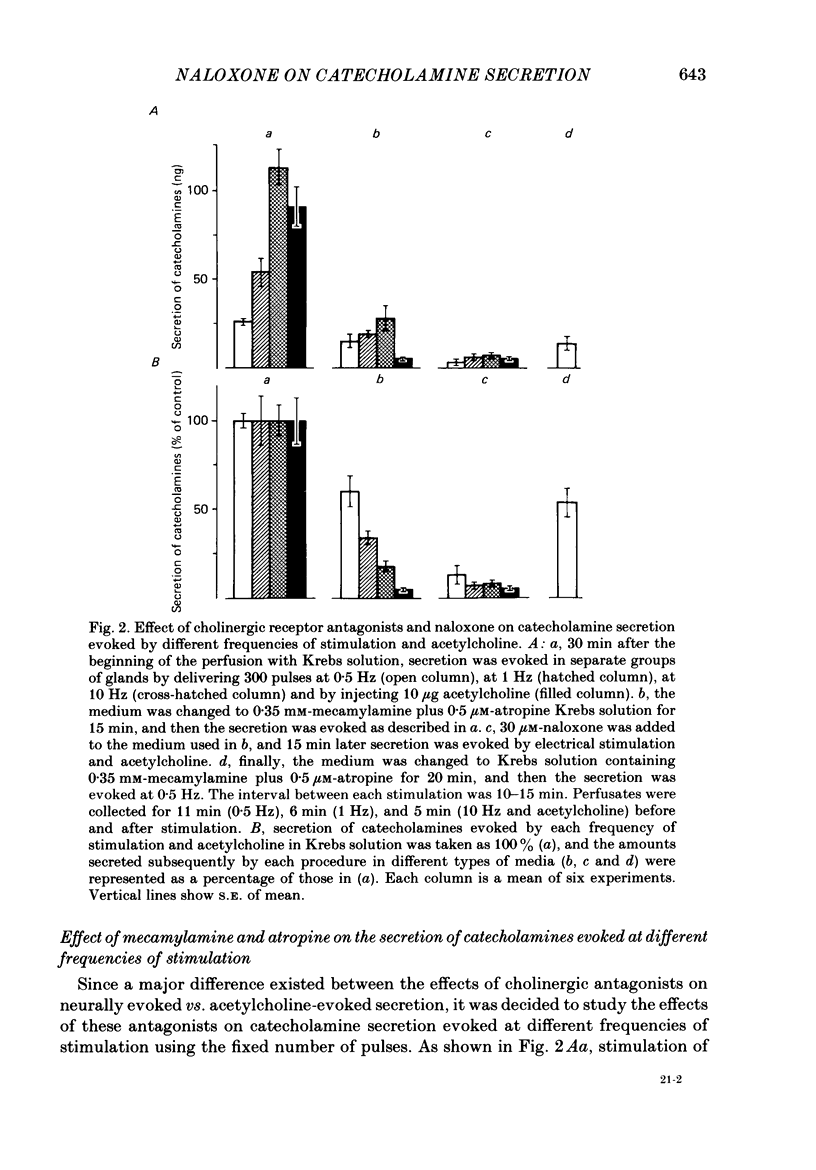
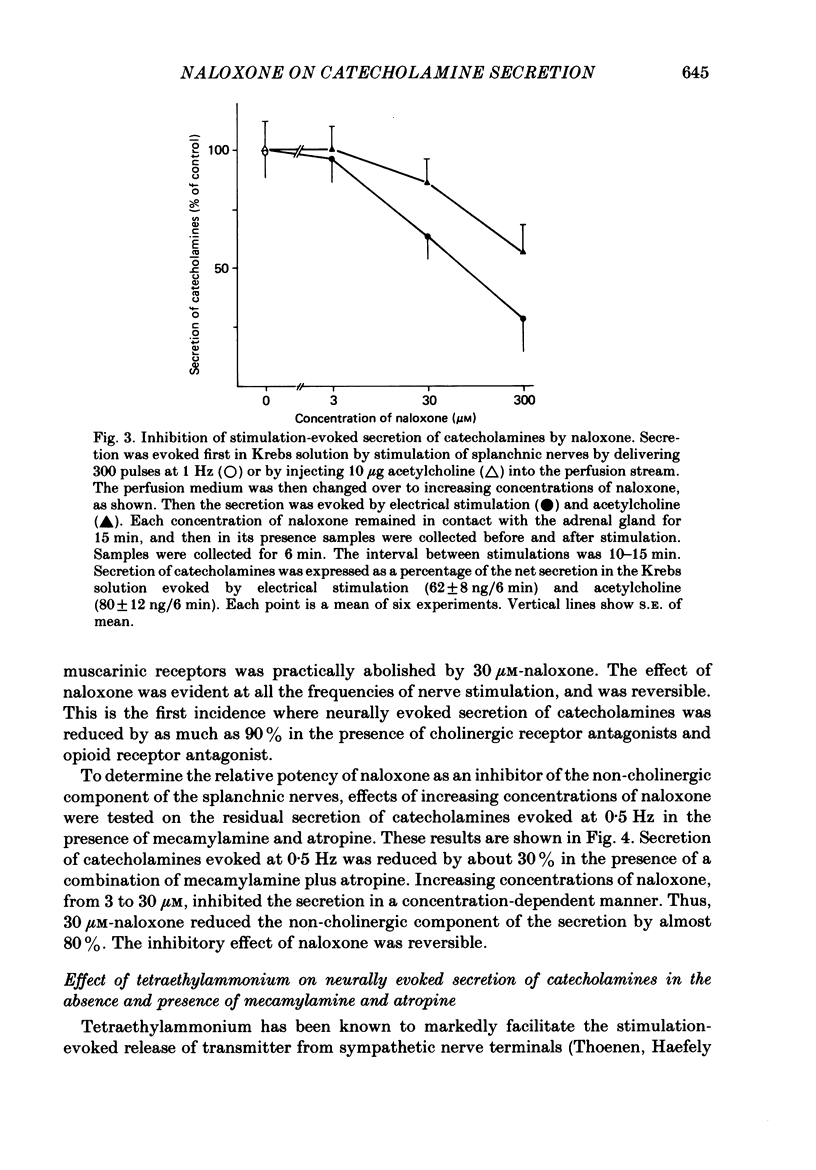
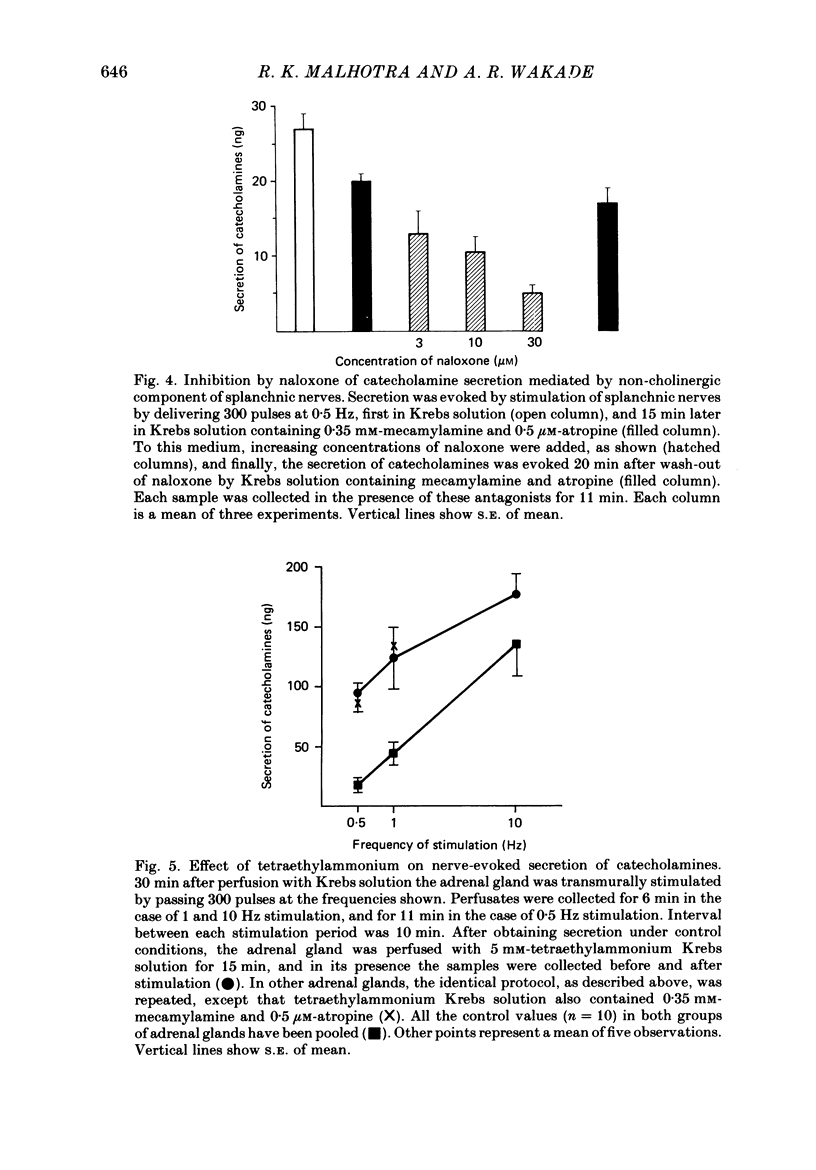
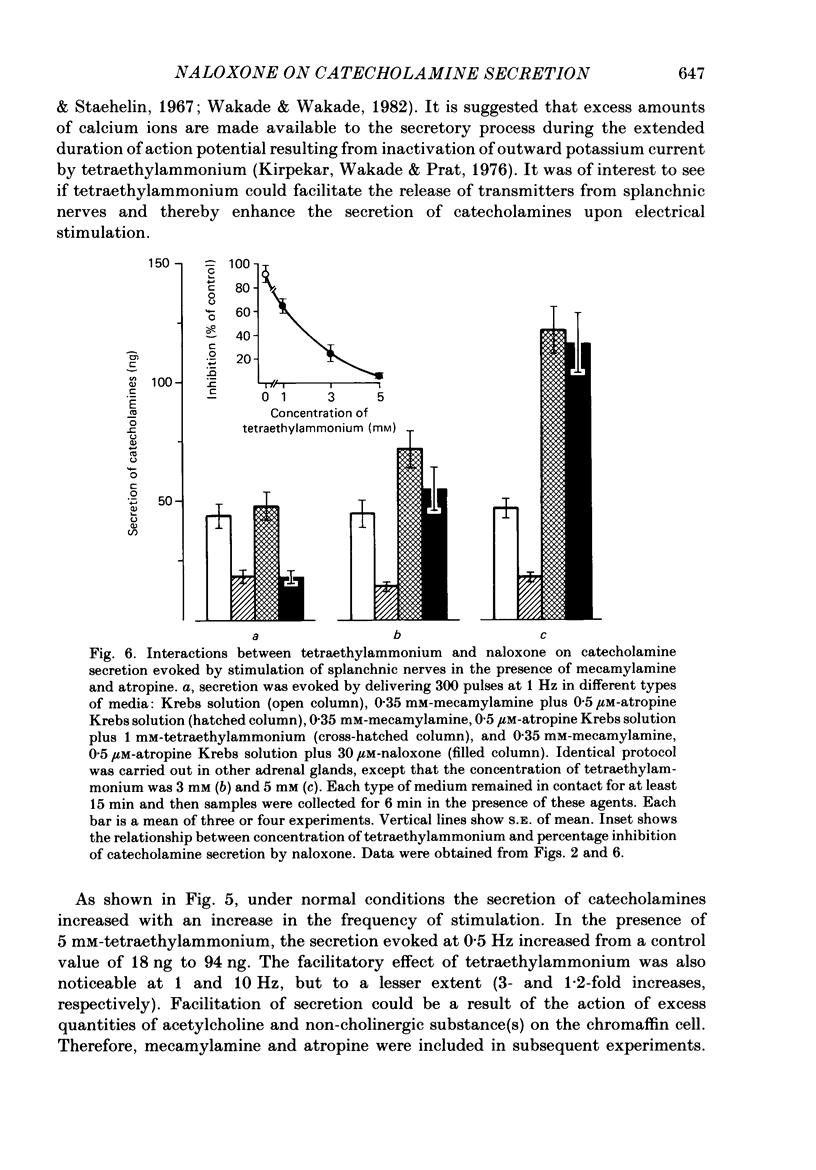
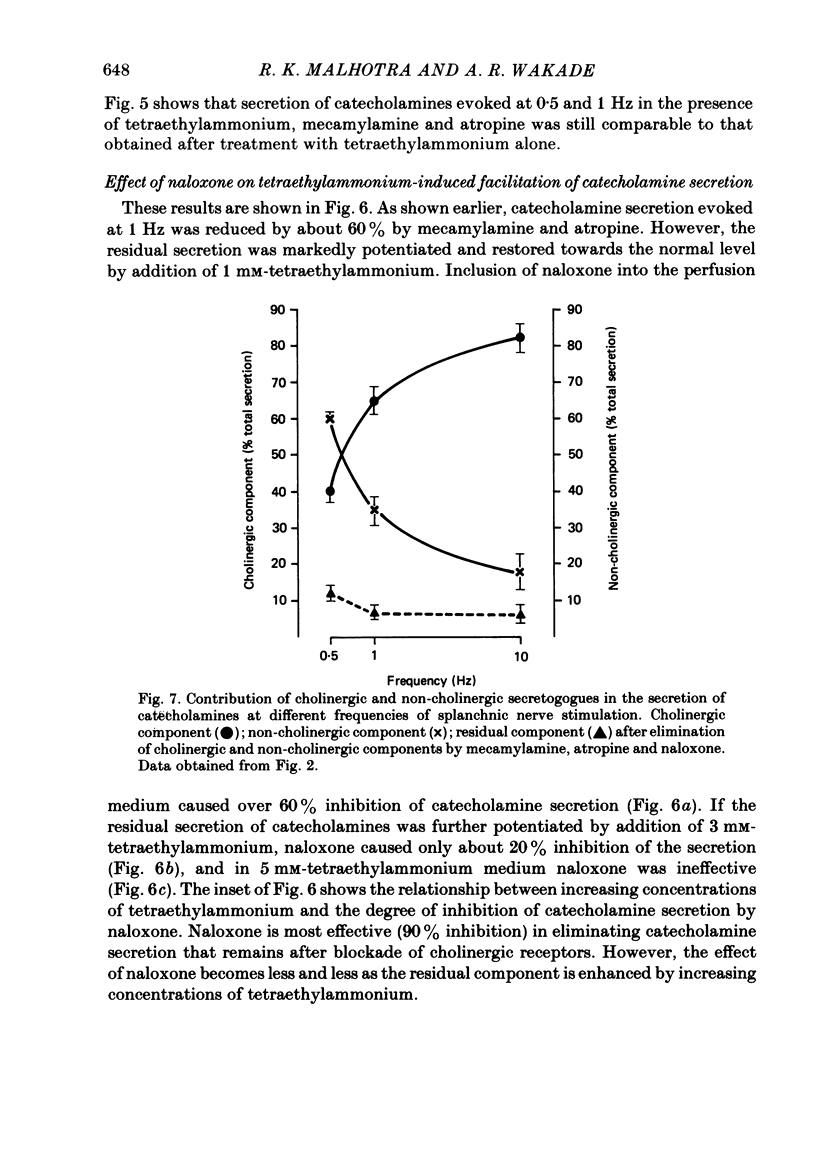
1. Effects of nicotinic (mecamylamine) and muscarinic (atropine) receptor antagonists were investigated on the secretion of catecholamines evoked by stimulation of splanchnic nerve terminals and acetylcholine in the isolated perfused adrenal gland of the rat to determine whether non-cholinergic substances released from nerve terminals participate in the secretion of catecholamines. 2. Increasing the frequency of stimulation from 0.5 to 10 Hz (300 pulses) caused enhanced secretion of catecholamines (26-110 ng/collection period). After blockade of nicotinic and muscarinic receptors with mecamylamine and atropine, the secretion was reduced by 40, 65 and 80% at 0.5, 1 and 10 Hz, respectively. Acetylcholine-evoked secretion of catecholamines, which was roughly equivalent to that produced by stimulation at 10 Hz, was blocked by over 90% by the cholinergic antagonists. 3. Naloxone (3-300 microM) caused a concentration-dependent inhibition of catecholamine secretion evoked by stimulation of splanchnic nerves (1 Hz); acetylcholine-evoked secretion was much less affected by naloxone. 4. The secretion of catecholamines that remained after blockade of cholinergic receptors at different frequencies of stimulation (see 2 above) was almost completely inhibited by inclusion of 30 microM-naloxone in the medium. The inhibitory effect of naloxone was concentration dependent (3-30 microM) and reversible. 