Abstract
Background
The surgical treatment for esophageal achalasia has evolved over the years, with laparoscopic Heller myotomy (LHM) and partial fundoplication becoming widely used worldwide. More recently, an increased interest in the robotic Heller myotomy (RHM) has arisen.
Purpose
Compare short-term and functional outcomes of RHM vs. LHM.
Methods
Systematic review and meta-analysis. PubMed, MEDLINE, Scopus, Web of Science, Cochrane Central Library, and ClinicalTrials.gov were queried. Primary outcome was esophageal perforation (EP). Risk ratio (RR), standardized mean difference (SMD), and 95% confidence intervals (95% CI) were effect size and relative inference measures. PROSPERO Registration Number: CRD42024512644.
Results
Fourteen observational studies (12962 patients) were included. Of those, 2503 (19.3%) underwent RHM. The patient age ranged from 34 to 66 years and 51.7% were males. EP occurred in 259 patients (1.99%). The cumulative incidence of EP was 1.67% for RHM and 2.07% for LHM. Compared to LHM, RHM was associated with a reduced risk of EP (RR: 0.31; 95% CI 0.16–0.59). No differences were found in term of dysphagia requiring reoperation or additional endoscopic procedures (RR: 0.47; 95% CI 0.20–1.09) and postoperative Eckardt score (SMD: -0.42; 95% CI -0.94, 0.11). Blood loss, conversion to open, operative time, and hospital length of stay were comparable.
Conclusions
RHM may be associated with a reduced risk of EP compared to LHM. However, because of selection bias, diverse surgeon expertise, variations in surgical technique, and prior endoscopic procedures these findings should not be viewed as conclusive while the superiority of one approach over the other remains to be established.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00423-025-03648-1.
Keywords: Esophageal achalasia, Dysphagia, Myotomy, Laparoscopy, Robotic, Esophageal perforation
Introduction
Achalasia is a rare primary disorder of esophageal motility characterized by the inability of the lower esophageal sphincter (LES) to relax and absence of peristalsis in the esophageal body [1–3]. As the condition progresses, patients commonly experience dysphagia along with chest pain, food regurgitation, airway aspiration, and malnutrition [4, 5]. Three distinct manometric subtypes of achalasia have been described by the Chicago International Study Group, with different prognosis in terms of symptom resolution after surgical or endoscopic therapy [6, 7].
The treatment approaches for achalasia have evolved over the years, with laparoscopic Heller myotomy (LHM) and partial fundoplication becoming the most widely used surgical technique globally [8–10]. More recently, due to the better 3-D visualization, instrument stability, and superior dexterity provided by the robotic platforms, there has been an increased interest in the robotic Heller myotomy (RHM) [11–16]. It has been hypothesized that RHM may offer better clinical outcomes compared to LHM, particularly in terms of reduced postoperative pain, shorter hospital length of stay (HLOS), and decreased risk of intraoperative mucosal esophageal perforation (EP) [14, 17–23]. While few studies and meta-analyses have been published, conclusive evidence supporting the superiority of one treatment over the other is still unavailable [14, 18, 24, 25].
Hence, this updated systematic review and meta-analysis aims to compare outcomes of RHM vs. LHM for the treatment of esophageal achalasia in term of short-term and functional outcomes.
Materials and methods
This study was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement and Meta-analyses Of Observational Studies in Epidemiology (MOOSE) guidelines [26, 27]. Institutional Review Board approval was not required. Multiple databases, including PubMed, Scopus, MEDLINE, Web of Science, ClinicalTrials.gov, Cochrane Central Library, and Google Scholar, were utilized for the search process. A combination of the following MeSH (Medical Subject Headings) terms was utilized for the literature search: “robotic,” “robot*,” “laparoscopic,” “laparosc*”, “Heller myotomy,” “myotomy”, “achalasia” AND “esophageal achalasia”. The complete search strategy is described in Appendix A. The last date of the search was 1st July 2024. Two authors (RD, AA) reviewed the title of each study and extracted relevant abstracts. The search was further enhanced by examining the references of each article. The study protocol has been registered with the international prospective register of systematic reviews (PROSPERO registration number: CRD42024618855).
Eligibility criteria
The criteria for inclusion were (a) clinical studies reporting a comparison between RHM and LHM in adult patients (> 17 years) diagnosed with esophageal achalasia related symptoms; (b) English written; (c) in cases where multiple studies were based on the same dataset, the study with the longest follow-up period or the largest sample size was selected, and (d) for duplicate studies, only the study with the most complete dataset was included for quantitative analysis. The exclusion criteria were (a) non-English articles; (b) studies with a lack of clear outcome comparison for LHM vs. RHM; (c) studies not reporting the pre-defined primary outcome; (d) case series and case reports.
Data extraction
The following variables were collected: the authors, year of publication, country, study design, the number of patients, age, sex, body mass index (BMI), American Society of Anesthesiologists (ASA) score, type of achalasia according to the Chicago classification, previous endoscopic treatment, myotomy length (cm), technical details repairing, the method for myotomy, type of fundoplication, short-term outcomes, functional outcomes, and need for redo surgery of endoscopic dilation. Three investigators (RD, AA, MM) independently gathered and analyzed the data, which were compared at the conclusion of the review. Discrepancies were resolved through discussion with a senior author (LB).
Outcomes
Primary outcome was EP. When specified, the timing of EP diagnosis was defined as intraoperative or postoperative. Secondary outcomes were operative time (minutes), estimated intraoperative blood loss (ml), hospital length of stay (days), post-operative Eckardt score, conversion to open, postoperative dysphagia requiring reoperation or endoscopic procedures.
Quality assessment
Two authors (RD, AA) performed separate and independent evaluations of the methodological quality of each study. The ROBINS-I tool was used, taking into account confounding bias, selection bias, classification bias, intervention bias, missing data bias, outcome measurement bias, and the bias in study reporting [28]. Each domain was rated as “yes”, “probably yes”, “probably no”, or “no”, leading to overall risk judgments categorized as a low, moderate, serious, or critical bias risk. The quality of the overall evidence from the studies was evaluated using the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) tool (https://www.gradepro.org; accessed on 15th September 2024) [29, 30].
