Abstract
Dysbiosis is a clinical condition marked by altered gut microbiota resulting from external and internal host factors. It is strongly associated with gastrointestinal and extraintestinal alterations, so its symptomatology is broad and nonspecific. To date, gaps remain that limit professionals from making a timely diagnosis and prescribing the appropriate treatment. We aim to synthesize existing literature regarding clinical parameters for the early detection of patients with intestinal dysbiosis and the clinical events in which the use of probiotics as adjuvant therapy is most frequently reported. A scoping review of the literature was conducted in PubMed, Embase, Cochrane, and BVS (Biblioteca Virtual en Salud in Spanish) databases for articles published in the last 5 years. Primary studies and literature reviews related to clinical presentation, dysbiosis screening, and probiotics as adjuvant therapy for adult and pediatric patients were included. Twenty-three articles were retrieved in which the most frequently reported symptoms were abdominal distension, abdominal pain, and diarrhea. Chronic and metabolic diseases where the conditions most strongly associated with dysbiosis. Depending on symptomatology and etiology, dysbiosis is often treated with probiotics. Dysbiosis, often linked to diarrhea, should be considered with other symptoms like abdominal distension and pain, along with predisposing conditions and patient risk factors. Probiotics are commonly used as co-adjuvant treatments for antibiotic-associated diarrhea, irritable bowel syndrome, and childhood allergic diseases. The most commonly used probiotics were Weizmannia coagulans (formerly B. coagulans), Alkalihalobacillus clausii (formerly Bacillus clausii), Lacticaseibacillus rhamnosus, Limosilactobacillus reuteri, and Saccharomyces boulardii.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12602-024-10353-w.
Keywords: Dysbiosis, Gut microbiota, Symptoms, Probiotics, Therapeutic uses
Introduction
Since birth, the human gastrointestinal tract (GIT) is colonized by a diverse community of microorganisms, collectively known as microbiota. This community, which includes protists, bacteria, archaea, fungi, and viruses, reaches its adult composition around the age of 3 [1]. Metabolites generated by this microbiota play an important role in human physiology. The GIT connects to the brain, endocrine glands, and various organs, such as the heart and lungs, via bidirectional axes. These axes serve as routes of transmission of microbial metabolism, which has consequences on health and sometimes even disease [2].
An individual’s microbiota diversity is influenced by several factors, such as host genetics, route of delivery, dietary habits (including human breast milk consumption), age, medication use (e.g., antibiotics or proton pump inhibitors (PPIs)), alcohol or sugar consumption, and elevated levels of stress and anxiety [3].
Alteration of this diversity has been associated with numerous human conditions, a phenomenon known as dysbiosis. In dysbiosis, there is a disruption in the natural interaction of microorganisms, which has implications for different organs through the transmission axes of microbial metabolism [4, 5]. Despite the diverse nature of these conditions, some common features of microbiota alteration, such as reduced diversity and increased facultative anaerobes (such as Enterobacteriaceae), commonly lead to uncontrolled local and systemic inflammation [6]. Furthermore, they are associated with diseases such as asthma, Crohn’s disease (CD), ulcerative colitis, non-alcoholic fatty liver disease, obesity, Alzheimer’s disease, ankylosing spondylarthritis, rheumatoid arthritis, hypertension, and hepatitis [7].
Until the time of this review, the tools available for the identification of intestinal dysbiosis remain scarce. Although this condition has been described as a predisposing factor and associated with different diseases, it has not been studied as a pathological condition [3]. Furthermore, research into probiotics as promising adjuvant treatments to control and improve symptoms of various bowel diseases has sparked increasing interest [8]. The ability of probiotics, defined as living microorganisms that, in sufficient quantities, produce a beneficial effect on the host, to influence patients with dysbiosis remains debatable.
Materials and Methods
A scoping review was performed following the methodological guidelines by Arksey and O’Malley [9]. After the protocol was established, a literature search was carried out, including primary studies and literature reviews that explored the symptomatology of patients with dysbiosis and the clinical events in which probiotics were reported as adjuvant therapy. The population was adult and pediatric patients with confirmed or suspected dysbiosis. Studies conducted in animals or those with a topic that was not strongly related to the objectives of this study were excluded.
The search was conducted between August and September 2023 in the PubMed, Embase, Cochrane, and BVS (Biblioteca Virtual en Salud in Spanish) databases. MeSH terms and Boolean operators were used to ensure a sensitive search, including “Dysbiosis” and “Gut microbiota dysbiosis.” Subsequently, additional strategies with up to 16 terms were built to refine the search, applying filters such as publication date (2018–2023) and language (English and Spanish). The detailed search strategy can be found in Supplementary Materials.
After removing duplicates, retrieved results were screened with the Rayyan® tool. In the following step, the primary screening of studies was performed according to relevance. This screening and the final selection were performed by two epidemiologists, experts in literature review and synthesis of evidence (E1: JRZ; E2: ICC). When there were disagreements between the screening and selection results, consensus was sought for the final decision. The documents recommended by the Joanna Briggs Institute (JBI) were used for the quality evaluation of the analytical studies and systematic reviews [10, 11]. The Scale for the Quality Assessment of Narrative Review Articles (SANRA) was used for narrative reviews [12].