5. Splanchnic nerve-evoked secretion of catecholamines was facilitated by 400% in the presence of tetraethylammonium or tetraethylammonium plus mecamylamine and atropine. The facilitatory effect of tetraethylammonium was inversely related to the frequency of stimulation. 6. The residual secretion of catecholamines obtained after blockade of cholinergic receptors was facilitated by increasing concentrations of tetraethylammonium (1-5 mM). 30 microM-naloxone antagonized the facilitatory effects of tetraethylammonium at 1 and 3 mM by 60% and 25%, respectively, but failed at 5 mM-tetraethylammonium; higher concentrations of naloxone (100 microM) were also ineffective. 7. It is concluded that neurally evoked secretion of catecholamines is mediated by acetylcholine and a non-cholinergic substance(s); the contribution of non-cholinergic substance(s) predominates at low neuronal activity, whereas that of acetylcholine is maximum at high neuronal activity. Blockade of the non-cholinergic component by naloxone suggests that an opioid peptide may be involved in the secretion of catecholamines in the rat adrenal medulla.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANTON A. H., SAYRE D. F. A study of the factors affecting the aluminum oxide-trihydroxyindole procedure for the analysis of catecholamines. J Pharmacol Exp Ther. 1962 Dec;138:360–375. [PubMed] [Google Scholar]