Statistical analysis
The findings of this study were synthesized using frequentist random-effect meta-analysis, aggregating risk ratios (RR). The DerSimonian–Laird estimator was employed to determine the variance of the true effect size (τ2), alongside an inverse-variance method [31, 32]. Heterogeneity across studies was assessed through the I [2] index and Cochran’s Q test [33]. Levels of statistical heterogeneity were categorized as low, moderate, and high for I [2] values of 25, 50, and 75%, respectively, with significance set at p < 0.10. [34, 35] Confidence intervals (CI) at 95% were computed using the Wald-type method for pooled measurements, while the Clopper-Pearson method was applied for individual intervals. The 95% CI for the I [2] index followed Higgins and Thompson’s approach [36]. Prediction intervals for the treatment effect of new studies were calculated based on Borenstein et al. [33]. Sensitivity analysis was conducted by iteratively excluding one study at a time to ensure the robustness of the overall results, considering varying sample sizes across studies. Statistical significance was defined as a two-sided p value of less than 0.05. All analyses and visualizations were performed using R software version 3.2.2 [37]. Consistent with Cochrane guidelines, publication bias assessment was not conducted for outcomes including less than 10 studies per data comparison.
Results
Systematic review
The PRISMA flow chart is reported in Fig. 1. A total of 404 publications were identified. After duplicates were excluded, 220 titles were screened, and abstracts were reviewed. After full text evaluation of 15 articles, 14 studies published between 2005 and 2023 met the inclusion/exclusion criteria and were comprised in the quantitative synthesis. All studies were observational while the specific quality of each study is depicted in Table 1 [15–17, 19–25, 38–41].
Fig. 1.
The preferred reporting items for systematic reviews and meta-analyses (PRISMA) diagram
Table 1.
Demographic, clinical, and operative data for patients undergoing RHM and LHM
| Author, year, country, study design | No. pts | Surgical procedure & No. pts | Age (yrs) | Gender (M) | BMI (kg/m2) |
ASA ≥ 3 | Classification | Previous endoscopic treatment | Fundoplicatio | Tenchique for myotomy | Myotomy lenght (cm) | Risk of Bias* | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type I | Type II | Type III | BTX | PD | BTX + PD | POEM | D | T | N | None | Mono | Bipo | Blunt | Total | Gastric | |||||||||
| Horgan et al., 2005, USA & Argentina, Ret [23] | 121 | LHM | 62 | 48 ± 19 | 29 | nr | nr | nr | 1 | 16 | 0 | 0 | 62 | 0 | ● | 8–9 | 2–3 | Serious | ||||||
| RHM | 59 | 42 ± 19 | 29 | nr | 4 | 17 | 5 | 0 | 59 | 0 | ● | 8–9 | 2–3 | |||||||||||
|
Huffmanm et al. 2007, USA, Pros [20] |
61 | LHM | 37 | nr | 23 | nr | nr | nr | nr | 2 | 32 | 0 | 3 | ● | 5–7 | 0.5-1 | Serious | |||||||
| RHM | 24 | 10 | nr | nr | 16 | 6 | 0 | 2 | ● | 5–7 | 0.5-1 | |||||||||||||
| Sanchez et al. 2012, Venezuela, Pros [38] | 31 | LHM | 18 | 40.7 | nr | nr | nr | nr | 0 | 1 | 0 | 0 | 18 | 0 | 0 | 0 | ● | 8 | 2 | Serious | ||||
| RHM | 13 | 38 | nr | 0 | 0 | 0 | 0 | 13 | 0 | 0 | 0 | ● | 8 | 2 | ||||||||||
| Perry et al. 2014, USA, Ret [39] | 75 | LHM | 19 | 47.8 ± 14 | 11 | 25.7 ± 5 | 3 | nr | nr | 4 | 13 | 0 | 2 | ● | 8 | > 2 | Moderate | |||||||
| RHM | 56 | 47.5 ± 16 | 28 | 26.3 ± 6 | 18 | nr | nr | 1 | 55 | 0 | 0 | ● | 8–10 | > 2 | ||||||||||
| Kim et al. 2019, USA, Ret [40] | 72 | LHM | 35 | 59.5 | 20 | nr | nr | nr | 11 | 7 | 0 | 0 | 27 | 0 | 0 | 8 | nr | 8.2 | 2.7 | Serious | ||||
| RHM | 37 | 61.7 | 16 | nr | 17 | 8 | 0 | 0 | 29 | 3 | 0 | 5 | 8.8 | 2.9 | ||||||||||
| Alì et al. 2020, USA, Ret [17] | 84 | LHM | 40 | 55 | 22 | nr | nr | nr | nr | 40 | 0 | 0 | nr | nr | nr | Moderate | ||||||||
| RHM | 44 | 57.5 | 28 | nr | nr | 44 | 0 | 0 | nr | nr | ||||||||||||||
| Arcerito et al. 2022, USA, Ret [15] | 111 | LHM | 96 | nr | nr | nr | nr | nr | 0 | 0 | 19 | 0 | 96 | 0 | 0 | 0 | ● | 7–8 | 2-2.5 | Moderate | ||||
| RHM | 15 | nr | 0 | 0 | 2 | 0 | 15 | 0 | 0 | 0 | ● | 7–8 | 2-2.5 | |||||||||||
| Chacko et al. 2022, USA, Ret [41] | 11,562 | LHM | 9703 | nr | nr | nr | nr | nr | nr | nr | nr | nr | Serious | |||||||||||
| RHM | 1859 | |||||||||||||||||||||||
| Engwall-Gill et al. 2022, USA, Ret [19] | 59 | LHM | 14 | 43 | 9 | nr | nr | nr | 4 | 0 | 0 | 0 | 7 | 0 | 0 | 7 | ● | 8 | 2 | Moderate | ||||
| RHM | 45 | 54 | 17 | nr | 4 | 0 | 0 | 0 | 11 | 0 | 0 | 34 | ● | 8 | 2 | |||||||||
| Gass et al. 2022, Switzerland, Pros [24] | 43 | LHM | 32 | 54.9 | 23 | 23.7 | 4 | 10 | 15 | 4 | 0 | 6 | 0 | 0 | 32 | 0 | 0 | 0 | ● | > 10 | nr | Moderate | ||
| RHM | 11 | 60.5 | 5 | 23.1 | 5 | 2 | 9 | 0 | 0 | 1 | 0 | 0 | 11 | 0 | 0 | 0 | ● | > 10 | nr | |||||
| Raja et al. 2022, USA, Ret PM [22] | 447 | LHM | 277 | 49 ± 16 | 141 | 26 ± 5 | 159 | 59 | 170 | 12 | 36 | 26 | 0 | 0 | 277 | 0 | 0 | 0 | ● | > 7 | > 3 | Moderate | ||