Results
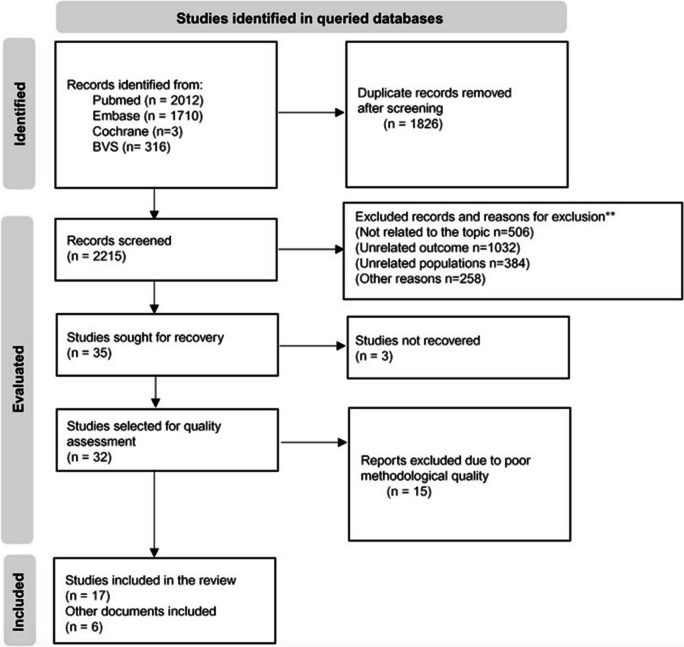
A total of 4041 records were obtained, and 2215 were screened after removing duplicates. After the screening, 17 articles were selected. Furthermore, six studies that were not part of the initial search but were considered clinically relevant were included. Overall, 23 articles were considered for full-text reading and data extraction. The flow of information through the stages of a scoping review is illustrated in the PRISMA-ScR flowchart [13] (Fig. 1).
Fig. 1.
Flow of information through the stages of the scoping review
Our results are presented according to the guiding questions of the scoping review.
Various interpretations of “dysbiosis” were found in the literature review. After a cautious and detailed appraisal, the following definition was considered, since it fits the objectives and context of this scoping review: Dysbiosis is defined as the alteration or imbalance in microbial communities’ structure, composition, and function. This definition requires considering that, due to the wide inter-individual variation of these communities, there is no gold standard for determining the ideal composition of healthy gut microbiota [4, 14, 15].
Question No. 1: What are the Clinical Parameters Reported in the Literature Suggesting the Presence of Intestinal Dysbiosis?
Symptoms Associated with Dysbiosis
Table 1 presents the articles that reported symptomatology associated with dysbiosis. Thirty percent (7/23) reported symptoms suggestive of dysbiosis, especially related to irritable bowel syndrome (IBS); however, the level of certainty in the evidence is low since they are mostly narrative reviews. To identify the degree of confidence in the evidence produced from such narrative reviews, the CERQual instrument was applied to each study [16]. The table describes the authors, type of study, symptoms reported as suggestive of dysbiosis, and level of confidence in the qualitative results for each document.
Table 1.
Findings related to symptomatology of dysbiosis
| Author/s and year | Document type | Reported symptoms | Assessment of the degree of confidence in the results based on CERQual |
|---|---|---|---|
| Netto Candido TL, Bressan J, Alfenas RCG (2018) [17] | Systematic review | Relationship to obesity, abdominal distension, diarrhea | Moderate confidence |
| Pérez N, Dorsen C, Squires A (2020) [14] | Systematic review | Increased sensitivity to pain, fatigue, anxiety, depression, sleep disturbances, memory loss, behavioral changes | High confidence |
| Dale-Fjeldheim H, Arslan G (2020) [18] | Narrative review | Abdominal pain, distension, flatulence | Moderate confidence |
| Hawort J, Boyle N, Vales A, Hobson A (2021) [19] | Retrospective cohort | Test for hydrogen and methane breath, distension, eructation | Moderate confidence |
| Tziatzios G, Gkolfakis P, Papanikolau I et al. (2020) [20] | Narrative review | Abdominal pain, distension, diarrhea | Low confidence |
| Saffouri G, Shields R, Chen J et al. (2019) [21] | Narrative review | Abdominal pain, diarrhea/soft watery stools, abdominal distension, heartburn, lack of appetite | Moderate confidence |
| Rhoads J, Collins J et al. (2018) [22] | Cases and controls nested in a cohort | Infant colic (defined as 3 h of crying and agitation for more than 3 days a week) | Moderate confidence |
According to these findings, the most frequently reported symptoms in the literature related to dysbiosis are abdominal distension, abdominal pain, and diarrhea. These findings have a moderate confidence level.
Additionally, the review found three additional tools for the diagnosis of dysbiosis that involve taking samples and laboratory tests: The fecal microbiota profile [15, 21], the Dysbiosis Index, which studies the proportion of the total number of proteobacterial strains relative to the total number of bacterial strains [4] and the Microbiome Health Index (MHI), which considers bacterial distributions within the phylum Bacillota, Bacteroidota, and Pseudomonadota (formerly called Firmicutes, Bacteroidetes, Proteobacteria) [4].
Another test described in the literature is the breath test [15], which measures the hydrogen and methane in breath. These gases are derived almost exclusively from the anaerobic microbial fermentation of carbohydrates in the large intestine and are used as a non-invasive substitute marker of colonic fermentation activity.
Studies developed in Latin America have proposed using the QRM Questionnaire (Questionário de Rastreamento Metabólico, Metabolic Tracking Questionnaire) as an alternative to identifying and classifying patients with symptomatology suggestive of dysbiosis; however, no validation studies in the diagnosis of dysbiosis [23] were found.
Finally, we explored the occurrence of any event leading to the reported symptoms. Some studies mention that high levels of stress and anxiety [24], poor mealtime regulation, unbalanced dietary habits [4], frequent travel [25], and use of PPIs [18] may predispose to dysbiosis. However, these findings are incidental and do not seem to influence the symptom’s onset.
Associated Comorbidities
Dysbiosis has been linked to the development of inflammatory, autoimmune, metabolic, neoplastic, and behavioral disorders. Although under many of these conditions, a clear temporal relationship between exposures and health effects on human populations has not been established, experimental fecal microbiota transplantation (FMT) models have revealed cause-and-effect relationships between microbiota alteration and certain diseases like obesity, where the obese microbiome has an increased capacity to harvest energy from the diet [24, 26].