- Chaminade M., Foutz A. S., Rossier J. Co-release of enkephalins and precursors with catecholamines from the perfused cat adrenal gland in situ. J Physiol. 1984 Aug;353:157–169. doi: 10.1113/jphysiol.1984.sp015329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavkin C., Cox B. M., Goldstein A. Stereospecific opiate binding in bovine adrenal medulla. Mol Pharmacol. 1979 May;15(3):751–753. [PubMed] [Google Scholar]
- Dean D. M., Lemaire S., Livett B. G. Evidence that inhibition of nicotine-mediated catecholamine secretion from adrenal chromaffin cells by enkephalin, beta-endorphin, dynorphin (1-13), and opiates is not mediated via specific opiate receptors. J Neurochem. 1982 Mar;38(3):606–614. doi: 10.1111/j.1471-4159.1982.tb08674.x. [DOI] [PubMed] [Google Scholar]
- Dumont M., Lemaire S. Opioid receptors in bovine adrenal medulla. Can J Physiol Pharmacol. 1984 Oct;62(10):1284–1291. doi: 10.1139/y84-215. [DOI] [PubMed] [Google Scholar]
- Feldberg W., Minz B., Tsudzimura H. The mechanism of the nervous discharge of adrenaline. J Physiol. 1934 Jun 9;81(3):286–304. doi: 10.1113/jphysiol.1934.sp003136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hökfelt T., Lundberg J. M., Schultzberg M., Fahrenkrug J. Immunohistochemical evidence for a local VIP-ergic neuron system in the adrenal gland of the rat. Acta Physiol Scand. 1981 Dec;113(4):575–576. doi: 10.1111/j.1748-1716.1981.tb06944.x. [DOI] [PubMed] [Google Scholar]
- Kirpekar S. M., Prat J. C., Wakade A. R. Effect of calcium on the relationship between frequency of stimulation and release of noradrenaline from the perfused spleen of the cat. Naunyn Schmiedebergs Arch Pharmacol. 1975;287(2):205–212. doi: 10.1007/BF00510451. [DOI] [PubMed] [Google Scholar]