| RHM | 170 | 50 ± 16 | 92 | 26 ± 5 | 134 | 22 | 116 | 3 | 13 | 2 | 0 | 0 | 170 | 0 | 0 | 0 | ● | > 7 | > 3 | |||||
| Ilie et al. 2023, Romania, Ret [25] | 152 | LHM | 69 | 51.2 | 38 | nr | nr | nr | 0 | 0 | 0 | 0 | 36 | 2 | 0 | 31 | nr | nr | nr | Moderate | ||||
| RHM | 83 | 47.1 | 40 | nr | 0 | 0 | 0 | 0 | 64 | 2 | 3 | 14 | nr | nr | ||||||||||
| Jiang et al. 2023, China, Ret [16] | 66 | LHM | 26 | 44.8 ± 11.4 | 14 | 20.8 ± 3 | nr | 7 | 14 | 2 | 0 | 7 | 0 | 0 | 26 | 0 | 0 | 0 | ● | 5–6 | 2 | Moderate | ||
| RHM | 40 | 43.1 ± 18.9 | 18 | 22.1 ± 3 | 10 | 22 | 3 | 0 | 7 | 0 | 2 | 40 | 0 | 0 | 0 | ● | 5–6 | 2 | ||||||
| Rabe et al. 2023, Germany, Ret [21] | 78 | LHM | 31 | 43.5 | 13 | 24 | nr | 16 | 13 | 2 | 3 | 19 | 0 | 6 | 31 | 0 | 0 | 0 | ● | 7–9 | 2–3 | Moderate | ||
| RHM | 47 | 53 | 25 | 24 | 16 | 30 | 1 | 2 | 15 | 0 | 0 | 47 | 0 | 0 | 0 | ● | 7–9 | 2–3 | ||||||
Ret retrospective; pros prospective; PM propensity score matching; pts patients; yrs years; M male sex; BMI body mass index; ASA American Society of Anesthesiology classification; BTX botulin toxin injection; PD pneumatic dilation; POEM peroral endoscopic myotomy; D Dor; T toupet; N Nissen; Mono monopolar energy device; Bipo bipolar energy device; Blunt blunt dissection with no utilization of electrocautery device. Data are reported as numbers, mean ± standard deviation, and median (range). Nr not reported. * the quality of included studies was assessed with the ROBINS-I tool for observational studies
Overall, 12,962 patients were included (Table 1). Of those, 2503 (19.3%) patients underwent RHM. The patient age ranged from 34 to 66 years, 51.7% were males, and the preoperative BMI ranged from 20.8 to 31 kg/m2. The specific achalasia subtype according to the Chicago Classification system was detailed in four studies: type I (25.5%), type II (69.7%), and type III (4.8%) [16, 21, 22, 24]. Only two studies detailed and reported and included data for patients diagnosed with stage III or stage IV disease according to the radiological classification [15, 22]. Ten studies detailed previous endoscopic intervention in 22.1% of patients that underwent botulin toxin injection (36.4%) [19, 21–23, 40], pneumatic dilation (50.6%) [16, 21–24, 38, 40], both (9.9%) [15, 23], and peroral endoscopic myotomy (POEM) (3.1%)[16, 21]. Overall, 62.1% of previous endoscopic procedures were performed in patients undergoing LHM. In total, 11 studies reported on the length of esophageal and gastric myotomy (Table 1) [15, 16, 19–24, 38–40]. Among these, 3 studies detailed the use of intraoperative endoscopy to assess the myotomy length, while only one study mentioned the use of intraoperative manometry to assess the resolution of the LES high-pressure zone [16, 20, 24]. None of the studies included in the analysis discussed the use of EndoFLIP technology. Additionally, three studies addressed the integrity of the mucosa after myotomy through intraoperative endoscopy with hydropneumatic bubbling test (2 studies) or dye blue methylene test (1 study) [15].
Overall, 10 studies described the surgical technique for esophageal myotomy detailing the utilization, for both LHM and RHM, of monopolar energy devices (6 studies) [15, 16, 19, 23, 24, 39], bipolar energy device (1 study) [22], and blunt dissection (2 studies) [21, 38]. Huffmann et al. [20] described blunt dissection in LHM and monopolar dissection for RHM patients. None of the studies described the power of energy device (wattage) or utilization of ultrasound or radiofrequency devices for LHM. EP was diagnosed in 259 patients; however, none of the studies provided specific data on defect size. Eleven studies reported the timing of diagnosis for 58 EP, with 96.5% being diagnosed intraoperatively [15, 17, 19–24, 38–40]. In cases of intraoperative diagnosis, the EP was closed using interrupted absorbable sutures and reinforced with the anterior fundoplication. Fundoplication after myotomy was detailed in 13 studies; [15–17, 19–25, 38–40] Dor fundoplication was performed in 81.4% of patients, followed by Toupet fundoplication (9.4%), no fundoplication (8.8%), and Nissen fundoplication (0.4%). The type of robotic platform was specified in eight studies [15, 16, 20–24, 38] reporting the experience with the DaVinci S, DaVinci Si, and DaVinci Xi technologies. None of the included studies detailed the individual operating surgeon proficiency for both the laparoscopic and robotic approach.
Postoperative follow-up varied from 1 to 120 months, showing a trend of longer follow-up durations in the LHM group. Postoperative patients’ quality of life was detailed heterogeneously in seven studies [16, 20, 23, 24, 38, 39] with questionnaires (SF-36, GERD-HRQL, GIQLI) and institutional satisfaction scales (Supplementary Table 1).