Table 2 presents the microbiota findings, the confidence of the evidence, and related references for each pathological condition. Findings regarding comorbidities indicate that disruption of the gut microbiota compromises the immune system, contributing to the generation of chronic and metabolic diseases. The confidence level of these findings is variable, as studies reporting these associations have been developed using different methodologies.
Table 2.
Findings related to dysbiosis-associated comorbidities
| Co-morbidities | Review result | Confidence level — CERQual | References |
|---|---|---|---|
| Irritable bowel syndrome | Studies have confirmed an increase in Bacillota (Firmicutes) (Clostridium, Ruminococcus, and Dorea) and a decrease in Ruminococcus albus, Bacteroides fragilis, B. vulgates, and R. callidus in patients with IBS | High confidence |
Gomaa 2020 [27]; International Life Science Institute-ILSI 2013 [28]; World Gastroenterology Organization (2023) [29] Fjeldhheim 2020 [18] |
| Autoimmune diseases | An increase in the amount of Streptococcus, Prevotella, and Eggerthella is associated with antibody production in autoimmune diseases | Low confidence | Gomaa 2020 [27] |
| Metabolic diseases | In people with obesity and type 2 diabetes mellitus (T2DM,), an abundance of Porphyromonas, Campylobacter, Bacteroides, Staphylococcus, Parabacteroides, Dialister, and Ruminococcus and a low proportion of Lactobacillus, Bifidobacterium, Faecalibacterium, Akkermansia, Methanobrevibacter, and Coprococcus have been identified | High confidence |
World Gastroenterology Organization (2023) [29] Magne 2020 [30] Sekirov 2020 [31] |
| Cancer | Bacteroides fragilis, Enterococcus faecalis, and Helicobacter hepaticus have been identified to contribute to carcinogenesis; Fusobacterium nucleatum is associated with the maintenance of a proinflammatory microenvironment | Moderate confidence |
Gomaa 2020 [27]; Bidell 2022 [4] |
| Neuropsychiatric diseases | Evidence showed that Campylobacter jejuni infection increases anxiety, depression, and cognitive impairment behavior by activating C-Fos proteins, which are markers of neuronal activation | Moderate confidence |
Luca 2020 [5]; Xu 2019 [32]; Hughes 2018 [33] |
| Allergies and asthma in children | An increase in Pseudomonadota (formerly called Proteobacteria) and Ruminococcus gnavus compared to Bifidobacterium and Bacteroides has been documented, causing alterations in intestinal permeability and favoring pro-inflammatory states | Moderate confidence |
Rhoads 2018 [22]; International Life Science Institute-ILSI 2013 [28]; World Gastroenterology Organization (2023) [29] |
Irritable Bowel Syndrome (IBS)
Evidence establishes IBS as a pathological condition resulting from dysbiosis, for which its symptomatology is assumed to be similar. IBS presents with a change in stool characteristics and associated abdominal discomfort. It can manifest as constipation, diarrhea, or also with alternating bowel habits between the two, called IBS with mixed bowel habits. More than a third of patients are often associated with bacterial overgrowth (SIBO, small intestinal bacterial overgrowth) [34].
Autoimmune Diseases
A relationship between autoimmune diseases and the presence of dysbiosis was frequently found. Of these, the most frequently reported were inflammatory bowel disease (IBD, specifically CD), rheumatoid arthritis, and lupus. The pathogenesis of these diseases is multifactorial, including disruption of the intestinal barrier, which triggers an abnormal immune response to the passage of toxic substances into the bloodstream, and alteration in the production of regulatory T cells, which are critical in regulating the immune response [35].
Metabolic Diseases
We found that diet is a critical factor in establishing gut microbiota. Thus, dietary-associated alterations of the microbiota may produce various microbial metabolites that are, in turn, associated with alterations in insulin regulation and related clinical conditions, such as T2DM [36].
Cancer
Numerous studies have studied the role of gut microbiota in the pathogenesis of cancer, and results have shown that, although a specific bacterial genus has not been identified as the primary cause, there is a partial association of members of the Fusobacterium genus with the development of cancer, especially colorectal. Fusobacterium is currently considered to contribute to the activation of the inflammatory cascade that leads to tumor growth.
Neuropsychiatric Diseases
The literature reports extensively how the microbiota-intestinal-brain axis has a profound impact on the behavior of people, which has already been physiologically explained. The most frequently reported conditions are depression, dementia, and Alzheimer’s disease [37].
Allergies and Asthma in Children
The prevalence of allergies and asthma in children was described in 2018 as close to 5% but has experienced an increase in recent years, especially in Western populations [35]. Current evidence has identified relationships between allergies and asthma in the infant population with the composition and function of the microbiome. In infants who develop adverse food reactions and progress to broncho-obstructive episodes in childhood, there is less diversity in the gut microbiota. For example, elevated amounts of Pseudomonadota (formerly called Proteobacteria) and Ruminococcus gnavus have been found in the analysis of fecal samples from allergic children compared to non-allergic children [36]. However, further study of these associations is still needed.
Question No. 2: What are the Most Commonly Reported Clinical Events for the Use of Probiotics as Adjuvant Therapy?
Gastrointestinal events, specifically antibiotic-associated diarrhea (AAD) and IBS, stood out as the most frequently reported clinical occurrences during probiotics’ utilization as adjuvant therapy. There is also good evidence for use in other gastrointestinal conditions and allergic diseases in children. Research involving probiotics in diverse populations with varying conditions has adhered to stricter methodologies, resulting in a higher level of certainty in the evidence, as determined by the CERQual assessment [16]. Table 3 provides a detailed description of the clinical applications, review outcomes, confidence levels, and associated references.
Table 3.