- Kirpekar S. M., Wakade A. R., Prat J. C. Effect of tetraethylammonium and barium on the release of noradrenaline from the perfused cat spleen by nerve stimulation and potassium. Naunyn Schmiedebergs Arch Pharmacol. 1976 Jul;294(1):23–29. doi: 10.1007/BF00692781. [DOI] [PubMed] [Google Scholar]
- Kumakura K., Karoum F., Guidotti A., Costa E. Modulation of nicotinic receptors by opiate receptor agonists in cultured adrenal chromaffin cells. Nature. 1980 Jan 31;283(5746):489–492. doi: 10.1038/283489a0. [DOI] [PubMed] [Google Scholar]
- Lemaire S., Day R., Dumont M., Chouinard L., Calvert R. Dynorphin and enkephalins in adrenal paraneurones. Opiates in the adrenal medulla. Can J Physiol Pharmacol. 1984 Apr;62(4):484–492. doi: 10.1139/y84-078. [DOI] [PubMed] [Google Scholar]
- Linnoila R. I., Diaugustine R. P., Hervonen A., Miller R. J. Distribution of [Met5]- and [Leu5]-enkephalin-, vasoactive intestinal polypeptide- and substance P-like immunoreactivities in human adrenal glands. Neuroscience. 1980;5(12):2247–2259. doi: 10.1016/0306-4522(80)90141-4. [DOI] [PubMed] [Google Scholar]
- Lord J. A., Waterfield A. A., Hughes J., Kosterlitz H. W. Endogenous opioid peptides: multiple agonists and receptors. Nature. 1977 Jun 9;267(5611):495–499. doi: 10.1038/267495a0. [DOI] [PubMed] [Google Scholar]