Meta-analysis- esophageal perforation (EP)
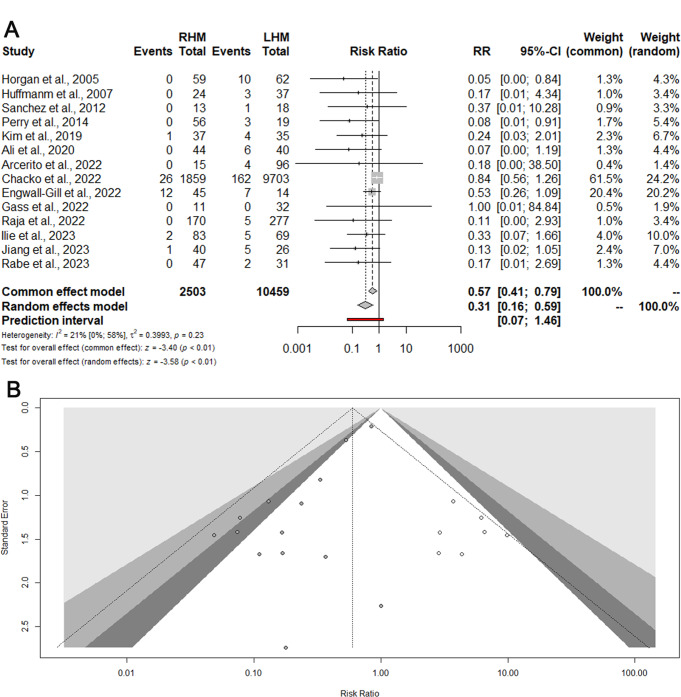
EP was reported in 14 studies (12962 patients) [15–17, 19–25, 38–41]. The cumulative incidence of EP was 1.67% for RHM and 2.07% for LHM. Compared to LHM, RHM was associated to a significantly reduced EP risk (RR: 0.31; 95% CI 0.16–0.59) (Fig. 2A). The prediction lower and upper limits were 0.07 and 1.46, respectively. The heterogeneity was low (I2 = 21%) and τ2 = 0.39. The Funnel plot (Fig. 2B) and the Egger test did not show evidence of publication bias. The sensitivity analysis showed that omitting the study by Chacko et al. [41] the heterogeneity decreased to 0.0% with a preserved statistically significant risk reduction for RHM (RR: 0.25; 95% CI 0.13–0.48).
Fig. 2.
Esophageal perforation. Forrest (A) and Funnel (B) plot. RR: Risk ratio; 95% CI: Confidence Interval
Secondary outcomes
Operative time, intraoperative blood loss, HLOS, and conversion rates to open were comparable between RHM vs. LHM (Table 2).
Table 2.
Summary of the analysis of the categorical and continuous outcomes comparing RHM vs. LHM
| Outcomes | No. Studies | No. pts | Heterogeneity | ||
|---|---|---|---|---|---|
| I2 (%) | 95% CI | ||||
| Dysphagia requiring reintervention or endoscopic procedures [15, 16, 22, 23, 25, 39, 40] | 7 | 1044 | 0.47 (0.20–1.09) ♦ | 54 | 0–80 |
| Conversion to open [15–17, 20, 21, 23–25, 38, 40] | 11 | 894 | 0.36 (0.13–1.05) ♦ | 0.0 | 0–60 |
| Post operative Eckardt score [16, 21, 25, 40] | 4 | 368 | -0.42 (-0.94, 0.11) * | 83 | 57–93 |
| Estimated intraoperative blood loss (ml) [16, 20, 23, 39, 40] | 5 | 395 | -0.46 (-0.98, 0.08) * | 80 | 54–92 |
| Operative time (min) [15–17, 20–25, 38–40] | 12 | 1341 | -0.34 (-1.90, 1.21) * | 98 | 97–99 |
| HLOS (day) [17, 19, 20–25, 38–41] | 12 | 12,785 | -0.25 (-0.43, 0.06) * | 63 | 31–80 |
♦ Risk ratio, * Standardized mean difference, (95% Confidence Interval), I [2] Heterogeneity
Functional outcomes
Dysphagia requiring reoperation or additional endoscopic procedures was detailed in 7 studies (1044 patients) [15, 16, 22, 23, 25, 39, 40], with no significant differences for RHM vs. LHM (RR: 0.47; 95% CI 0.20–1.09; I2 = 54%) (Fig. 3). The sensitivity analysis showed that omitting the study by Raja et al. [22] the heterogeneity decreases to 0.0% with a preserved nonsignificant result (RR: 0.69; 95% CI 0.35–1.34). The postoperative Eckardt score was reported in four studies (368 patients) [16, 21, 25, 40]. No significant differences were found for RHM vs. LHM (SMD: -0.42; 95% CI -0.94, 0.11; I2 = 83%) (Fig. 4). The sensitivity analysis showed the robustness of findings in term of point estimation, 95% CI, and heterogeneity. The Funnel plot (Fig. 2B) and the Egger test did not show evidence of publication bias for both functional outcomes. Using the GRADE tool, we rated the quality of evidence for the primary and secondary outcomes supporting each outcome as moderate mostly due to the limitations in study design (Supplementary Table 2).
Fig. 3.
Dysphagia requiring reoperation or additional endoscopic procedures. Forrest plot. RR: Risk ratio; 95% CI: Confidence Interval
Fig. 4.
Postoperative Eckardt score. Forrest plot. SMD: standardized mean difference; 95% CI: Confidence Interval
Discussion
This meta-analysis shows that the overall risk of EP related to surgical myotomy for the treatment of achalasia is about 2%. RHM may be associated with a reduced risk of EP compared to LHM however, caution is mandatory because the hidden impact of several confounders such as outcome reporting (detection bias), surgeon expertise, impact of prior endoscopic procedures at the LES, and variations of surgical techniques (i.e., type of energy devices, wattage, type of dissection, type of fundoplication etc.). Both LHM and RHM have comparable functional effect on postoperative dysphagia requiring reoperation or additional endoscopic procedures and decrease of postoperative Eckard score.