Findings related to clinical events in which the use of probiotics as adjuvant therapy is most frequently reported
| Type of use | Review result | CERQual assessment | References |
|---|---|---|---|
| Antibiotic-associated diarrhea (AAD) | Combined results from clinical trials showed that Lacticaseibacillus rhamnosus, Saccharomyces boulardii, and Bacillus clausii have shown potential for decreased frequency and duration of AAD | High confidence |
Blaabjerg (2108) [38] Seon-Kyun (2019) [39] Kesavelu (2023) [40] WGO (2023) [29] |
| Irritable bowel syndrome | The evidence found that the use of Bacillus coagulans is useful for the treatment of symptoms in patients with IBS. Additionally, beneficial effects were greater when using multiple supplements for more than 8 weeks | High confidence |
Zhang (2022) [41] Dale (2019) [42] Seon-Kyun [39] (2019) WGO (2023) [29] |
| Crohn’s disease | No significant differences have been found for disease remission with the use of probiotics and placebo. Treatment discontinuation has been reported due to intolerance | Low confidence |
Limketkai (2020) [43] Seon-Kyun (2019) [39] WGO (2023) [29] |
| Colorectal cancer | Evidence shows that quantitative and qualitative changes in gut microbiota as well as changes in metabolism have been associated with the prevention of colorectal cancer. The effect depends on the bacterial strain as well as the dose administered | Low confidence |
Eslami (2019) [44] Molska (2019) [45] Seon-Kyun (2019) [39] |
| Acute gastroenteritis, prevention of AAD, colic, Helicobacter pylori infection, asthma, and childhood allergies | In children, the evidence indicates that Lacticaseibacillus rhamnosus, Limosilactobacillus reuteri, and Saccharomyces boulardii are recommended for the management of acute gastroenteritis; the latter is also recommended in the management of Helicobacter pylori infection. Regarding the use of Bacillus clausii in children, there is a controversy because some studies have shown very low efficacy [46] | High confidence |
Kesavelu (2023) [40] Szajewska (2023) [46] WGO (2023) [29] |
Use of Probiotics in AAD
Antibiotic use is strongly associated with diarrhea. Clostridioides (formerly Clostridium) may cause infections in the large intestine, which is one of the most serious side effects of antibiotic use due to the alteration of the intestinal microbiota. Studies have shown that the use of probiotics, including their use as complementary therapy, can help prevent the risk of AAD in outpatients of all ages, with an effectiveness of up to 51% [38].
Irritable Bowel Syndrome
IBS is a chronic, relapsing condition that causes abdominal pain and changes in bowel rhythm. An unbalanced diet and high levels of stress are thought to contribute to the occurrence of IBS. Evidence suggests that gut microbiota may be involved in the pathogenesis of IBS, as differences in its composition between healthy individuals and patients with IBS have been documented; therefore, maintaining a balanced gut microbiota is believed to prevent IBS or decrease its severity [41, 47].
Crohn’s Disease
CD is an IBD that affects the entire gastrointestinal tract and is accompanied by abdominal pain, diarrhea, fever, fatigue, and weight loss. Although the cause of CD remains unknown, it has been hypothesized that microbiological, genetic, and environmental factors play a role in its development. Medications such as steroids to reduce intestinal inflammation and immunosuppressants to reduce immune system activity have been used for the management of CD [48]. Similarly, the use of probiotics as an alternative adjuvant treatment to improve symptomatology has been explored; however, at the time, this study was conducted; evidence of its effectiveness was conflicting [39].
Colorectal Cancer
People with a history of IBS, IBD, or CD have shown an increased incidence of colorectal cancer. Therefore, given their potential immunomodulatory mechanisms, probiotics have been considered to play a role in regulating gut microbiota. Results from early studies indicate that in patients undergoing surgical resection, probiotics have favored the epithelial barrier’s function by improving mucosal structure [44].
Diarrhea, Asthma, and Allergies in Children
The use of probiotics in children has been commonly studied, given the high prevalence of asthma and allergic rhinitis and the high occurrence of AAD as a consequence of infectious diseases, especially in early childhood. These studies have concluded that Lacticaseibacillus rhamnosus, Limosilactobacillus reuteri, and Saccharomyces boulardii have shown positive effects in reducing the frequency and duration of AAD and potentially in preventing food and respiratory allergies, especially in younger children [40, 49]. Regarding the use of probiotics in gastrointestinal conditions, in 2023, the European Society for Pediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) formulated a number of updated recommendations for the use of probiotics for the prevention of various gastrointestinal disorders in the pediatric population [46], including acute gastroenteritis, colic, and Helicobacter pylori infection [50]; however, the level of evidence and degree of certainty in the recommendations is low.
Discussion
The findings of this review suggest that understanding dysbiosis as a common, multi-causal, and impactful phenomenon in the well-being of patients should evolve into more concrete elements that provide practical tools for the health professional, to identify and manage patients with this condition. Until this study was conducted, no documents with sufficient evidence that would allow a more accurate classification and diagnosis of dysbiosis based on a clinical assessment were found.
Although a causal relationship between dysbiosis and different diseases has not been established with certainty, mechanisms connecting the GIT and different body systems have been described, including the gut-brain and gut-lung axes, amongst others, where individuals with gut microbiota changes present with allergic, metabolic, and other diseases described above. These relationships lead to changes in health and disease conditions, which has described possible associations between dysbiosis and multisystemic diseases [4, 27–29]. In this regard, it is relevant for health professionals to identify elements that guide clinical suspicion for the diagnosis and the timely management of related symptoms.
The documents analyzed in this review describe a common symptomatology, including abdominal distension, abdominal pain, and diarrhea [14, 17, 18, 21]. These symptoms are nonspecific and may confound the clinical diagnostic path of dysbiosis. Additionally, available studies are mostly narrative revisions; therefore, their findings have a moderate confidence level. This has implications in clinical practice for informed decision-making by healthcare professionals and provides an opportunity for consensus development to help standardize some symptoms suggestive of dysbiosis and facilitate the detection and incorporation of patients into adjuvant regimens, thereby reducing the disease’s impact on well-being.