- Lundberg J. M., Hamberger B., Schultzberg M., Hökfelt T., Granberg P. O., Efendić S., Terenius L., Goldstein M., Luft R. Enkephalin- and somatostatin-like immunoreactivities in human adrenal medulla and pheochromocytoma. Proc Natl Acad Sci U S A. 1979 Aug;76(8):4079–4083. doi: 10.1073/pnas.76.8.4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Role L. W., Perlman R. L. Both nicotinic and muscarinic receptors mediate catecholamine secretion by isolated guinea-pig chromaffin cells. Neuroscience. 1983 Nov;10(3):979–985. doi: 10.1016/0306-4522(83)90236-1. [DOI] [PubMed] [Google Scholar]
- Saito H., Saito S., Ohuchi T., Oka M., Sano T., Hosoi E. Co-storage and co-secretion of somatostatin and catecholamine in bovine adrenal medulla. Neurosci Lett. 1984 Nov 23;52(1-2):43–47. doi: 10.1016/0304-3940(84)90348-3. [DOI] [PubMed] [Google Scholar]
- Sawynok J., Pinsky C., LaBella F. S. On the specificity of naloxone as an opiate antagonist. Life Sci. 1979 Nov 5;25(19):1621–1632. doi: 10.1016/0024-3205(79)90403-x. [DOI] [PubMed] [Google Scholar]
- Schultzberg M., Lundberg J. M., Hökfelt T., Terenius L., Brandt J., Elde R. P., Goldstein M. Enkephalin-like immunoreactivity in gland cells and nerve terminals of the adrenal medulla. Neuroscience. 1978;3(12):1169–1186. doi: 10.1016/0306-4522(78)90137-9. [DOI] [PubMed] [Google Scholar]
- Thoenen H., Haefely W., Staehelin H. Potentiation by tetraethylammonium of the response of the cat spleen to postganglionic sympathetic nerve stimulation. J Pharmacol Exp Ther. 1967 Sep;157(3):532–540. [PubMed] [Google Scholar]