Minimally invasive Heller myotomy is the preferred surgical option for treating symptomatic achalasia [4, 8, 9, 42–45]. Numerous studies indicate that LHM is both safe and effective in the medium to long term follow-up, achieving symptom relief in 80–100% of patients after five years and 75% after 15 years [8, 9, 42, 46]. The risk of complications with LHM is approximately 6.3%, while the mortality risk is just 0.1% [8, 47–51]. Since its introduction in 2001, RHM has gained widespread acceptance worldwide due to its improved three-dimensional visualization, greater range of motion of robotic arms, and enhanced precision during the procedure, potentially lowering the risk of iatrogenic full-thickness perforations [11, 20, 23]. EP during myotomy can occur in 1–3% of patients and may arise from direct injury to the esophageal mucosa during blunt dissection or from thermal damage due to electrocautery or energy devices. Thermal injury may lead to immediate or late perforation caused by fall of eschar at the myotomy site. When promptly identified intraoperatively, the defect should be sutured with interrupted stitches and patched with an anterior fundoplication [19, 49]. Esophageal myotomy remains challenging because of the difficult identification of the correct mucosal plane particularly in patients with esophageal dilation and mucosal redundancy and the possible presence of adhesions between the mucosal and the inner circular muscular layer in patients with previous endoscopic treatments [19, 47, 52]. For these reasons, preoperative esophageal lavage with removal of food debris, surgeon expertise, and cautious surgical dissection can minimize the risk of EP [50]. In our study, the pooled cumulative risk of EP for RHM and LHM was 1.67% and 2.07%, respectively, consistent with literature data. The quantitative analysis suggested a significantly reduced EP risk for RHM vs. LHM (RR: 0.31; 95% CI 0.16–0.59). This result was also supported by the sensitivity 0% heterogeneity analysis (RR: 0.25; 95% CI 0.13–0.48). A previous meta-analysis by Milone et al. including 6 studies (2625 patients), showed a significantly reduced odds for EP in RHM (OR: 0.13; p < 0.001) [14]. Similarly, Ataya et al. reported a significantly reduced odds for EP in patients undergoing RHM compared to LHM (OR: 0.36; p = 0.02) [18]. Also, Raja et al. in their retrospective single center analysis of 447 patients reported a lower EP rate for RHM vs. LHM (0% vs. 1.8%) [22]. These results highlight the benefits of robotic surgery that offer a wide range of motion, motion scaling with tremor filtration, high-definition magnification, binocular three-dimensional visualization of the surgical area, and enhanced ergonomic comfort for the surgeon. These features of the robot-assisted system likely contribute to improved clinical outcomes by facilitating safer dissections, enhancing visualization, and increasing instrument maneuverability. In the setting of the Heller myotomy, the advanced 3D imaging and the stability of the surgical field allows a more precise identification of both longitudinal and circular esophageal muscle fibers. Furthermore, the magnification helps the surgeon to better identify the submucosal plane and to safeguard against perforation [11–14]. Despite the low or 0% heterogeneity, several factors should be considered when interpreting our results. First, the proficiency of the individual operating surgeons was not reported in any of the included studies. Second, the techniques used for esophageal myotomy were inconsistently documented, with none of the studies mentioning the use of ultrasound or radiofrequency instruments and details such as the wattage of the monopolar devices. No specific details were reported about the technique of blunt dissection in the body of the esophagus and at the esophagogastric junction. Third, although RHM is a newer approach compared to LHM, it is possible that confounding bias exists, as RHM is often performed by skilled minimally invasive surgeons who have already mastered the LHM technique. Fourth, data on the degree of esophageal dilation was limited and heterogeneous across the included studies, with only two of these providing details on stage III-IV patients, who present greater challenges due to mucosal redundancy. Fifth, a higher proportion of patients undergoing LHM had a history of previous endoscopic treatment, which may be associated with submucosal-mucosal adhesions, complicating surgical dissection and increasing the risk of mucosal violation [52]. Finally, 96.5% EP were identified during surgery, promptly sutured and patched, thus limiting the postoperative clinical significance of such events with no impact on postoperative HLOS. Future studies should likely focus more closely on delayed perforations, which have a greater impact on clinical outcomes, overall morbidity, and longer length of stay.
Persistent or recurrent dysphagia after Heller myotomy is observed in up to 10% of patients and may necessitate revisional procedures such as endoscopic dilation, POEM, or redo myotomy [52–57]. Dysphagia can result from incomplete myotomy, tissue fibrosis and scarring, peptic stricture, diverticulum at the myotomy site, twisted fundoplication, hiatal hernia, progressive esophageal dilation leading to a sigmoid shape esophagus, or long-term cancer development [53–55, 58–60]. Importantly, our study found no significant differences in functional outcomes regarding dysphagia necessitating additional endoscopic interventions or reoperations and the postoperative Eckardt score. This aligns with findings from Milone et al. and Ataya et al., which reported similar functional outcomes for LHM and RHM [14, 18]. This may be attributed to the fact that functional outcomes following myotomy depend more on factors such as the length of myotomy, preoperative manometric classification, degree of esophageal dilation, the surgeon’s experience, and follow-up, rather than the surgical approach itself [9, 14, 18, 24, 25, 61]. In our analysis the follow-up duration ranged from 1 to 120 months. Notably, the follow-up was generally longer in the LHM group compared to the RHM group, which may have introduced a temporal bias in the recorded quality of life and satisfaction outcomes. Finally, no notable differences were observed between treatment methods concerning operating time, HLOS, intraoperative blood loss, or conversion to open surgery. In terms of postoperative quality of life, data were inconsistently reported across different follow-up time points, making robust quantitative analysis difficult. However, Huffmann et al. [20]. noted a significantly higher quality of life postoperatively for RHM compared to LHM, while other studies reported no differences [20]. Future research focusing on quality of life and patient-reported outcomes is needed to further explore this important aspect of the procedure.