When considering probiotics as adjunctive therapy, our investigation referenced clinical practice guidelines and literature reviews [29, 46]. Among the frequently utilized probiotics mentioned were Weizmannia coagulans (Formerly Bacillus coagulans), Alkalihalobacillus clausii (Formerly Bacillus clausii), Lacticaseibacillus rhamnosus, Limosilactobacillus reuteri, and Saccharomyces boulardii; however, the quality of evidence in stand-alone studies is low and, therefore, despite consensus and recommendations published by recognized bodies (such as the World Gastroenterology Organization) [29], the final formulation decision remains in the hands of the physician through analysis tools that may sometimes be limited.
Two main limitations of this review are acknowledged: The restriction to studies published only in English and Spanish may hinder the discovery of pertinent information in comparable settings, such as the progression of knowledge in countries like Brazil. Additionally, since most of the documents found in the searches were narrative reviews, constructed with a special interest in describing the relationship between the microbiome and some specific pathological conditions, the level of certainty in the evidence was moderate to low.
One of this study’s strengths lies in its rigorous methodology, which involves a structured approach to locating documents in the literature and analyzing and extracting pertinent information to address the initial questions posed at the start of the review. This meticulous methodology enhances the usability of the results for healthcare professionals across various domains.
Conclusion
Although dysbiosis is often and predominantly related to diarrhea, other symptoms such as abdominal distension and pain must be considered when making a diagnosis, including predisposing conditions and patient risk factors. Probiotics have proven useful in both ameliorating symptoms and reducing the impact of an altered gut microbiota, being AAD, IBS, and childhood allergic diseases the most frequently disease stated where they are employed. The most commonly used probiotics in adults and children were Weizmannia coagulans (formerly B. coagulans), Alkalihalobacillus clausii (formerly Bacillus clausii), Lacticaseibacillus rhamnosus, Limosilactobacillus reuteri, and Saccharomyces boulardii.
The results of this review underscore the need to develop educational actions targeting patients and the general population on the importance of identifying a comprehensive clinical history and modifiable predisposing events of dysbiosis, such as diet imbalance and high stress levels. This is crucial given the substantial evidence supporting the role of diet in shaping the composition, structure, and functional activity of the gut microbiota. Such education efforts should particularly emphasize the gastrointestinal well-being of children during the critical window of microbiota development.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Editorial support was provided by Julio Ricardo Zuluaga Peña, from Odds Epidemiology SAS.
Author Contribution
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Isabel Cristina Casas and Julio Ricardo Zuluaga Peña. The first draft of the manuscript was written by Isabel Cristina Casas and Julio Ricardo Zuluaga Peña and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript. The idea of the article, literature search, data analysis, draft and revision of the work was performed by all authors.
Funding
This study was funded by Sanofi.
Data Availability
No datasets were generated or analysed during the current study.
Declarations
Ethical Approval
Not applicable.
Competing Interests
MCC is currently an employee of Sanofi and may hold shares and/or stock options in the company.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author Submission Declaration
The authors formally declare that the content of this paper is the original work of themselves. It has not been published previously in any media including journals, conferences, or websites. It is not being reviewed by any editorial office of publishers. All cited materials have been properly credited with citations in the contexts and the “References” section.
References
- 1.Meng X, Zhang G, Cao H, Yu D, Fang X, de Vos WM, et al. (2020) Gut dysbacteriosis and intestinal disease: mechanism and treatment. J Appl Microbiol [Internet]. 2020 Oct 1 [cited 2024 Aug 11];129(4):787–805. Available from: https://pubmed-ncbi-nlm-nih-gov.udea.lookproxy.com/32277534/ [DOI] [PMC free article] [PubMed]
- 2.Thursby E, Juge N (2017) Introduction to the human gut microbiota. Biochem J [Internet]. 2017 Jun 1 [cited 2024 Aug 11];474(11):1823–36. Available from: https://pubmed-ncbi-nlm-nih-gov.udea.lookproxy.com/28512250/ [DOI] [PMC free article] [PubMed]