- Tsujimoto A., Nishikawa T. Further evidence for nicotinic and muscarinic receptors and their interaction in dog adrenal medulla. Eur J Pharmacol. 1975 Dec;34(2):337–344. doi: 10.1016/0014-2999(75)90260-5. [DOI] [PubMed] [Google Scholar]
- Viveros O. H., Diliberto E. J., Jr, Hazum E., Chang K. J. Enkephalins as possible adrenomedullary hormones: storage, secretion, and regulation of synthesis. Adv Biochem Psychopharmacol. 1980;22:191–204. [PubMed] [Google Scholar]
- Wakade A. R. Studies on secretion of catecholamines evoked by acetylcholine or transmural stimulation of the rat adrenal gland. J Physiol. 1981;313:463–480. doi: 10.1113/jphysiol.1981.sp013676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakade A. R., Wakade T. D. Contribution of nicotinic and muscarinic receptors in the secretion of catecholamines evoked by endogenous and exogenous acetylcholine. Neuroscience. 1983 Nov;10(3):973–978. doi: 10.1016/0306-4522(83)90235-x. [DOI] [PubMed] [Google Scholar]
- Wakade A. R., Wakade T. D. Mechanism of presynaptic actions of adenosine and acetylcholine on noradrenaline release in the guinea-pig heart. Neuroscience. 1982;7(9):2267–2276. doi: 10.1016/0306-4522(82)90137-3. [DOI] [PubMed] [Google Scholar]
- Wilson S. P., Kirshner N. Isolation and characterization of plasma membranes from the adrenal medulla. J Neurochem. 1976 Dec;27(6):1289–1298. doi: 10.1111/j.1471-4159.1976.tb02606.x. [DOI] [PubMed] [Google Scholar]
- Wilson S. P., Kirshner N. The acetylcholine receptor of the adrenal medulla. J Neurochem. 1977 Apr;28(4):687–695. doi: 10.1111/j.1471-4159.1977.tb10615.x. [DOI] [PubMed] [Google Scholar]