We recognize that our paper has several limitations, primarily related to the retrospective designs of the included studies, which may introduce preoperative selection and reporting biases. In the quantitative analysis, the study by Chacko et al. [41] included a substantial number of patients, which may have impacted the overall effect globally. Nevertheless, the use of random effects analysis in the quantitative assessment moderated the influence of this large study. Additionally, only a few studies provided information on the operating surgeon’s proficiency, which could significantly affect myotomy-related outcomes. Furthermore, there was a lack of detailed technical information about the surgical methodology for myotomy (such as wattage and whether a bottom-up or up-down technique was used), with none of the studies reporting on myotomy performed with an ultrasonic scalpel. The functional outcomes and quality of life measures were reported inconsistently across different follow-up time points. Importantly, even when studies reported on patients’ symptoms, it remains challenging to assess therapeutic success based solely on postoperative symptoms, due to potential exaggeration of symptom improvement and altered swallowing perceptions. The absence of objective postoperative assessments in most studies (i.e., esophageal emptying) might contribute to the potential discordance between esophageal emptying and symptoms. Lastly, related costs were heterogeneously reported in few studies thus impeding a robust quantitative assessment.
Conclusions
Our analysis suggests that RHM may be linked to a lower risk of EP compared to LHM. However, because of preoperative selection bias, variations in surgeon expertise, prior endoscopic procedures, and the techniques employed for the myotomy, the current findings should not be viewed as conclusive, and the superiority of one approach over the other remains to be established.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
None.
Author contributions
All authors played a role in the study’s planning and conceptual framework. The task of gathering and analyzing data was carried out by AA, RD and MM. AA, GB, and FC managed data and image processing. AA and RD prepared a draft manuscript, which DB, AB, and LB critically reviewed and revised to improve its intellectual content. Every writer gave feedback on previous drafts and gave their approval for the final manuscript.
Funding
Open access funding provided by Università degli Studi di Milano within the CRUI-CARE Agreement.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethical approval
Not applicable.
Consent for publication
Not applicable.
Disclosures
Drs Alberto Aiolfi, Riccardo Damiani, Michele Manara, Francesco Cammarata, Gianluca Bonitta, Antonio Biondi, Davide Bona, and Luigi Bonavina have no conflicts of interest or financial ties to disclose.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pandolfino JE, Gawron AJ, Achalasia (2015) JAMA 313(18):1841. 10.1001/jama.2015.2996 [DOI] [PubMed] [Google Scholar]
- 2.Schlottmann F, Patti MG (2018) Esophageal achalasia: current diagnosis and treatment. Expert Rev Gastroenterol Hepatol 12(7):711–721. 10.1080/17474124.2018.1481748 [DOI] [PubMed] [Google Scholar]
- 3.Khashab MA, Vela MF, Thosani N et al (2020) ASGE guideline on the management of achalasia. Gastrointest Endosc 91(2):213–227e6. 10.1016/j.gie.2019.04.231 [DOI] [PubMed] [Google Scholar]
- 4.Nguyen NT, Clarke JO, Lipham JC et al The AFS textbook of foregut disease. AFS Textb Foregut Dis April 2023:1–625. 10.1007/978-3-031-19671-3/COVER
- 5.Stavropoulos SN, Friedel D, Modayil R, Parkman HP (2016) Diagnosis and management of esophageal achalasia. BMJ September i2785. 10.1136/bmj.i2785 [DOI] [PubMed]
- 6.Yadlapati R, Kahrilas PJ, Fox MR et al (2021) Esophageal motility disorders on high-resolution manometry: Chicago classification version 4.0 ©. Neurogastroenterol Motil 33(1). 10.1111/nmo.14058 [DOI] [PMC free article] [PubMed]
- 7.Salvador R, Costantini M, Zaninotto G et al (2010) The Preoperative Manometric Pattern predicts the outcome of Surgical Treatment for Esophageal Achalasia. J Gastrointest Surg 14(11):1635–1645. 10.1007/s11605-010-1318-4 [DOI] [PubMed] [Google Scholar]
- 8.Bonavina L, Nosadini A, Bardini R, Baessato M, Peracchia A (1992) Primary treatment of esophageal achalasia. Long-term results of myotomy and Dor fundoplication. Arch Surg 127(2):222–227. 10.1001/ARCHSURG.1992.01420020112016 [DOI] [PubMed] [Google Scholar]
- 9.Bonavina L (2006) Minimally invasive surgery for esophageal achalasia. World J Gastroenterol 12(37):5921. 10.3748/wjg.v12.i37.5921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eckardt AJ, Eckardt VF (2009) Current clinical approach to achalasia. World J Gastroenterol 15(32):3969. 10.3748/wjg.15.3969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galvani C, Gorodner MV, Moser F, Baptista M, Donahue P, Horgan S (2006) Laparoscopic Heller myotomy for achalasia facilitated by robotic assistance. Surg Endosc 20(7):1105–1112. 10.1007/s00464-005-0272-9 [DOI] [PubMed] [Google Scholar]
- 12.Melvin WS, Needleman BJ, Krause KR et al (2002) Computer-enhanced robotic telesurgery. Surg Endosc 16(12):1790–1792. 10.1007/s00464-001-8192-9 [DOI] [PubMed] [Google Scholar]
- 13.Maeso S, Reza M, Mayol JA et al (2010) Efficacy of the Da Vinci Surgical System in abdominal surgery compared with that of Laparoscopy. Ann Surg 252(2):254–262. 10.1097/SLA.0b013e3181e6239e [DOI] [PubMed] [Google Scholar]