- 3.Lin TY, Wu PH, Lin YT, Hung SC (2021) Gut dysbiosis and mortality in hemodialysis patients. NPJ Biofilms Microbiomes [Internet]. 2021 Dec 1 [cited 2024 Aug 11];7(1). Available from: https://pubmed-ncbi-nlm-nih-gov.udea.lookproxy.com/33658514/ [DOI] [PMC free article] [PubMed]
- 4.Bidell MR, Hobbs ALV, Lodise TP(2022) Gut microbiome health and dysbiosis: a clinical primer. Pharmacotherapy [Internet]. 2022 Nov 1 [cited 2024 Aug 11];42(11):849–57. Available from: https://pubmed-ncbi-nlm-nih-gov.udea.lookproxy.com/36168753/ [DOI] [PMC free article] [PubMed]
- 5.Luca M, Chattipakorn SC, Sriwichaiin S, Luca A (2020) Cognitive-behavioural correlates of dysbiosis: a review. Int J Mol Sci [Internet]. 2020 Jul 2 [cited 2024 Aug 11];21(14):1–14. Available from: https://pubmed-ncbi-nlm-nih-gov.udea.lookproxy.com/32650553/ [DOI] [PMC free article] [PubMed]
- 6.Yoo JY, Groer M, Dutra SVO, Sarkar A, McSkimming DI (2020) Gut microbiota and immune system interactions. Microorganisms [Internet]. 2020 Oct 1 [cited 2024 Aug 11];8(10):1–22. Available from: https://pubmed-ncbi-nlm-nih-gov.udea.lookproxy.com/33076307/ [DOI] [PMC free article] [PubMed]
- 7.Zhao M, Chu J, Feng S, Guo C, Xue B, He K, et al. (2023) Immunological mechanisms of inflammatory diseases caused by gut microbiota dysbiosis: a review. Biomed Pharmacother [Internet]. 2023 Aug 1 [cited 2024 Aug 11];164. Available from: https://pubmed-ncbi-nlm-nih-gov.udea.lookproxy.com/37311282/ [DOI] [PubMed]
- 8.Di Stefano M, Santonocito S, Polizzi A, Mauceri R, Troiano G, Lo Giudice A, et al. (2023) A reciprocal link between oral, gut microbiota during periodontitis: the potential role of probiotics in reducing dysbiosis-induced inflammation. Int J Mol Sci [Internet]. 2023 Jan 1 [cited 2024 Aug 11];24(2). Available from: https://pubmed-ncbi-nlm-nih-gov.udea.lookproxy.com/36674600/ [DOI] [PMC free article] [PubMed]
- 9.Arksey H, O’Malley L (2005) Scoping studies: towards a methodological framework. Int J Soc Res Methodol [Internet]. 2005 Feb [cited 2024 Aug 11];8(1):19–32. Available from: https://www.tandfonline.com/doi/abs/10.1080/1364557032000119616
- 10.Aromataris E, Fernandez R, Godfrey CM, Holly C, Khalil H, Tungpunkom P (2015) Summarizing systematic reviews: methodological development, conduct and reporting of an umbrella review approach. Int J Evid Based Healthc [Internet]. 2015 Sep 1 [cited 2024 Aug 11];13(3):132–40. Available from: https://pubmed-ncbi-nlm-nih-gov.udea.lookproxy.com/26360830/ [DOI] [PubMed]
- 11.Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K, Sfetcu R, et al. (2017) Chapter 7: Systematic reviews of etiology and risk. Joanna Briggs Institute reviewer’s manual The Joanna Briggs Institute 5:217–69
- 12.Baethge C, Goldbeck-Wood S, Mertens S (2019) SANRA-a scale for the quality assessment of narrative review articles. Res Integr Peer Rev [Internet]. 2019 Dec [cited 2024 Aug 11];4(1). Available from: https://pubmed-ncbi-nlm-nih-gov.udea.lookproxy.com/30962953/ [DOI] [PMC free article] [PubMed]
- 13.Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. (2018) PRISMA Extension for Scoping Reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med [Internet]. 2018 Oct 2 [cited 2024 Aug 11];169(7):467–73. Available from: https://pubmed-ncbi-nlm-nih-gov.udea.lookproxy.com/30178033/ [DOI] [PubMed]
- 14.Perez NB, Dorsen C, Squires A (2019) Dysbiosis of the gut microbiome: a concept analysis. J Holist Nurs [Internet]. 2020 Jun 1 [cited 2024 Aug 11];38(2):223–32. Available from: https://pubmed-ncbi-nlm-nih-gov.udea.lookproxy.com/31603019/ [DOI] [PubMed]
- 15.Wei S, Bahl MI, Baunwall SMD, Hvas CL, Licht TR (2021) Determining gut microbial dysbiosis: a review of applied indexes for assessment of intestinal microbiota imbalances. Appl Environ Microbiol [Internet]. 2021 Jun 1 [cited 2024 Aug 11];87(11):1–13. Available from: https://pubmed-ncbi-nlm-nih-gov.udea.lookproxy.com/33741632/ [DOI] [PMC free article] [PubMed]
- 16.Wainwright M, Zahroh RI, Tunçalp Ö, Booth A, Bohren MA, Noyes J, et al. (2023) The use of GRADE-CERQual in qualitative evidence synthesis: an evaluation of fidelity and reporting. Health Res Policy Syst [Internet]. 2023 Dec 1 [cited 2024 Aug 11];21(1). Available from: https://pubmed-ncbi-nlm-nih-gov.udea.lookproxy.com/37491226/ [DOI] [PMC free article] [PubMed]
- 17.Cândido TLN, Bressan J, Alfenas R de CG (2018) Dysbiosis and metabolic endotoxemia induced by high-fat diet. Nutr Hosp [Internet]. 2018 Nov 1 [cited 2024 Aug 11];35(6):1432–40. Available from: https://pubmed-ncbi-nlm-nih-gov.udea.lookproxy.com/30525859/ [DOI] [PubMed]
- 18.Fjeldheim Dale H, Arslan Lied G (2020) Gut microbiota and therapeutic approaches for dysbiosis in irritable bowel syndrome: recent developments and future perspectives. Turk J Med Sci [Internet]. 2020 [cited 2024 Aug 11];50(SI-2):1632–41. Available from: https://pubmed-ncbi-nlm-nih-gov.udea.lookproxy.com/32222124/ [DOI] [PMC free article] [PubMed]