- 14.Milone M, Manigrasso M, Vertaldi S et al (2019) Robotic versus laparoscopic approach to treat symptomatic achalasia: systematic review with meta-analysis. Dis Esophagus 32(10):1–8. 10.1093/dote/doz062 [DOI] [PubMed] [Google Scholar]
- 15.Arcerito M, Jamal MM, Perez MG, Kaur H, Sundahl A, Moon JT (2022) Esophageal achalasia: from laparoscopic to Robotic Heller Myotomy and Dor Fundoplication. JSLS J Soc Laparosc Robot Surg 26(3). e2022.00027 [DOI] [PMC free article] [PubMed]
- 16.Jiang X, Ye C, Jiang L et al (2023) Single-center experience of transitioning from video-assisted laparoscopic to robotic Heller myotomy with Dor fundoplication for esophageal motility disorders. BMC Surg 23(1):341. 10.1186/s12893-023-02202-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ali AB, Khan NA, Nguyen DT et al (2020) Robotic and per-oral endoscopic myotomy have fewer technical complications compared to laparoscopic Heller myotomy. Surg Endosc 34(7):3191–3196. 10.1007/s00464-019-07093-2 [DOI] [PubMed] [Google Scholar]
- 18.Ataya K, Bsat A, Aljaafreh A, Bourji H, Al Ayoubi AR, Hassan N (2023) Robot-assisted Heller Myotomy Versus Laparoscopic Heller Myotomy: a systematic review and Meta-analysis. Cureus November. 10.7759/cureus.48495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Engwall-Gill AJ, Soleimani T, Engwall SS (2022) Heller myotomy perforation: robotic visualization decreases perforation rate and revisional surgery is a perforation risk. J Robot Surg 16(4):867–873. 10.1007/s11701-021-01307-3 [DOI] [PubMed] [Google Scholar]
- 20.Huffmanm LC, Pandalai PK, Boulton BJ et al (2007) Robotic Heller myotomy: a safe operation with higher postoperative quality-of-life indices. Surgery 142(4):613–620. 10.1016/j.surg.2007.08.003 [DOI] [PubMed] [Google Scholar]
- 21.Rabe SM, Burmeister E, Niebisch S, Gockel I (2023) Clinical and functional outcome following robotic Heller-myotomy with partial fundoplication in patients with achalasia. J Robot Surg 17(4):1689–1696. 10.1007/s11701-023-01557-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raja S, Adhikari S, Blackstone EH et al (2022) A comparative study of robotic and laparoscopic approaches to Heller myotomy. J Thorac Cardiovasc Surg 164(6):1639–1649e7. 10.1016/j.jtcvs.2022.04.046 [DOI] [PubMed] [Google Scholar]
- 23.Horgan S, Galvani C, Gorodner MV et al (2005) Robotic-assisted Heller Myotomy Versus Laparoscopic Heller Myotomy for the treatment of esophageal achalasia: Multicenter Study. J Gastrointest Surg 9(8):1020–1030. 10.1016/j.gassur.2005.06.026 [DOI] [PubMed] [Google Scholar]
- 24.Gass J-M, Cron L, Mongelli F et al (2022) From laparoscopic to robotic-assisted Heller myotomy for achalasia in a single high-volume visceral surgery center: postoperative outcomes and quality of life. BMC Surg 22(1):391. 10.1186/s12893-022-01818-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ilie V-C, Manciu S, Lacatus M et al (2023) Achalasia Treatment. Robotic Approach or Laparoscopy? Chirurgia (Bucur) 118(3):272. 10.21614/chirurgia.2023.v.118.i.3.p.272 [DOI] [PubMed] [Google Scholar]
- 26.Page MJ, McKenzie JE, Bossuyt PM et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed]
- 27.Brooke BS, Schwartz TA, Pawlik TM (2021) MOOSE Reporting guidelines for Meta-analyses of Observational studies. JAMA Surg 156(8):787–788. 10.1001/JAMASURG.2021.0522 [DOI] [PubMed] [Google Scholar]
- 28.Sterne JA, Hernán MA, Reeves BC et al (2016) ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 355. 10.1136/BMJ.I4919 [DOI] [PMC free article] [PubMed]
- 29.Schünemann H, Brożek J, Guyatt G, Oxman A (eds) (2013) GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations. Updated October 2013. (The GRADE Working Group, ed.).; Available from guidelinedevelopment.org/handbook
- 30.McMaster University and Evidence Prime GRADEpro GDT: GRADEpro Guideline Development Tool [Software]. Available from gradepro.org. Published 2024
- 31.DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7(3):177–188. 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 32.Aiolfi A, Lombardo F, Matsushima K et al (2021) Systematic review and updated network meta-analysis of randomized controlled trials comparing open, laparoscopic-assisted, and robotic distal gastrectomy for early and locally advanced gastric cancer. Surgery 170(3):942–951. 10.1016/j.surg.2021.04.014 [DOI] [PubMed] [Google Scholar]
- 33.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR (2010) A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods 1(2):97–111. 10.1002/jrsm.12 [DOI] [PubMed] [Google Scholar]
- 34.Higgins JPT, Thompson SG, Spiegelhalter DJ (2009) A re-evaluation of Random-effects Meta-Analysis. J R Stat Soc Ser Stat Soc 172(1):137–159. 10.1111/j.1467-985X.2008.00552.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rausa E, Zappa MA, Kelly ME et al (2019) A standardized use of intraoperative anastomotic testing in colorectal surgery in the new millennium: is technology taking over? A systematic review and network meta-analysis. Tech Coloproctol 23(7):625–631. 10.1007/s10151-019-02034-6 [DOI] [PubMed] [Google Scholar]
- 36.Higgins JPT (2003) Measuring inconsistency in meta-analyses. BMJ 327(7414):557–560. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.R Development Core Team (2015) R: A Language and Environment for Statistical Computing. (R Foundation for Statistical Computing, ed.). Vienna
- 38.Sánchez A, Rodríguez O, Nakhal E et al (2012) Robotic-assisted Heller myotomy versus laparoscopic Heller myotomy for the treatment of esophageal achalasia: a case–control study. J Robot Surg 6(3):213–216. 10.1007/s11701-011-0294-3 [DOI] [PubMed] [Google Scholar]
- 39.Perry KA, Kanji A, Drosdeck JM et al (2014) Efficacy and durability of robotic heller myotomy for achalasia: patient symptoms and satisfaction at long-term follow-up. Surg Endosc 28(11):3162–3167. 10.1007/s00464-014-3576-9 [DOI] [PubMed] [Google Scholar]
- 40.Kim SS, Guillen-Rodriguez J, Little AG (2019) Optimal surgical intervention for achalasia: laparoscopic or robotic approach. J Robot Surg 13(3):397–400. 10.1007/s11701-018-0865-7 [DOI] [PubMed] [Google Scholar]
- 41.Chacko J, Leeds SG, Aladegbami BG, Ogola GO, Ward MA (2022) Overall complications following robotic Heller Myotomy are lower compared with Laparoscopy. Surg Laparosc Endosc Percutan Tech 32(3):319–323. 10.1097/SLE.0000000000001041 [DOI] [PubMed] [Google Scholar]
- 42.Asti E, Sironi A, Lovece A et al (2017) Health-related quality of life after laparoscopic Heller myotomy and Dor fundoplication for achalasia. Surgery 161(4):977–983. 10.1016/j.surg.2016.10.023 [DOI] [PubMed] [Google Scholar]
- 43.Aiolfi A, Asti E, Bonitta G, Bonavina L (2018) Esophagectomy for End-Stage Achalasia: systematic review and Meta‐analysis. World J Surg 42(5):1469–1476. 10.1007/s00268-017-4298-7 [DOI] [PubMed] [Google Scholar]
- 44.Aiolfi A, Asti E, Bonitta G, Siboni S, Bonavina L (2018) Esophageal resection for end-stage Achalasia. Am Surg 84(4):506–511. http://www.ncbi.nlm.nih.gov/pubmed/29712597 [PubMed] [Google Scholar]
- 45.Aiolfi A, Bona D, Riva CG et al (2020) Systematic Review and bayesian network Meta-analysis comparing Laparoscopic Heller Myotomy, Pneumatic Dilatation, and Peroral Endoscopic Myotomy for Esophageal Achalasia. J Laparoendosc Adv Surg Tech 30(2):147–155. 10.1089/lap.2019.0432 [DOI] [PubMed] [Google Scholar]
- 46.Boeckxstaens GE, Annese V, Varannes SB (2011) Pneumatic dilation versus Laparoscopic Heller’s myotomy for idiopathic achalasia. N Engl J Med 364(19):1807–1816. 10.1056/NEJMoa1010502 [DOI] [PubMed] [Google Scholar]
- 47.Lynch KL, Pandolfino JE, Howden CW, Kahrilas PJ (2012) Major Complications of Pneumatic Dilation and Heller Myotomy for Achalasia: single-center experience and systematic review of the literature. Am J Gastroenterol 107(12):1817–1825. 10.1038/ajg.2012.332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ross SW, Oommen B, Wormer BA et al (2015) National outcomes of laparoscopic Heller myotomy: operative complications and risk factors for adverse events. Surg Endosc 29(11):3097–3105. 10.1007/s00464-014-4054-0 [DOI] [PubMed] [Google Scholar]
- 49.Souma Y, Nakajima K, Taniguchi E et al (2017) Mucosal perforation during laparoscopic surgery for achalasia: impact of preoperative pneumatic balloon dilation. Surg Endosc 31(3):1427–1435. 10.1007/s00464-016-5133-1 [DOI] [PubMed] [Google Scholar]
- 50.Boeckxstaens GE, Zaninotto G, Richter JE, Achalasia (2014) Lancet 383(9911):83–93. 10.1016/S0140-6736(13)60651-0 [DOI] [PubMed] [Google Scholar]
- 51.Pohl D, Tutuian R (2007) Achalasia: an overview of diagnosis and treatment. J Gastrointestin Liver Dis 16(3):297–303. http://www.ncbi.nlm.nih.gov/pubmed/17925926 [PubMed] [Google Scholar]
- 52.Bonavina L, Incarbone R, Reitano M, Antoniazzi L, Peracchia A (2000) Does previous endoscopic treatment affect the outcome of laparoscopic Heller myotomy? Ann Chir 125(1):45–49. http://www.ncbi.nlm.nih.gov/pubmed/10921184 [PubMed] [Google Scholar]
- 53.Milito P, Siboni S, Lovece A, Andreatta E, Asti E, Bonavina L (2022) Revisional Therapy for recurrent symptoms after Heller Myotomy for Achalasia. J Gastrointest Surg 26(1):64–69. 10.1007/s11605-021-05098-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weche M, Saad AR, Richter JE, Jacobs JJ, Velanovich V (2020) Revisional procedures for recurrent symptoms after Heller Myotomy and per-oral endoscopic myotomy. J Laparoendosc Adv Surg Tech A 30(2):110–116. 10.1089/LAP.2019.0277 [DOI] [PubMed] [Google Scholar]
- 55.Smith KE, Saad AR, Hanna JP et al (2020) Revisional surgery in patients with recurrent Dysphagia after Heller Myotomy. J Gastrointest Surg 24(5):991–999. 10.1007/S11605-019-04264-3 [DOI] [PubMed] [Google Scholar]
- 56.Saleh CMG, Ponds FAM, Schijven MP, Smout AJPM, Bredenoord AJ (2016) Efficacy of pneumodilation in achalasia after failed Heller myotomy. Neurogastroenterol Motil 28(11):1741–1746. 10.1111/NMO.12875 [DOI] [PubMed] [Google Scholar]
- 57.O’Neill OM, Achalasia (2013) A review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol 19(35):5806. 10.3748/wjg.v19.i35.5806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Capovilla G, Salvador R, Provenzano L et al (2021) Laparoscopic revisional surgery after failed Heller Myotomy for Esophageal Achalasia: long-term outcome at a single Tertiary Center. J Gastrointest Surg 25(9):2208–2217. 10.1007/S11605-021-05041-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tustumi F, Bernardo WM, da Rocha JRM et al (2017) Esophageal achalasia: a risk factor for carcinoma. A systematic review and meta-analysis. Dis Esophagus 30(10):1–8. 10.1093/dote/dox072 [DOI] [PubMed] [Google Scholar]
- 60.Salvador R, Caruso V, Costantini M et al (2015) Shorter myotomy on the gastric site (≤ 2.5 cm) provides adequate relief of dysphagia in achalasia patients. Dis Esophagus 28(5):412–417. 10.1111/dote.12226 [DOI] [PubMed] [Google Scholar]
- 61.Aiolfi A, Foschi D, Zappa MA et al (2021) Laparoscopic Heller Myotomy and Dor Fundoplication for the treatment of esophageal Achalasia after Sleeve Gastrectomy—a video vignette. Obes Surg 31(3):1392–1394. 10.1007/s11695-020-05114-x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.