- 19.Haworth JJ, Boyle N, Vales A, Hobson AR (2021) The prevalence of intestinal dysbiosis in patients referred for antireflux surgery. Surg Endosc [Internet]. 2021 Dec 1 [cited 2024 Aug 11];35(12):7112–9. Available from: https://pubmed-ncbi-nlm-nih-gov.udea.lookproxy.com/33475845/ [DOI] [PMC free article] [PubMed]
- 20.Tziatzios G, Gkolfakis P, Papanikolaou IS, Mathur R, Pimentel M, Giamarellos-bourboulis EJ, et al. (2020) Gut microbiota dysbiosis in functional dyspepsia. Microorganisms [Internet]. 2020 May 1 [cited 2024 Aug 11];8(5). Available from: https://pubmed-ncbi-nlm-nih-gov.udea.lookproxy.com/32397332/ [DOI] [PMC free article] [PubMed]
- 21.Saffouri GB, Shields-Cutler RR, Chen J, Yang Y, Lekatz HR, Hale VL, et al. (2019) Small intestinal microbial dysbiosis underlies symptoms associated with functional gastrointestinal disorders. Nat Commun [Internet]. 2019 Dec 1 [cited 2024 Aug 11];10(1). Available from: https://pubmed-ncbi-nlm-nih-gov.udea.lookproxy.com/31043597/ [DOI] [PMC free article] [PubMed]
- 22.Rhoads JM, Collins J, Fatheree NY, Hashmi SS, Taylor CM, Luo M, et al. (2018) Infant colic represents gut inflammation and dysbiosis. J Pediatr [Internet]. 2018 Dec 1 [cited 2024 Aug 11];203:55–61.e3. Available from: https://pubmed-ncbi-nlm-nih-gov.udea.lookproxy.com/30177353/ [DOI] [PMC free article] [PubMed]
- 23.Reis AC, da Carvalho GS, da Sousa NLS, dos Santos GM, de Sousa PVL, dos Barros NVA (2022) Sinais e sintomas sugestivos de disbiose intestinal na população brasileira: uma revisão de literatura. Res Soc Dev 11(9):e56111932094 [Google Scholar]
- 24.Álvarez J, Fernández Real JM, Guarner F, Gueimonde M, Rodríguez JM, Saenz de Pipaon M, et al. (2021) Gut microbes and health. Gastroenterol Hepatol [Internet]. 2021 Aug 1 [cited 2024 Aug 11];44(7):519–35. Available from: https://pubmed-ncbi-nlm-nih-gov.udea.lookproxy.com/33652061/ [DOI] [PubMed]
- 25.Riddle MS, Connor BA (2016) The traveling microbiome. Curr Infect Dis Rep [Internet]. 2016 Sep 1 [cited 2024 Aug 11];18(9). Available from: https://pubmed-ncbi-nlm-nih-gov.udea.lookproxy.com/27447891/ [DOI] [PubMed]
- 26.Álvarez-Calatayud G, Guarner F, Requena T, Marcos A (2018) Diet and microbiota. Impact on health. Nutr Hosp [Internet]. 2018 Sep 1 [cited 2024 Aug 11];35(Spec No6):11–5. Available from: https://pubmed-ncbi-nlm-nih-gov.udea.lookproxy.com/30351154/ [DOI] [PubMed]
- 27.Gomaa EZ (2020) Human gut microbiota/microbiome in health and diseases: a review. Antonie Van Leeuwenhoek [Internet]. 2020 Dec 1 [cited 2024 Aug 11];113(12):2019–40. Available from: https://pubmed-ncbi-nlm-nih-gov.udea.lookproxy.com/33136284/ [DOI] [PubMed]
- 28.ILSI Europe (2024) Probiotics, prebiotics and the gut microbiota [Internet]. 2024 [cited 2024 Aug 11]. Available from: https://ilsi.eu/publication/probiotics-prebiotics-and-the-gut-microbiota/
- 29.World Gastroenterology Organisation Global Guidelines (2023) Probiotics and prebiotics [Internet]. J Clin Gastroenterol 42. Available from: https://journals.lww.com/00004836-200809001-00006 [DOI] [PubMed]
- 30.Magne F, Gotteland M, Gauthier L, Zazueta A, Pesoa S, Navarrete P, et al. (2020) The Firmicutes/Bacteroidetes ratio: a relevant marker of gut dysbiosis in obese patients? Nutrients [Internet]. 2020 May 1 [cited 2024 Aug 11];12(5). Available from: https://pubmed-ncbi-nlm-nih-gov.udea.lookproxy.com/32438689/ [DOI] [PMC free article] [PubMed]
- 31.Sekirov I, Russell SL, Caetano M Antunes L, Finlay BB(2010) Gut microbiota in health and disease. Physiol Rev [Internet]. 2010 Jul [cited 2024 Aug 11];90(3):859–904. Available from: https://pubmed-ncbi-nlm-nih-gov.udea.lookproxy.com/20664075/ [DOI] [PubMed]
- 32.Xu R, Tan C, Zhu J, Zeng X, Gao X, Wu Q, et al. (2019) Dysbiosis of the intestinal microbiota in neurocritically ill patients and the risk for death. Crit Care [Internet]. 2019 May 31 [cited 2024 Aug 11];23(1). Available from: https://pubmed-ncbi-nlm-nih-gov.udea.lookproxy.com/31151471/ [DOI] [PMC free article] [PubMed]
- 33.Hughes HK, Rose D, Ashwood P (2018) The gut microbiota and dysbiosis in autism spectrum disorders. Curr Neurol Neurosci Rep [Internet]. 2018 Nov 1 [cited 2024 Aug 11];18(11). Available from: https://pubmed-ncbi-nlm-nih-gov.udea.lookproxy.com/30251184/ [DOI] [PMC free article] [PubMed]
- 34.Ghoshal UC, Shukla R, Ghoshal U (2017) Small intestinal bacterial overgrowth and irritable bowel syndrome: a bridge between functional organic dichotomy. Gut Liver [Internet]. 2017 Mar 1 [cited 2024 Aug 11];11(2):196–208. Available from: https://pubmed-ncbi-nlm-nih-gov.udea.lookproxy.com/28274108/ [DOI] [PMC free article] [PubMed]
- 35.Caparrós E, Wiest R, Scharl M, Rogler G, Gutiérrez Casbas A, Yilmaz B, et al. (2021) Dysbiotic microbiota interactions in Crohn’s disease. Gut Microbes [Internet]. 2021 [cited 2024 Aug 11];13(1). Available from: https://pubmed-ncbi-nlm-nih-gov.udea.lookproxy.com/34313550/ [DOI] [PMC free article] [PubMed]
- 36.Zhou Z, Sun B, Yu D, Zhu C (2022) Gut microbiota: an important player in type 2 diabetes mellitus. Front Cell Infect Microbiol [Internet]. 2022 Feb 15 [cited 2024 Aug 11];12. Available from: https://pubmed-ncbi-nlm-nih-gov.udea.lookproxy.com/35242721/ [DOI] [PMC free article] [PubMed]
- 37.Liu S, Gao J, Zhu M, Liu K, Zhang HL (2020) Gut microbiota and dysbiosis in Alzheimer’s disease: implications for pathogenesis and treatment. Mol Neurobiol [Internet]. 2020 Dec 1 [cited 2024 Aug 11];57(12):5026–43. Available from: https://pubmed-ncbi-nlm-nih-gov.udea.lookproxy.com/32829453/ [DOI] [PMC free article] [PubMed]
- 38.Blaabjerg S, Artzi DM, Aabenhus R (2017) Probiotics for the prevention of antibiotic-associated diarrhea in outpatients-a systematic review and meta-analysis. Antibiotics (Basel) [Internet]. 2017 Dec 1 [cited 2024 Aug 11];6(4). Available from: https://pubmed-ncbi-nlm-nih-gov.udea.lookproxy.com/29023420/ [DOI] [PMC free article] [PubMed]
- 39.Kim SK, Guevarra RB, Kim YT, Kwon J, Kim H, Cho JH, et al. (2019) Role of probiotics in human gut microbiome-associated diseases. J Microbiol Biotechnol [Internet]. 2019 [cited 2024 Aug 11];29(9):1335–40. Available from: https://pubmed-ncbi-nlm-nih-gov.udea.lookproxy.com/31434172/ [DOI] [PubMed]
- 40.Kesavelu D, Jog P (2023) Current understanding of antibiotic-associated dysbiosis and approaches for its management. Ther Adv Infect Dis [Internet]. 2023 Jan 1 [cited 2024 Aug 11];10. Available from: https://pubmed-ncbi-nlm-nih-gov.udea.lookproxy.com/36860273/ [DOI] [PMC free article] [PubMed]
- 41.Zhang T, Zhang C, Zhang J, Sun F, Duan L (2022) Efficacy of probiotics for irritable bowel syndrome: a systematic review and network meta-analysis. Front Cell Infect Microbiol [Internet]. 2022 Apr 1 [cited 2024 Aug 11];12. Available from: https://pubmed-ncbi-nlm-nih-gov.udea.lookproxy.com/35433498/ [DOI] [PMC free article] [PubMed]
- 42.Dale HF, Rasmussen SH, Asiller ÖÖ, Lied GA (2019) Probiotics in irritable bowel syndrome: an up-to-date systematic review. Nutrients [Internet]. 2019 Sep 1 [cited 2024 Aug 11];11(9). Available from: https://pubmed-ncbi-nlm-nih-gov.udea.lookproxy.com/31480656/ [DOI] [PMC free article] [PubMed]
- 43.Limketkai BN, Akobeng AK, Gordon M, Adepoju AA (2020) Probiotics for induction of remission in Crohn’s disease. Cochrane Database Syst Rev [Internet]. 2020 Jul 17 [cited 2024 Aug 11];7(7). Available from: https://pubmed-ncbi-nlm-nih-gov.udea.lookproxy.com/32678465/ [DOI] [PMC free article] [PubMed]
- 44.Eslami M, Yousefi B, Kokhaei P, Hemati M, Nejad ZR, Arabkari V, et al. (2019) Importance of probiotics in the prevention and treatment of colorectal cancer. J Cell Physiol [Internet]. 2019 Oct 1 [cited 2024 Aug 11];234(10):17127–43. Available from: https://pubmed-ncbi-nlm-nih-gov.udea.lookproxy.com/30912128/ [DOI] [PubMed]
- 45.Molska M, Reguła J (2019) Potential mechanisms of probiotics action in the prevention and treatment of colorectal cancer. Nutrients [Internet]. 2019 Oct 1 [cited 2024 Aug 11];11(10). Available from: https://pubmed-ncbi-nlm-nih-gov.udea.lookproxy.com/31615096/ [DOI] [PMC free article] [PubMed]
- 46.Szajewska H, Berni Canani R, Domellöf M, Guarino A, Hojsak I, Indrio F, et al. (2023) Probiotics for the management of pediatric gastrointestinal disorders: position paper of the ESPGHAN Special Interest Group on Gut Microbiota and Modifications. J Pediatr Gastroenterol Nutr [Internet]. 2023 Feb 1 [cited 2024 Aug 11];76(2):232–47. Available from: https://pubmed-ncbi-nlm-nih-gov.udea.lookproxy.com/36219218/ [DOI] [PubMed]
- 47.Simon E, Călinoiu LF, Mitrea L, Vodnar DC (2021) Probiotics, prebiotics, and synbiotics: implications and beneficial effects against irritable bowel syndrome. Nutrients [Internet]. 2021 Jun 1 [cited 2024 Aug 11];13(6). Available from: https://pubmed-ncbi-nlm-nih-gov.udea.lookproxy.com/34203002/ [DOI] [PMC free article] [PubMed]
- 48.Gade AK, Douthit NT, Townsley E (2020) Medical management of Crohn’s disease. Cureus [Internet]. 2020 May 29 [cited 2024 Aug 11];12(5). Available from: https://pubmed-ncbi-nlm-nih-gov.udea.lookproxy.com/32617224/ [DOI] [PMC free article] [PubMed]
- 49.Yachha S, Sarma M, Mohan N, Wadhwa N, Vt N, Srinivasan R, et al. (2022) Indian Academy of Pediatrics consensus guidelines for probiotic use in childhood diarrhea. Indian Pediatr [Internet]. 2022 Mar [cited 2024 Aug 11];59(7):543–51. Available from: https://pubmed-ncbi-nlm-nih-gov.udea.lookproxy.com/35273131/ [PubMed]
- 50.Ronan V, Yeasin R, Claud EC (2021) Childhood development and the microbiome-the intestinal microbiota in maintenance of health and development of disease during childhood development. Gastroenterology [Internet]. 2021 Jan 1 [cited 2024 Aug 11];160(2):495–506. Available from: https://pubmed-ncbi-nlm-nih-gov.udea.lookproxy.com/33307032/ [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.